Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 49(1); 2015 > Article
-
Review
Molecular Imaging in the Era of Personalized Medicine - Kyung-Ho Jung, Kyung-Han Lee
-
Journal of Pathology and Translational Medicine 2015;49(1):5-12.
DOI: https://doi.org/10.4132/jptm.2014.10.24
Published online: January 15, 2015
Department of Nuclear Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- Corresponding Author: Kyung-Han Lee, M.D. Department of Nuclear Medicine, Samsung Medical Center, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea Tel: +82-2-3410-2630 Fax: +82-2-3410-2639 E-mail: khnm.lee@samsung.com
• Received: October 24, 2014 • Accepted: October 24, 2014
© 2015 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Clinical imaging creates visual representations of the body interior for disease assessment. The role of clinical imaging significantly overlaps with that of pathology, and diagnostic workflows largely depend on both fields. The field of clinical imaging is presently undergoing a radical change through the emergence of a new field called molecular imaging. This new technology, which lies at the intersection between imaging and molecular biology, enables noninvasive visualization of biochemical processes at the molecular level within living bodies. Molecular imaging differs from traditional anatomical imaging in that biomarkers known as imaging probes are used to visualize target molecules-of-interest. This ability opens up exciting new possibilities for applications in oncologic, neurological and cardiovascular diseases. Molecular imaging is expected to make major contributions to personalized medicine by allowing earlier diagnosis and predicting treatment response. The technique is also making a huge impact on pharmaceutical development by optimizing preclinical and clinical tests for new drug candidates. This review will describe the basic principles of molecular imaging and will briefly touch on three examples (from an immense list of new techniques) that may contribute to personalized medicine: receptor imaging, angiogenesis imaging, and apoptosis imaging.
- Clinical imaging is the art (and technique) of creating visual representations of the interior of a body for clinical assessment of disease. Although distinctly separate disciplines, clinical imaging and pathology actually overlap substantially in their roles in medical practice, i.e., both are used to detect and diagnose diseases, identify therapeutic targets, and predict treatment responses and patient outcomes. As such, the routine diagnostic workflows for patients with various diseases are deeply dependent on both imaging and pathology. The hallmark of the pathologist’s trade is to reveal abnormalities in tissues removed from the body using a microscope. Similarly, medical imaging specialists visualize and assess abnormal lesions in living bodies using specialized instruments. Depending on the type of signal picked up from the body interior, these instruments include computed tomography (CT), magnetic resonance imaging (MRI), ultrasonography, gamma scintigraphy, and positron emission tomography (PET).
- Imaging and pathology contribute substantially to clinical practice by providing diagnoses and monitoring disease progression. Today, we are witnessing a radical shift in the way diseases are managed: from the present onefitsall approach to one that delivers medical care tailored to the needs of individual patients [1]. This includes the detection of disease predisposition, early diagnosis, prognosis assessment, measurement of drug efficacy and disease monitoring. A crucial key for the success of this new healthcare paradigm is powerful molecular diagnostics [2]. Thus, the introduction of personalized medicine is creating an unprecedented opportunity for new technology development in fields of diagnostic medicine including pathology and imaging. The essence of diagnostic medicine in the context of personalized medicine lies in the use of biomarkers, objective indicators of pathogenic processes or pharmacologic effects that can be incorporated to predict or monitor treatment responses. Whereas markers have come mostly from patient tissues or serum, the location and activity of important markers can also be tracked with new imaging technologies. Such imaging tests may characterize diseases and assess treatment efficacy, with the added advantage of noninvasive longitudinal monitoring at multiple time points. The recent explosion of molecular biology and imaging technologies is now allowing quantitative characterization of biological processes inside the body at the molecular and genetic level. This exciting new field is envisioned to transform the future of medicine on a massive scale and have an enormous impact on the advancement of targeted therapies for individualized medicine. This innovative new technique is referred to as “molecular imaging.”
