Gastrointestinal stromal tumor (GIST), which is associated with mutations in KIT or a platelet-derived growth factor receptor, alpha polypeptide (PDGFRA), is the most common mesenchymal tumor of the gastrointestinal tract[1]. A definitive diagnosis of GIST is important to ensure administration of effective drugs, such as imatinib mesylate, and immmunohistochemical staining for c-Kit or DOG1 is useful for the diagnosis. According to previous studies, cytokeratin (CK) expression is a rare event in GISTs[2-6], so they can easily be misdiagnosed as other epithelial or epithelioid mesenchymal tumors. In such cases, a diagnosis of GIST can be made when DOG1 immunoreactivity or mutation of KIT or PDGFRA are observed.
CASE REPORT
- A 44-year-old man was referred for evaluation of pain in his right foot. Laboratory test results, including electrolyte levels and blood-cell counts, were all within normal range. Computed tomographic (CT) angiography of the lower extremity showed a well circumscribed mass with central cystic changes in the pelvic cavity, measuring 7.3 cm×7.1 cm. The patient did not complain of abdominal discomfort, and he had no specific familial or medical history of GIST.
- The patient underwent a segmental resection and anastomosis of the small bowel. The mass abutted on the peritoneal surface of the small bowel, 150 cm apart from the ileocecal valve.
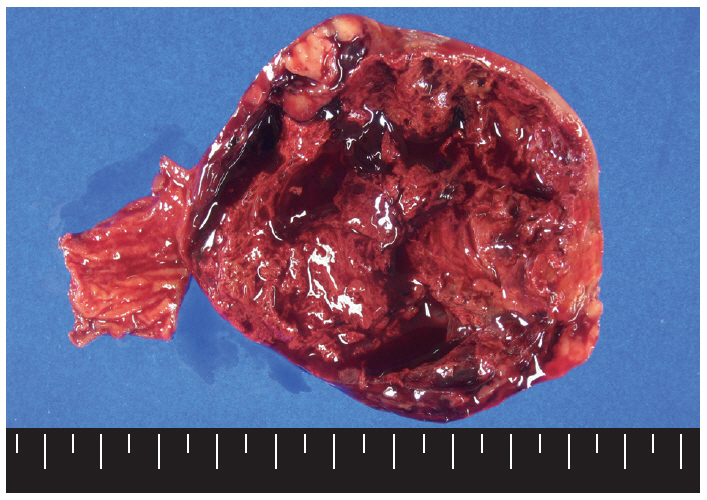
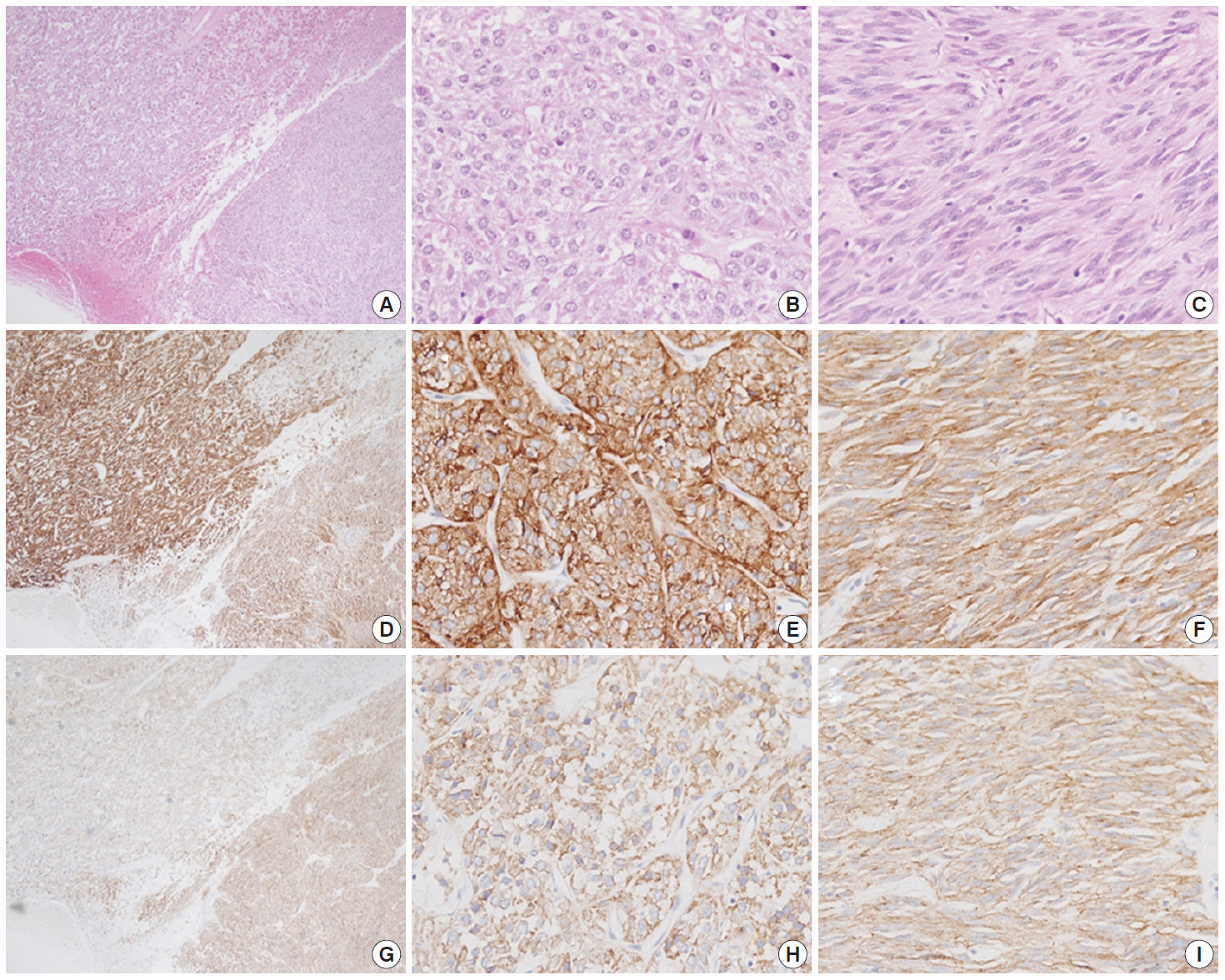
- Macroscopically, the mass was located on the serosal surface of the small bowel. It showed hemorrhage and necrosis in the center. The peripheral solid portion was tan-gray and soft in consistency. The central hemorrhagic and necrotic area was ragged in appearance (Fig. 1). Microscopically, the hemorrhagic and necrotic portion was exclusively composed of epithelioid cells. The peripheral solid portion revealed both epithelioid and spindle cells. There was no transition area between the epithelioid and spindle-cell areas (Fig. 2A). Mitotic figures were found at more than 10 per 50 high-power fields in both epithelioid and spindle-cell areas. According to the National Institutes of Health (NIH) consensus criteria[1], the lesion was graded as a high risk of aggressive behavior.
