Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(5); 2014 > Article
-
Case Study
Alveolar Soft Part Sarcoma of the Uterine Cervix: A Case Report and Review of the Literature - Hyun Ju Lee
-
Korean Journal of Pathology 2014;48(5):361-365.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.5.361
Published online: October 27, 2014
Department of Pathology, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea
- Corresponding Author: Hyun Ju Lee, M.D. Department of Pathology, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, 31 Suncheonhyang 6-gil, Dongnam-gu, Cheonan 330-721, Korea Tel: +82-41-570-3589 Fax: +82-41-570-3580 E-mail: c84103@schmc.ac.kr
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- A rare case of Alveolar Soft-Part Sarcoma in the Uterine cervix
Mei Du, Yanli Li, Xiaorong Fan, Han Gao, Jie Shi, Shiyu Cheng, Tingzhu Meng
Diagnostic Pathology.2025;[Epub] CrossRef - Primary Uterine Alveolar Soft Part Sarcoma in a Postmenopausal Woman: Histopathologic and Immunohistochemical Characteristics of a Rare Case
Anjali Gupta, Parikshaa Gupta, Amarjot Kaur, Snigdha Kumari, Gupta Nalini, Shalini Gainder
International Journal of Surgical Pathology.2024; 32(6): 1165. CrossRef - Alveolar Soft Part Sarcoma in the Female Genital Tract: Case Series with Literature Review and SEER Database Analysis
Xingtao Long, Qingming Jiang, Rengui Li, Dong Wang, Dongling Zou
International Journal of Women's Health.2024; Volume 16: 17. CrossRef - Alveolar soft part sarcoma: a clinicopathological and immunohistochemical analysis of 26 cases emphasizing risk factors and prognosis
Yi Zhang, Yuchen Huang, Yanzi Qin, Ningning Yang, Panpan Yang, Nan Li, Zhenzhong Feng
Diagnostic Pathology.2024;[Epub] CrossRef - Epithelioid and clear‐cell variant of Kaposi sarcoma: A rare histopathologic subtype
Kaitlyn M. Yim, Tom Liang, Esteban Gnass, Brittney DeClerck