CLINICAL IMAGING AND PATHOLOGY
- Molecular imaging emerged amidst the overflowing innovations from new discoveries made in the field of molecular biotechnology. Unlike conventional imaging that relies on visualizing late consequences of pathologic alterations, molecular imaging interrogates the very molecular events that drive disease processes [3,4]. It can thus be defined as “noninvasive imaging and quantification of molecular and biochemical events that occur at the cellular and molecular level in tissues in their normal surroundings inside living bodies.” Noninvasive examination of cells inside living subjects by molecular imaging is critically dependent on biomarker probes that target key proteins linked to disease processes. This is analogous to the dependence of immunohistochemical staining of extracted tissue on special antibodies that bind to proteinsofinterest. In both situations, biomarker probes are used to identify key molecules that provide a more complete diagnostic picture for the referring physician (Fig. 1).
- The clinical benefits of molecular imaging are immense. At the top of the list is the promise of early disease detection and prediction of treatment response, which will lead to optimal therapies for individual patients. Molecular imaging is already having a substantial impact on therapeutic decisions made by clinicians, and this will be even more apparent as individualized treatment becomes the norm of clinical practice. Another important contribution will be in drug development, which typically requires expensive and prolonged preclinical and clinical trials to bring new drugs to market. Molecular imaging has the ability to noninvasively monitor the pharmacokinetic properties and pharmacodynamic changes of candidate drugs in the living body [5]. This can substantially shorten the development phase of drug production for the pharmaceutical industry by assessing drug response substantially earlier than by using anatomic criteria.
EMERGENCE OF MOLECULAR IMAGING
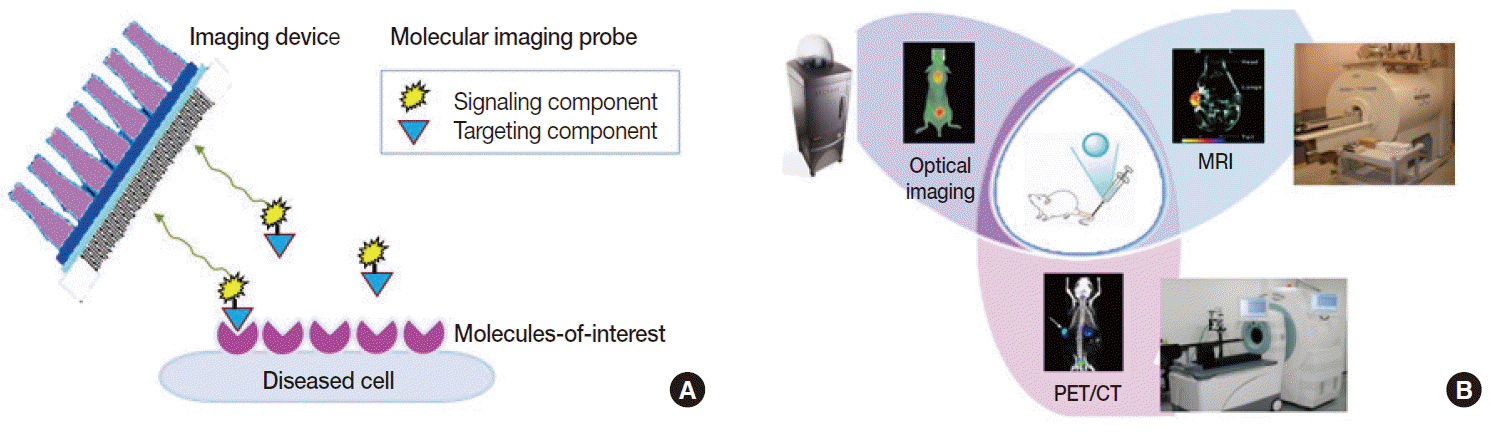
- Deriving noninvasive information regarding molecular events within the body can be done by tracking the temporal and spatial distribution of a marker probe that has high target specificity and behaves favorably in vivo. Therefore, the first and most pivotal step in molecular imaging research is the design and synthesis of superior probes that can interrogate important molecular targets [6]. Such probes are fundamentally different from contrast agents that are used in conventional imaging, which are nonspecific agents that increase the contrast of the blood pool. Molecular imaging probes are beacons that depict and enhance epitopes of key proteins that would otherwise be impossible to distinguish from surrounding tissue. Molecular imaging probes thus consist of a targeting component and a signaling component. Targeting moieties have traditionally been based on small molecules. More recently, however, advancements have introduced new classes of molecular imaging probes, including peptides, proteins, antibodies, aptamers, affibodies, and nanoparticles. Direction of the moieties to target molecules is provided by specific ligands that are included as part of the agents or are surfacedecorated. A signaling component is conjugated or labeled to the moieties so that they can be detected from outside of the body. Signals emitted can be radioactive, magnetic, echogenic, luminescent or fluorescent (Fig. 2A). Ideal molecular imaging probes should have low nonspecific binding, high selectivity for the processofinterest, high in vivo stability, and favorable in vivo pharmacokinetics. They should also have a good safety profile so that clinical translation is possible.