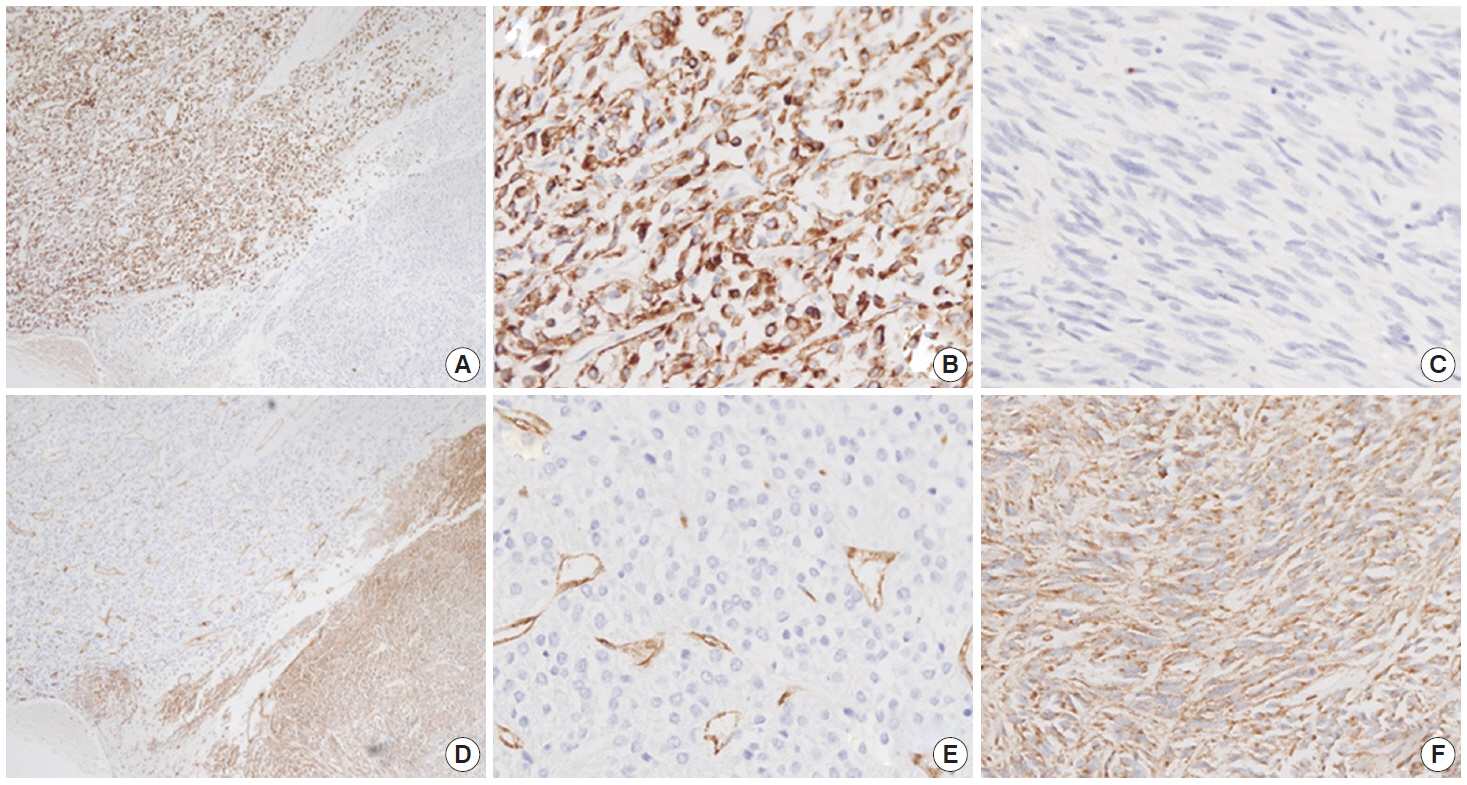
- Immunohistochemistry was performed in an autostainer (BOND-MAX, Leica Biosystems, Nussloch, Germany) using the following primary antibodies: anti-c-Kit (1:50, polyclonal, Dako, Glostrup, Denmark), anti-CD34 (1:200, QBEnd10, Dako), anti-CK (1:200, AE1/AE3, Dako), and anti-DOG1 (1:100, K9, Novocastra, Newcastle Upon Tyne, UK). The epithelioid cells were immunoreactive for CK, c-Kit, and DOG1 but not for CD34. The spindle cells were immunoreactive for c-Kit, DOG1, and CD34, but not for CK (Figs. 2, 3). There was a subtle difference in intensity of c-Kit staining between the epithelioid and spindle cells (Fig. 2D). Interestingly, the expressions of CD34 and CK were mutually exclusive between the epithelioid and spindle-cell areas. Direct sequencing of the KIT gene was performed in both sense and antisense directions in order to verify the results, using formalin-fixed, paraffin-embedded tissue from both areas and an ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Grand Island, NY, USA), following the supplier’s recommendations. Sequencing revealed mutations in exon 11 codon 557 of the KIT gene in both areas. At a two-month follow-up appointment, multiple liver nodules with low attenuation were detected by abdominal CT, which were thought to be metastatic lesions. At an 18-month follow-up appointment, abdominopelvic CT revealed no change in size or attenuation of the nodules. The patient had been treated regularly with imatinib mesylate, and there was no evidence of tumor progression at 78 months after the operation.