Journal of Cutaneous Pathology.2022; 49(4): 381. CrossRef - A Case of TFE3-positive Non-neoplastic Pseudodecidualized Endometrium Presenting as a Cervical Mass
Serenella Serinelli, Dana Hariri, Gustavo de la Roza, Daniel J. Zaccarini
Applied Immunohistochemistry & Molecular Morphology.2022; 30(6): e50. CrossRef - Alveolar Soft Part Sarcoma of the Uterus: Clinicopathological and Molecular Characteristics
Yurimi Lee, Kiyong Na, Ha Young Woo, Hyun-Soo Kim
Diagnostics.2022; 12(5): 1102. CrossRef - Alveolar soft part sarcoma in childhood and adolescence: Report of three cases and review of literature
Yudi Zhang, Ying Wang, Hao Wang, Chuan Wen, Xiaochuan Wu
Frontiers in Pediatrics.2022;[Epub] CrossRef - Exploring the Histogenesis and Diagnostic Strategy Using Immunoassay and RT-PCR in Alveolar Soft Part Sarcoma
Xinxin Ju, Kunming Sun, Ruixue Liu, Shugang Li, Gulinaer Abulajiang, Hong Zou, Jiaojiao Lan, Yan Ren, Jinfang Jiang, Weihua Liang, Lijuan Pang, Feng Li
Pathology & Oncology Research.2018; 24(3): 593. CrossRef - Alveolar Soft Part Sarcoma of the Female Genital Tract
J. Kenneth Schoolmeester, Joseph Carlson, Gary L. Keeney, Karen J. Fritchie, Esther Oliva, Robert H. Young, Marisa R. Nucci
American Journal of Surgical Pathology.2017; 41(5): 622. CrossRef - Recurrent alveolar soft part sarcoma of the uterine cervix
Aeli Ryu, Seong Taek Mun, Hyun Ju Lee, Nan-Seol Kim
Journal of Obstetrics and Gynaecology.2017; 37(8): 1099. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles
-
- Cytological characteristics of Müllerian adenosarcoma of the uterine corpus: a case report and literature review
- Metastatic choroidal melanoma in the breast: a case report and review of the literature
- Hepatic carcinoma expressing inhibin: case report of a proposed novel entity and review of the literature



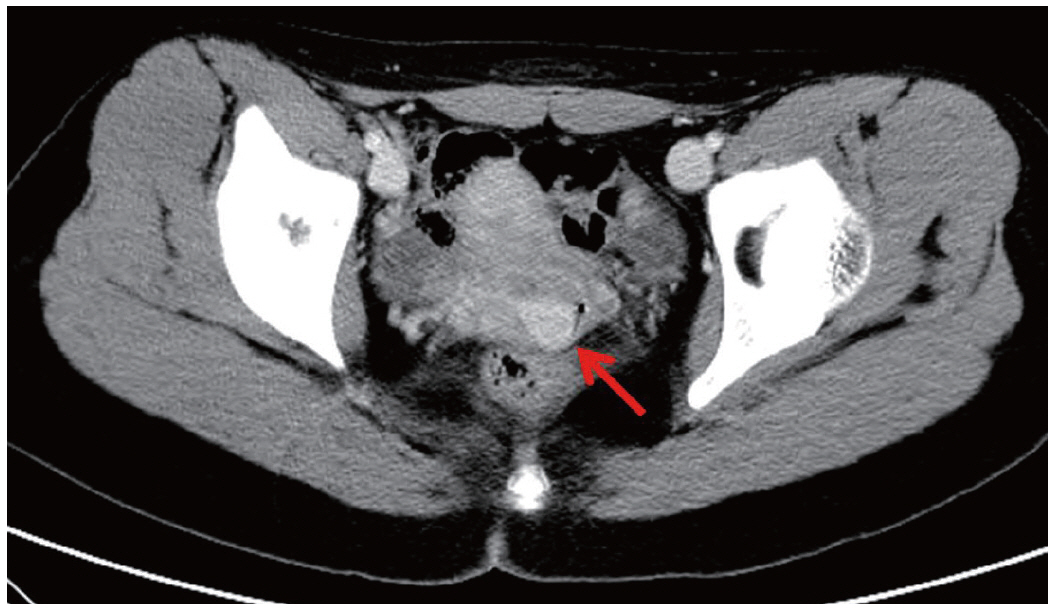
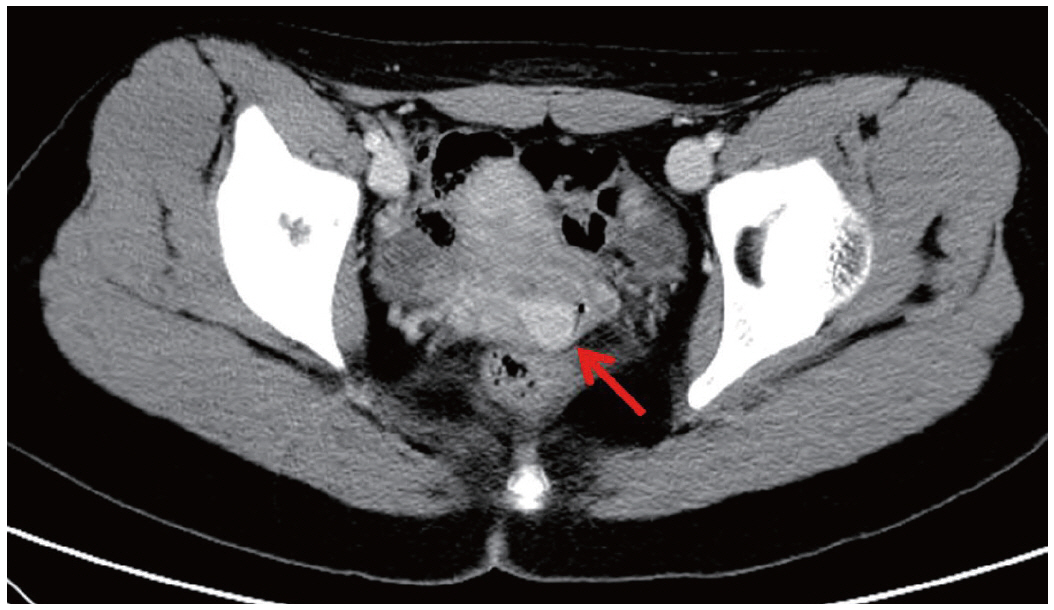
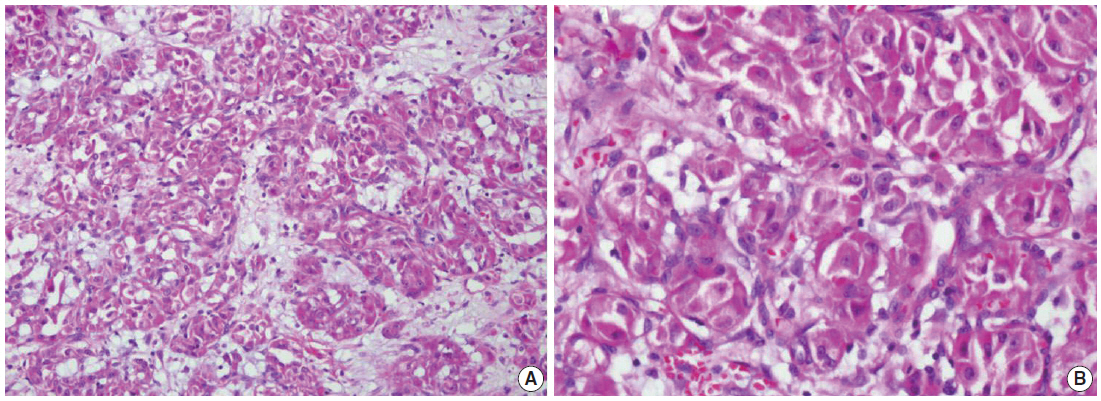
Fig. 1.