MOLECULAR IMAGING PROBES
- The second requirement for molecular imaging is specialized instruments that detect signals from probes in sensitive and accurate manners. Depending on the type of signal, modalities used in the clinic include CT, MRI, ultrasonography, optical imaging devices, gamma scintigraphy, and PET (Fig. 2B). Among these, clinical molecular imaging has traditionally been the domain of nuclear medicine. Indeed, molecular imaging has been in clinics for some time in the form of radionuclide imaging. Radioactive probes can track specific molecules in living bodies in a safe and hypersensitive manner. PET is already an indispensable clinical imaging tool and is the most mature molecular imaging technique in routine clinical use [7].
- PET has superior sensitivity and spatial resolution over gamma scintigraphy. It also has quantitative capabilities and high tissue penetration depth, making it the preferred molecular imaging modality in the clinic. The signaling component of PET probes is composed of positronemitting radioisotopes including 18F, 11C, and 15O, which are the major constituents of the building blocks of life. The positrons decay with a relatively short physical halflife of minutes to hours, and immediately collide with an electron to annihilate into two photons. These two photons proceed in exactly opposite trajectories but with identical energies, which makes it possible to pinpoint their location with high accuracy. Unlike PET, gamma scintigraphy detects probes that emit gammaray photons. Although more affordable than PET, it is less sensitive and has lower spatial resolution. Because PET can provide truly quantitative molecular information and is readily applicable to humans, it is a major driver of personalized medicine [7].
- Other imaging modalities are also speedily entering into molecular imaging research. Magnetic resonance (MR) is a versatile modality that uses powerful magnets and radiofrequency signals to provide images with high temporal and spatial resolution. MR images have excellent tissue contrast with an unlimited depth of tissue penetration and can simultaneously acquire anatomical structure and physiological function information. Limitations include weak magnetic signals and relatively low sensitivity, which requires administration of contrast agents and signal amplification strategies. Ferromagnetic agents such as superparamagnetic iron oxide reduce signals in T2weighted images (negative contrast), whereas paramagnetic agents such as gadolinium–diethylenetriamine pentaacetic acid increase signals in T1weighted images (positive contrast). MR probes have been developed that can interrogate molecular processes in a small number of cells [8]. A major problem for clinical translation of most of these techniques, however, is the issue of potential toxicity that arises from the relatively large doses of probe administration required.
- CT provides 3dimensional Xray images of structural anatomy. Although iodinated contrast agents improve soft tissue contrast, they require large quantities and are not targetspecific. In this sense, CT does not fit in the list of bonafide molecular imaging methods. However, recent small animal dedicated microCT instruments have provided ultrahigh resolution, and have transformed preclinical CT from visualization of the organ level to the molecular level [9]. CT is usually combined with PET for molecular imaging. Recent developments in CT contrast agents offer increasing potential, but further studies will be required to explore its molecular imaging capability.
- Optical imaging is based on light signals emitted from fluorescent or bioluminescent probes. It is safe, has high sensitivity, and is widely available. The main disadvantage is that low photon energies limit the penetration depth to a few centimeters, which makes it unsuitable for clinical use except for surface targets. However, this is not a problem for small experimental animals, where it is widely used for preclinical research and drug development [10]. Bioluminescence imaging exploits photons produced by enzymatic reaction of luciferase with its substrate. Several types of enzymes, including Firefly, Renilla and Gaussia luciferase, each require different conditions for reaction. Since mammalian tissues do not have endogenous bioluminescence, luminescent imaging has excellent signal to background ratios. Fluorescence imaging takes advantage of fluorescent signals that are typically emitted from genetically encoded reporter proteins. A celebrated example is the Nobelprize winning green fluorescence protein reporter. Fluorescence imaging can also be employed by administering fluorophoretagged probes to visualize molecular events in living cells. The strengths of fluorescence imaging include low cost, potential for multiplexed imaging, and, in the case of reporter protein, no need for probe administration.