DISCUSSION
- CK expression in GIST is a rare occurrence that may lead to diagnostic difficulty and errors[3,4]. Some authors described misdiagnosed epithelioid lesions, such as poorly differentiated carcinomas and angiosarcomas, which were eventually diagnosed as GIST[4,6]. If spindle-cell components or epithelioid cells with unusual morphology and immunophenotype are present, other potential diagnoses should be considered, and additional ancillary tests are needed to make an accurate diagnosis. Several reports have demonstrated that GIST may have CK-positivity with variable intensities and patterns[2-6]. CK expression in GIST using monoclonal CK antibodies, such as CK18 or CK8, has been evaluated in several studies, and these studies support the conclusion that CK-immunoreactivity is more frequently noted in the epithelioid area than in the spindle-cell area (Table 1). However, another study of 687 GIST cases reported that the frequency of CK expression does not depend on cell morphology[7]. Thus, it seems that a higher rate of CK-immunoreactivity in epithelioid GIST might be due to a selection bias. In practice, we usually conduct CK immunostaining for epithelioid lesions but not for spindle-cell lesions.
- In addition to GIST, CK-positivity has been observed in some mesenchymal tumors with epithelioid morphology, such as epithelioid angiosarcoma, epithelioid sarcoma, Ewing’s sarcoma/primitive neuroectodermal tumor, leiomyosarcoma, or schwannoma. Aberrant expression of CK is known to be a result of aberrant synthesis of CK by tumor cells or cross-reactivity to other intermediate filament proteins[8].
- In a study of biphasic (mixed pattern) GIST, CD34 immunoreactivity was shown to be weaker in the cellular and mitotically active epithelioid areas than in the spindle-cell areas[9]. Metastatic nodules of GIST tend to reveal epithelioid histomorphology with a higher mitotic index, whereas the primary lesions tend to have spindle-cell features with lower mitotic activities. Another study of a precursor lesion of sporadic GIST demonstrated that all minute GIST cases had spindle-cell patterns[10]. In the present case, the tumor was predominantly composed of epithelioid cells rather than spindle cells, and showed similar mitotic activity in both epithelioid and spindle-cell areas. Despite the lack of immunoreactivity for CD34 in the epithelioid area, there was no evidence that the epithelioid area implicates an advanced lesion.
- We presented a case of GIST with a biphasic (mixed epithelioid and spindle cells) phenotype and distinct immunohistochemical profiles. CK immunoreactivity in the epithelioid area can be a diagnostic challenge unless tumor cells reveal immunoreactivity for c-Kit and DOG1.
No potential conflict of interest relevant to this article was reported.
Fig. 1.The cut surface of the tumor shows a well circumscribed mass with extensive hemorrhagic necrosis at the center. Solid areas (tan-gray colored regions) are noted at the periphery.
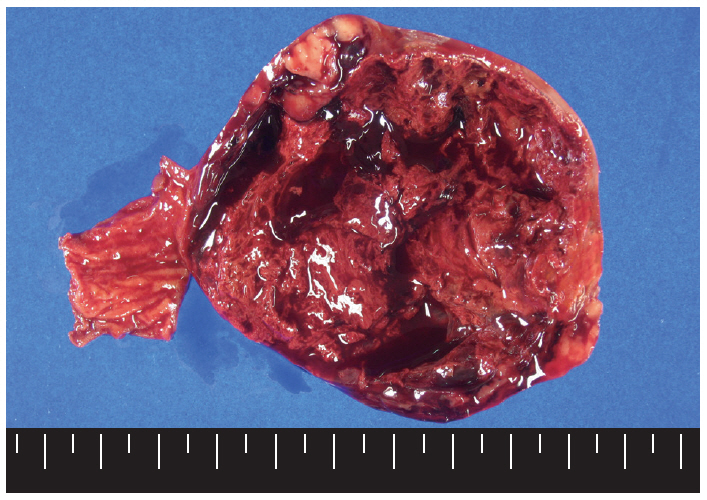
Fig. 2.A low-power view of the tumor shows an epithelioid area in the left upper portion and a spindle-cell area in the right lower portion (A, D, G). Both epithelioid (B, E, H) and spindle cell areas (C, F, I) are diffusely positive for c-Kit (D–F) and DOG1 (G–I).