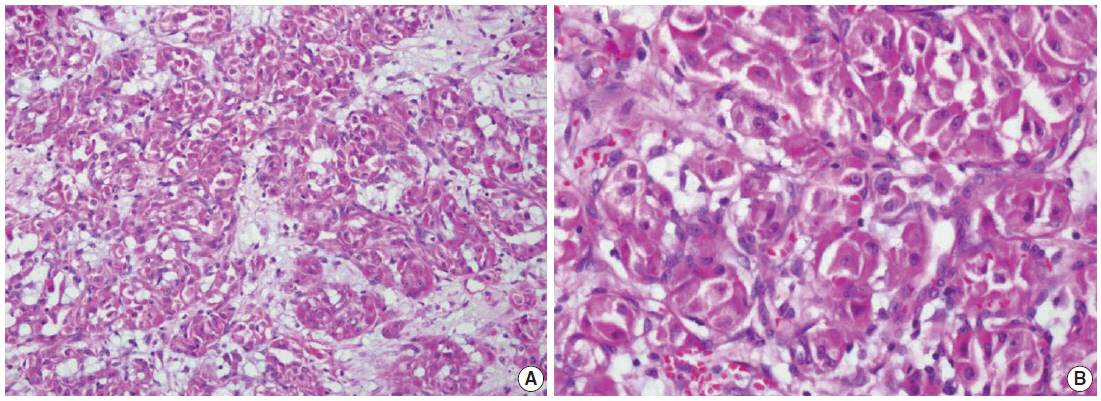
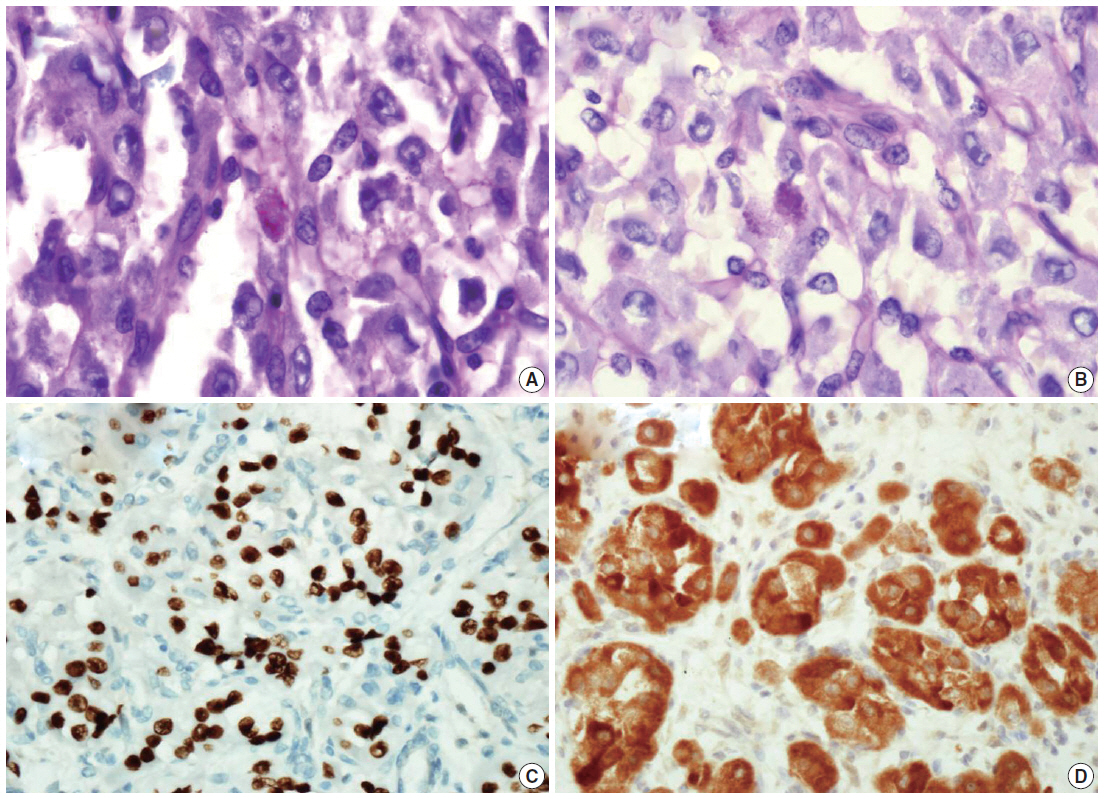
Fig. 2.