MOLECULAR IMAGING INSTRUMENTS
- While a vast number of molecular imaging techniques are in preclinical development or in the process of clinical translation, several have already entered the clinical arena. These techniques tend to use radiolabeled probes, which is due to their superior sensitivity and high safety profile. PET using radiolabeled fluorodeoxyglucose (FDG), a glucose analogue avidly taken up by cancer cells, is by far the most widely employed clinical molecular imaging test. FDG PET has virtually revolutionized cancer management, including tumor diagnosis, staging, and monitoring of treatment response [7]. Other examples include PET using radiolabeled 18Ffluorothymidine, a thymine analogue biomarker of cell proliferation, and PET using radiolabeled Pittsburgh compound B, a biomarker of amyloid plaques in Alzheimer’s disease.
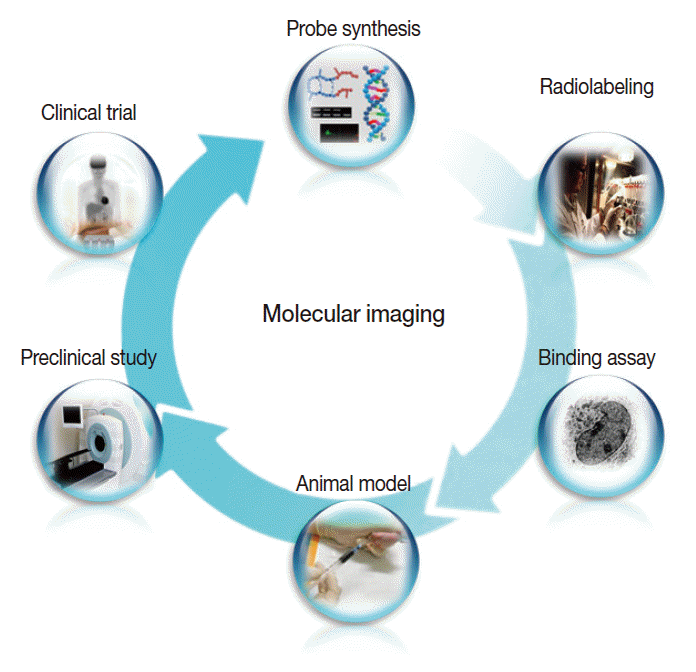
- In addition, a vast list of newer molecular imaging techniques are in the process of clinical translation for evaluation of malignant, neurologic, cardiovascular, and inflammatory diseases. In keeping with the overall molecular imaging development process illustrated in Fig. 3, various novel imaging targets have been identified for which arrays of imaging biomarkers are being designed and synthesized. Following verification of targetspecific binding and favorable in vivo pharmacokinetics, the probes are then tested for efficacy in preclinical animal models and finally in human subjects. As it is impossible to touch on all of these new techniques, which number in the hundreds, we will briefly look at just three examples among the many new molecular imaging techniques that are anticipated to contribute to personalized medicine. For other areas of molecular imaging, readers are asked to refer to the many excellent reviews on specific subjects.
- Tumor receptor imaging
- The next decade will see an individual’s tumor biology characterized at the molecular level by noninvasive imaging applications. An example is imaging of tumor receptor expression to identify promising therapeutic targets and delineate pharmacologic effects. The ability to measure receptor expression by imaging rather than by histologic inspection has the advantages of noninvasiveness, ability to assess sites difficult to sample, and potential for serial monitoring over time or after drug treatment. Importantly, it also allows assessment of the entire disease burden and avoids sampling errors from biopsies when receptor expression is heterogeneous. Overexpression of growth factor receptors in cancers leads to aberrant stimulation of growth signaling pathways, and their evaluation may thus help predict the efficacy of targeted drugs.