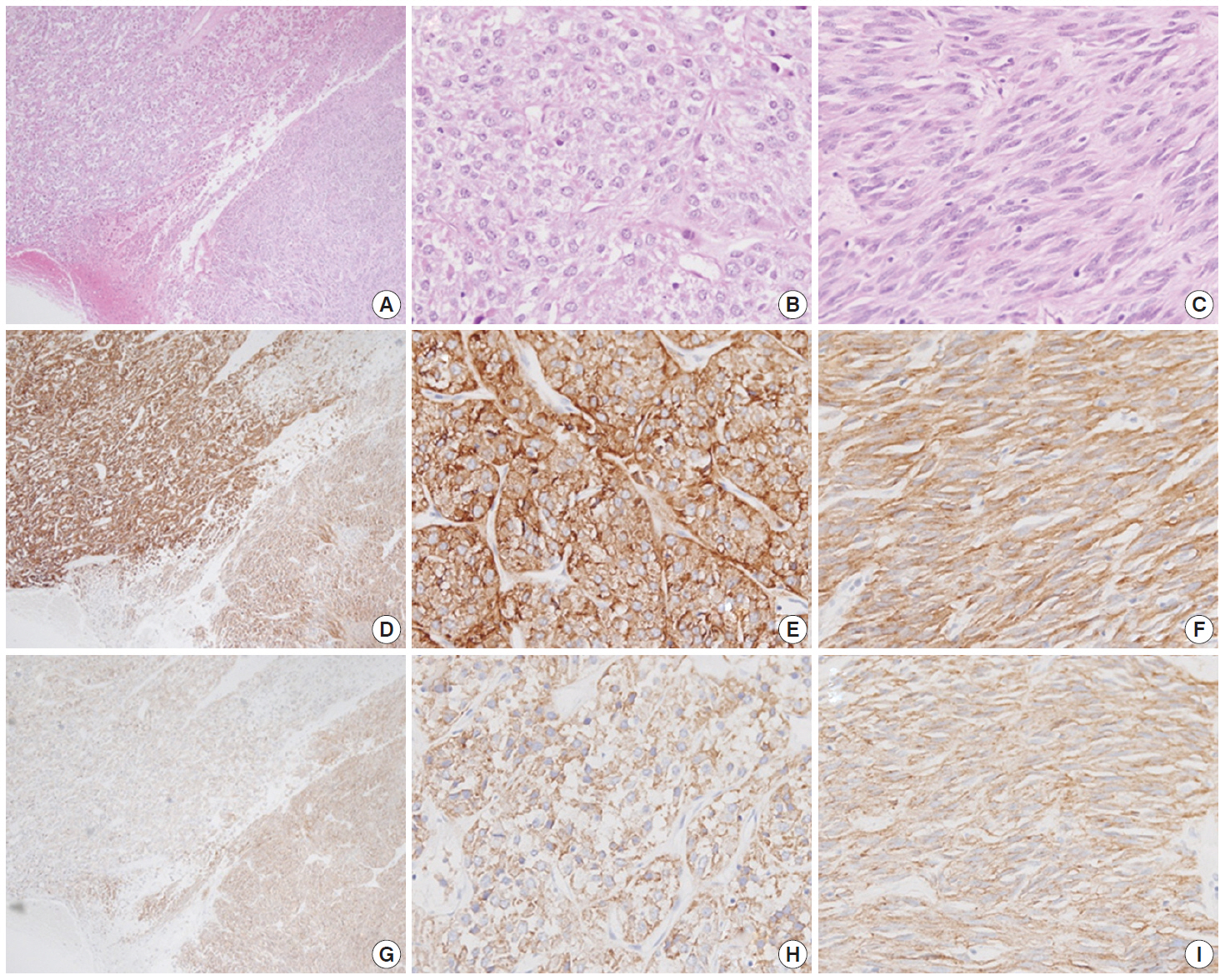
Fig. 3.The epithelioid lesion is immunopositive for CK (A, B) and immunonegative for CD34 (D, E). The spindle cell area is immunoreactive for CD34 (D, F) and immunonegative for CK (A, C).