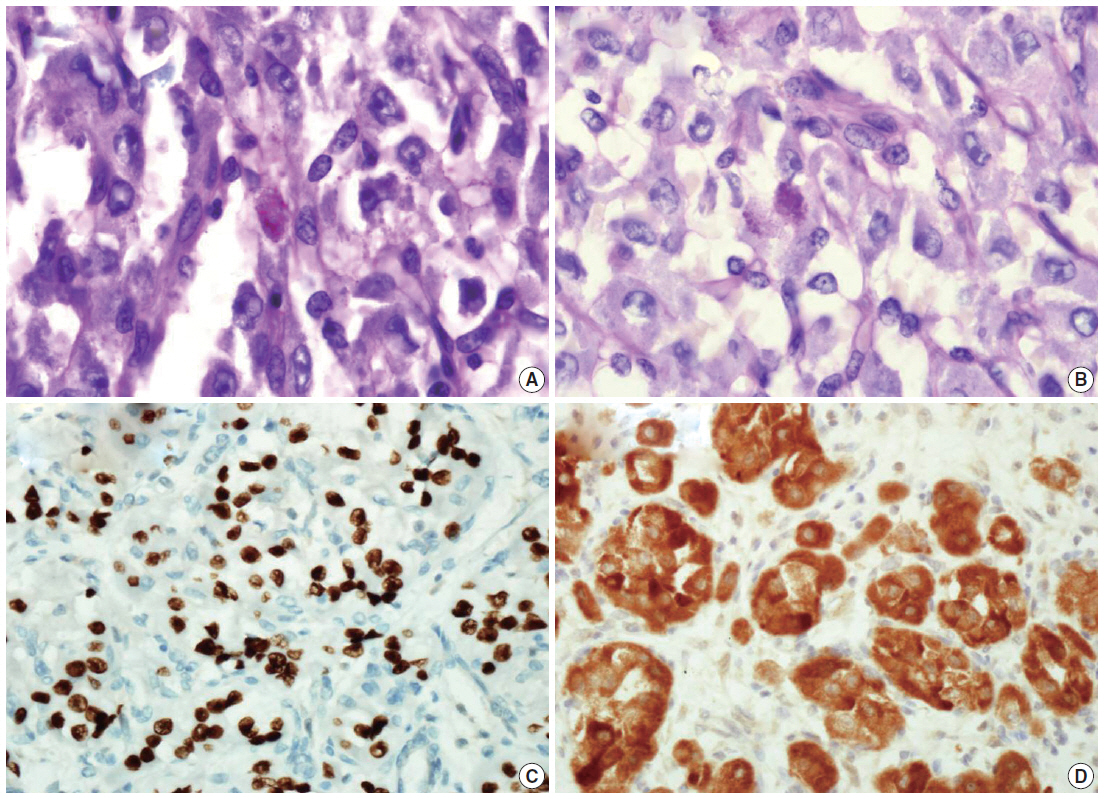
Fig. 3.
| Antibody | Staining result | Antibody | Staining result |
|---|---|---|---|
| TFE3 | P (6/6, 100) | CK | N (9/9, 100) |
| MyoD1 | P (5/5, 100) | HMWCK | N (1/1, 100) |
| Myoglobin | P (4/6, 67) | EMA | N (3/3, 100) |
| SMA | P (4/8, 50) | Syn | N (4/4, 100) |
| Desmin | P (3/9, 33) | Chromo | N (5/5, 100) |
| NSE | P (5/5, 100) | CD56 | N (2/2, 100) |
| S-100 | P (2/10, 20) | CD10 | N (3/3, 100) |
| CK8/18 | P (1/3, 33) | GFAP | N (1/1, 100) |
| VT | P (2/8, 25) | ||
| HMB45 | P (2/7, 29) | ||
| CD34 | P (1/2, 50) |
Values are presented as number, percent in parenthesis. TFE3, transcription factor E3; P, positive; CK, cytokeratin; N, negative; HMWCK, high molecular weight cytokeratin; EMA, epithelial membrane antigen; SMA, smooth muscle actin; Syn, synaptophysin; Chromo, chromogranin A; NSE, neuron-specific enolase; CK, cytokeratin; GFAP, glial fibrillary acidic protein; VT, vimentin; HMB45, human melanoma black 45.

 E-submission
E-submission