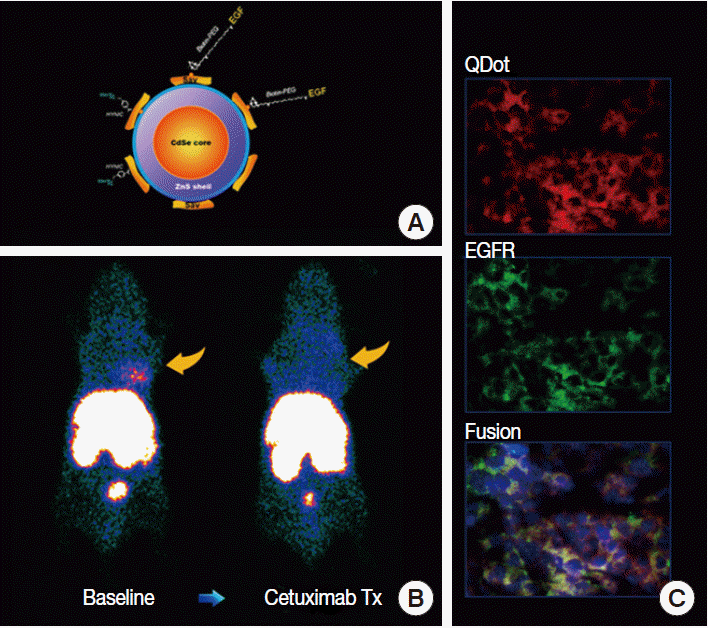
- Growth receptors have high binding affinities and can become saturated by even small molar quantities of binding ligands. Therefore, imaging probes need to have especially high signal density. The most success has been achieved with probes based on receptorspecific ligands or antibodies [11]. In comparison, probes based on tyrosine kinase inhibitors are challenging due to the ubiquity of tyrosine kinase expression and the requirement for intracellular transport. Radiolabeled fragments of trastuzumab have shown feasibility for imaging regional HER2 expression in animal models [12]. Our group demonstrated highcontrast epidermal growth factor (EGF) receptor imaging using a quantum dot probe multiplexed with EGF as a ligand for high affinity binding and radiolabels for scintigraphic imaging [13]. The technique was able to discriminate high from low receptor expression and monitor tumor response to targeted treatment (Fig. 4). Other studies include imaging of estrogen receptors and somatostatin receptors [11]. Similar technologies may be transferable to human subjects in the near future to monitor the adequacy of targeted anticancer therapies.
- Angiogenesis imaging
- Many pathologic processes involve inflammatory and ischemic responses that stimulate signaling for angiogenesis and apoptosis. Progression of tumor growth is highly dependent on remodeling of the vascular network through angiogenesis, as is the healing process following ischemic injury. As such, angiogenesis is receiving a large amount of attention as a potential target for treating tumors as well as ischemic diseases. Because the response to such targeted therapies cannot be readily assessed by conventional methods, there is a need for an imaging biomarker that can visualize and monitor changes in angiogenesis over time.
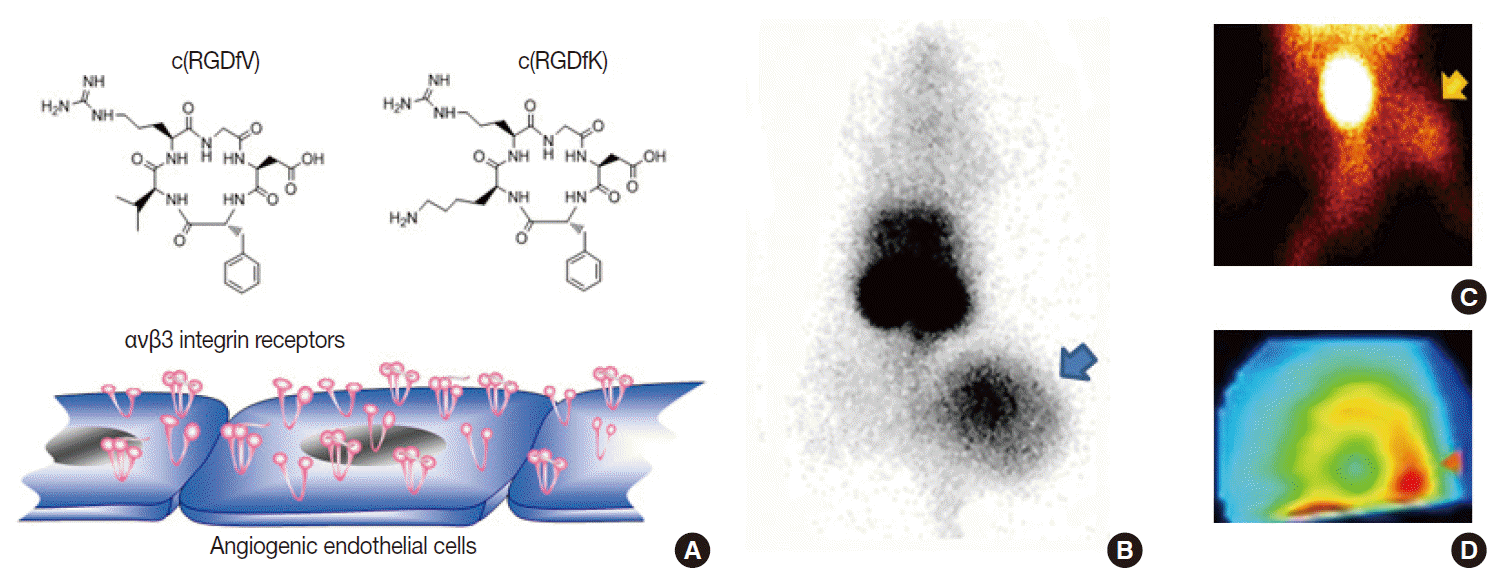
- Potential biologic targets for angiogenesis imaging include markers of activated endothelial cells and extracellular matrix [14,15]. Among various targets, including vascular endothelial growth factor receptors and matrix metalloproteinases, the most successful to date is the αvβ3 integrin receptor, a member of a family of heterodimeric cellsurface receptors highly upregulated at sites of neovascularization. These receptors recognize the tripeptide ArgGlyAsp (RGD) sequence