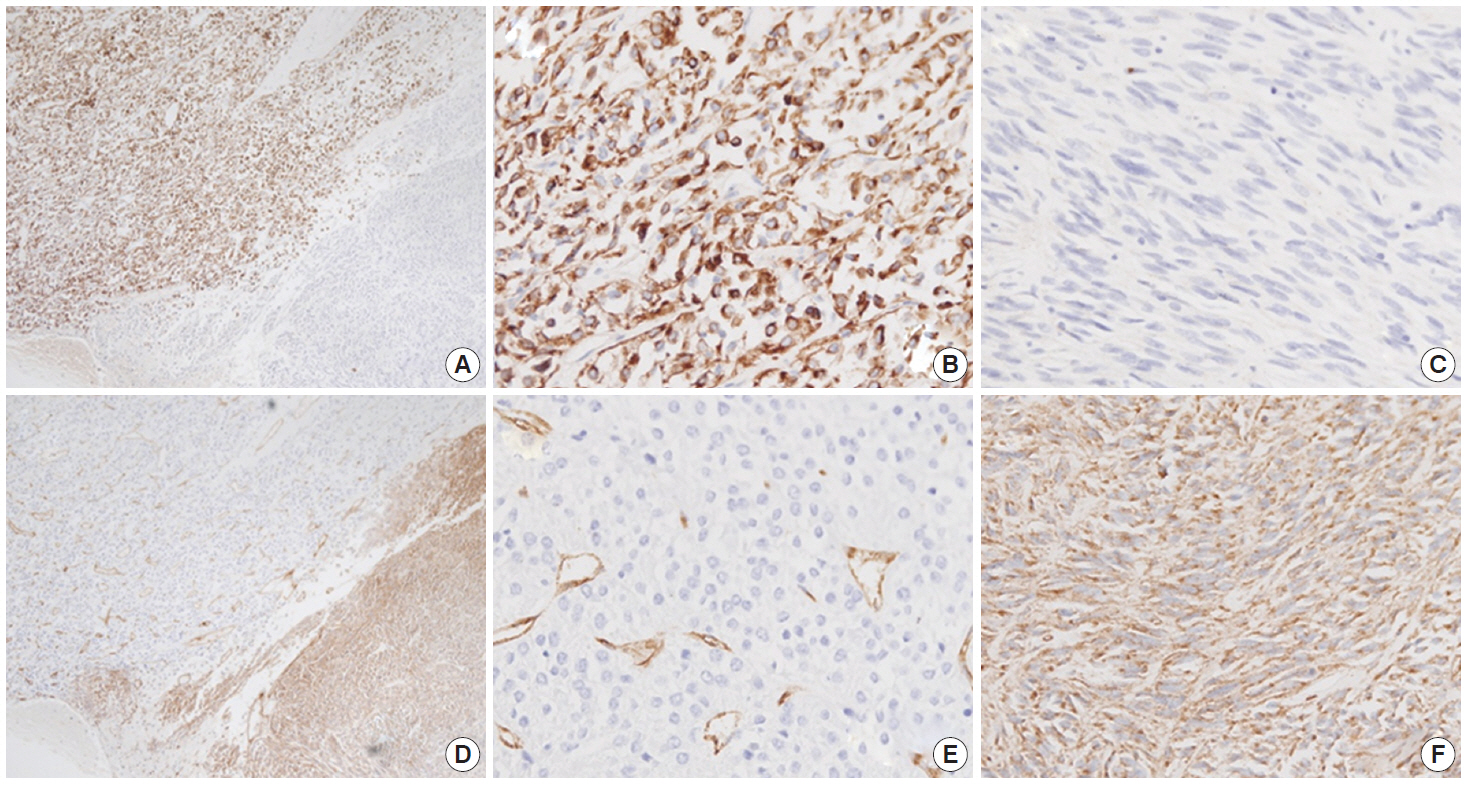
Table 1.Cytokeratin (CK) expression in a gastrointestinal stromal tumor: a literature review of single-case studies
|
Reference |
Age (yr) |
Primary site |
Histomorphology |
CK expression |
Antibodies |
Other IHC |
|
Lippai et al.[2]. |
45 |
Stomach |
Anaplastic epithelioid |
Diffuse positive |
AE1/AE3 |
CD34 (+), c-Kit (+) |
|
Laforga[3] |
57 |
Small intestine |
Epithelioid |
Focal positive |
AE1/AE3 |
c-Kit (+), CD34 (–) |
|
Rossi et al.[4]. |
32 |
Stomach |
Epithelioid cells |
Positive |
AE1/AE3 |
CAM5.2 (+), HMCK (–), CD34 (+), c-Kit (+) |
|
Nga et al.[5]. |
69 |
Stomach |
Elongated cells |
Perinuclear dot-like positivity |
CAM 5.2 |
EMA (–), AE1/AE3 (–), CD34 (+), c-Kit (+) |
|
Mourra and Dehni[6] |
65 |
Stomach |
Poorly cohesive cells and spindle cells (deep area) |
Positive in poorly cohesive cells |
KL1 |
CD34 (+), c-Kit (+) |
REFERENCES
- 1. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006; 23: 70-83. ArticlePubMed
- 2. Lippai N, Fule T, Németh T, et al. Keratin-positive gastrointestinal stromal tumor of the stomach mimicking gastric carcinoma: diagnosis confirmed by c-kit mutation analysis. Diagn Mol Pathol 2008; 17: 241-4. ArticlePubMed
- 3. Laforga JB. Malignant epithelioid gastrointestinal stromal tumors: report of a case with cytologic and immunohistochemical studies. Acta Cytol 2005; 49: 435-40. PubMed
- 4. Rossi G, Sartori G, Valli R, et al. The value of c-kit mutational analysis in a cytokeratin positive gastrointestinal stromal tumour. J Clin Pathol 2005; 58: 991-3. ArticlePubMedPMC