as their binding motif. Hence, a large series of cyclic RGDcontaining peptide compounds have been synthesized, labeled with different radioisotopes or fluorophores, and characterized as probes for angiogenesis imaging [14,15]. Our lab developed a radiolabeled RGD probe with high affinity binding to αvβ3 integrin, and imaging in mice showed high contrast tumor uptake that was reduced by antiangiogenic treatment in a manner correlating to reduced integrin expression and tumor growth retardation [16]. In addition, we were among the first to show that RGD imaging can also monitor angiogenic responses of ischemic limbs [17] and myocardial tissue (Figs. 5, 6). More recently, RGD PET has been moving toward clinical translation [15], with clinical studies taking place in Europe that are showing the feasibility and safety of radiolabeled galactoRGD and AH111585 for delineating integrinpositive tumors. Furthermore, clinical studies with radiolabeled FPPRGD2 have demonstrated good lesion contrast in cancer patients.
- Apoptosis imaging
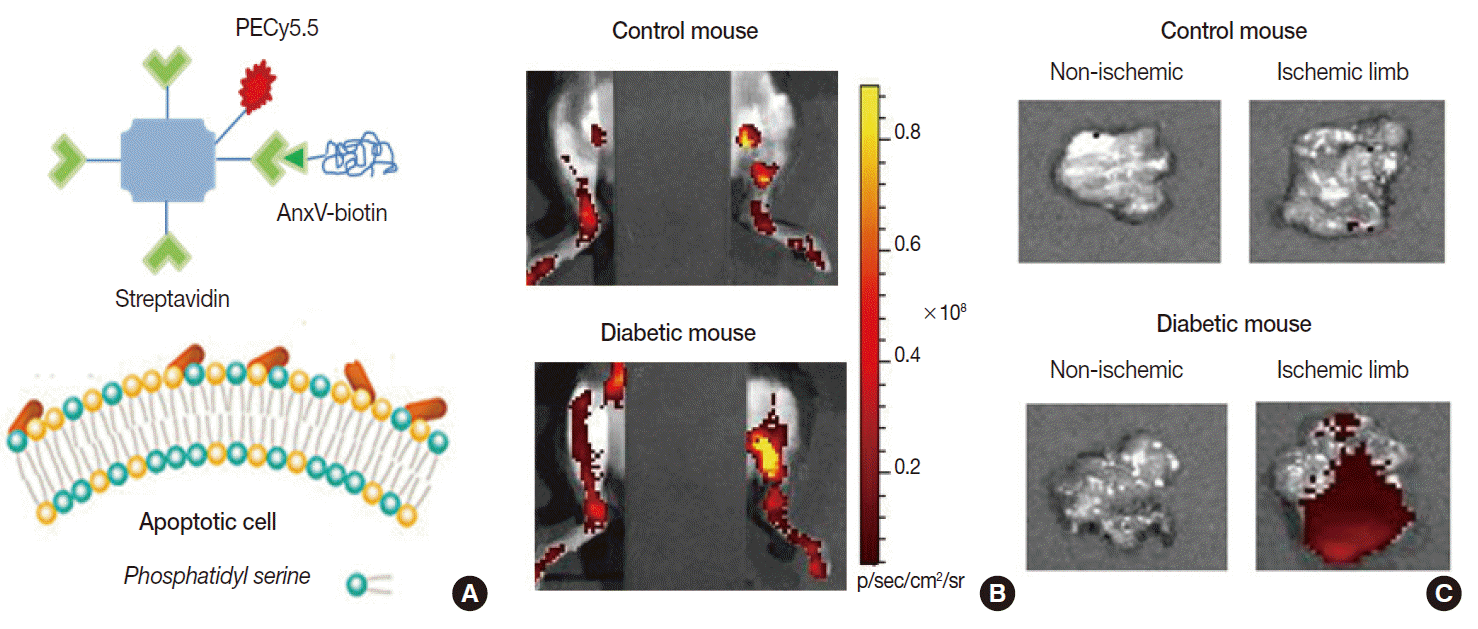
- Apoptosis is a finetuned biological process of programmed cell death that can be triggered by anticancer drugs and ionizing radiation as well as by various disease processes including ischemic injury. It is therefore being increasingly evaluated as a prognostic biomarker of treatment outcomes. Among several probes being investigated, the most extensively investigated is annexin-V, a peptide with highaffinity binding to phosphatidylserine [18]. This target normally resides in the inner leaflet of the plasma membrane, but scramblase activation by apoptotic stimuli flips it to the outer leaflet, exposing it to binding by annexin-V.
- Radiolabeled annexin-V compounds have been shown to enable imaging of apoptotic cells in animal models of anticancer therapy, allograft rejection, myocardial infarction, and infectious disease. Our group showed that fluorescentconjugated annexin-V probes can image ischemiainduced apoptosis in a mouse model of diabetic limb ischemia [19]. Annexin-V imaging has also been applied in clinical trials of cancer patients receiving chemotherapy, where its predictive potential was indicated by the increased probe accumulation in cases that later showed remission [20]. A limitation for annexin-V imaging is the possible difficulty in discriminating necrotic cell death, because phosphatidylserine of disrupted plasma membranes may also become accessible for binding.
- Other early changes in the apoptotic membrane, including loss of membrane potential, membrane acidification and activation of scramblase, may also serve as imaging targets. ML-10 shows promise as a PET probe for apoptotic imaging of tumors following therapy, where uptake correlated with breakdown of mitochondrial membrane potential and caspase activation. ML-10 PET was recently evaluated in human patients with brain metastases, and a significant correlation was found between early tumor uptake and late anatomical response [21]. Several groups have also developed PET probes that target caspase-3 activation, but found limited sensitivity despite specific caspase binding. Further studies are needed to evaluate the applicability of these apoptotic markers in clinical practice.
EXAMPLES OF MOLECULAR IMAGING TECHNIQUES
- Molecular imaging enables dynamic and quantitative visualization of specific biochemical and molecular events in living bodies. In recent years, this new technology has seen progress in early diagnosis, curative effect monitoring, and drug development. Many of these roles are similar to those pursued by molecular pathology, indicating significant overlap in mission and research agenda. Therefore, reinforcement of this trend may offer substantial synergic collaboration between pathologists and molecular imaging specialists, and pooling resources and strategic goals will have a powerful multiplier effect on future medicine.
- In conclusion, molecular imaging has emerged as a young but powerful new discipline. This paradigm shift in clinical imaging requires the rapid implementation of new validated imaging biomarkers. Although many aspects of molecular imaging are still in the early stages of development, the longrange idea is that medical professionals will be able to utilize the techniques to improve diagnoses, make better treatment choices, and predict patient outcomes. We foresee that the next decade will see even greater technological advances in molecular imaging methods, which in turn, will have a large impact on personalized medicine.
FUTURE PERSPECTIVES AND CONCLUSION
Acknowledgments
Fig. 1.Similarity of molecular imaging and pathology in utilizing probes to contribute to personalized medicine.