- 5. Nga ME, Wong AS, Wee A, Salto-Tellez M. Cytokeratin expression in gastrointestinal stromal tumours: a word of caution. Histopathology 2002; 40: 480-1. PubMed
- 6. Mourra N, Dehni N. Cytokeratin expression in GIST: a diagnostic pitfall in gastric biopsy. Appl Immunohistochem Mol Morphol 2010; 18: 486-8. Article
- 7. Lopes LF, Bacchi CE. Cytokeratin expression in gastrointestinal stromal tumor: a clinicopathologic and immunohistochemical study of 687 cases. Appl Immunohistochem Mol Morphol 2012; 20: 8-12. PubMed
- 8. Fanburg-Smith JC, Majidi M, Miettinen M. Keratin expression in schwannoma: a study of 115 retroperitoneal and 22 peripheral schwannomas. Mod Pathol 2006; 19: 115-21. ArticlePubMedPDF
- 9. Agaimy A, Haller F, Gunawan B, Wünsch PH, Füzesi L. Distinct biphasic histomorphological pattern in gastrointestinal stromal tumours (GISTs) with common primary mutations but divergent molecular cytogenetic progression. Histopathology 2009; 54: 295-302. ArticlePubMed
- 10. Agaimy A, Wünsch PH, Hofstaedter F, et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol 2007; 31: 113-20. ArticlePubMed
Citations
Citations to this article as recorded by

- CYTOKERATINS: NOT AN EPITHELIAL ENTITY ANYMORE?
Geetpriya Kaur, Devicharan Shetty, Seema Sikka, Aparna Pathak
INTERNATIONAL JOURNAL OF SCIENTIFIC RESEARCH.2022; : 15. CrossRef - Gastrointestinal stromal tumors of the stomach in a 10-year-old child
Saeed Nasher, Fayed Al-Yousofy, Faisal Ahmed
Journal of Pediatric Surgery Case Reports.2021; 74: 102044. CrossRef


 PubReader
PubReader ePub Link
ePub Link Cite this Article
Cite this Article




 E-submission
E-submission