Fig. 2.Basic principles of molecular imaging. (A) Molecular imaging probes containing targeting components that interact with molecules-of-interest and signaling components that allow detection from outside of the body. (B) Representative small animal-dedicated molecular imaging devices that visualize signals emitted from probes within the living bodies of animal models. PET-CT, positron emission tomography–computed tomography; MRI, magnetic resonance imaging.


Fig. 3.The overall process of molecular imaging research, which is similar to that of new drug development.


Fig. 4.Example of tumor receptor imaging. (A) Quantum dot (QDot) probe surface-conjugated with epidermal growth factor (EGF) for targeting and radioisotopes for signaling. (B) A human breast cancer xenograft shows high probe uptake at baseline that is blocked by cetuximab therapy (Tx). (C) Tumor tissue sections show co-localization of fluorescent signals from the QDot with that from fluorescent-conjugated antibodies against EGF receptors (EGFR), indicating receptor-specific probe targeting.


Fig. 5.Example of angiogenesis imaging. (A) Cyclic Arg-Gly-Asp (RGD) peptide probes can interrogate αvβ3 integrin receptors overexpressed on activated endothelial cells. (B–D) Examples of a xenografted tumor-bearing mouse model (B), a hindlimb ischemia mouse model (C), and a myocardial infarction rat model (D) showing increased uptake of radiolabeled RGD probes in lesions with angiogenesis.


Fig. 6.Example of apoptosis imaging. (A) Annexin-V (AnxV) based probes target phosphatidyl serine exposed to the surface of cells that are undergoing apoptotic death. Here, AnxV is bound to streptavidin conjugated with PECy5.5 for fluorescent signaling. (B, C) In vivo fluorescent images of hindlimbs of living mice (supine with torso covered to block background signals; B) and ex vivo images of extracted tissue (C). Probe uptake is significantly increased in the ischemic hindlimb of the diabetic mouse due to apoptosis.


- 1. Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med 2010; 363: 301-4. ArticlePubMed
- 2. Hoggatt J. Personalized medicine: trends in molecular diagnostics: exponential growth expected in the next ten years. Mol Diagn Ther 2011; 15: 53-5. ArticlePubMedPDF
- 3. Herschman HR. Molecular imaging: looking at problems, seeing solutions. Science 2003; 302: 605-8. ArticlePubMed
- 4. Kircher MF, Hricak H, Larson SM. Molecular imaging for personalized cancer care. Mol Oncol 2012; 6: 182-95. ArticlePubMedPMCPDF
- 5. Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov 2008; 7: 591-607. ArticlePubMedPDF
- 6. Reynolds F, Kelly KA. Techniques for molecular imaging probe design. Mol Imaging 2011; 10: 407-19. ArticlePubMedPMCPDF
- 7. Weber WA, Grosu AL, Czernin J. Technology Insight: advances in molecular imaging and an appraisal of PET/CT scanning. Nat Clin Pract Oncol 2008; 5: 160-70. ArticlePubMedPDF
- 8. Gore JC, Manning HC, Quarles CC, Waddell KW, Yankeelov TE. Magnetic resonance in the era of molecular imaging of cancer. Magn Reson Imaging 2011; 29: 587-600. ArticlePubMedPMC
- 9. Ritman EL. Molecular imaging in small animals: roles for micro-CT. J Cell Biochem Suppl 2002; 39: 116-24. ArticlePubMed
- 10. Hellebust A, Richards-Kortum R. Advances in molecular imaging: targeted optical contrast agents for cancer diagnostics. Nanomedicine (Lond) 2012; 7: 429-45. ArticlePubMedPMC
- 11. Mankoff DA, Link JM, Linden HM, Sundararajan L, Krohn KA. Tumor receptor imaging. J Nucl Med 2008; 49 Suppl 2: 149S-63S. Article
- 12. Smith-Jones PM, Solit DB, Akhurst T, Afroze F, Rosen N, Larson SM. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol 2004; 22: 701-6. ArticlePubMedPDF
- 13. Jung KH, Choe YS, Paik JY, Lee KH. 99mTc-Hydrazinonicotinamide epidermal growth factor-polyethylene glycol-quantum dot imaging allows quantification of breast cancer epidermal growth factor receptor expression and monitors receptor downregulation in response to cetuximab therapy. J Nucl Med 2011; 52: 1457-64. ArticlePubMed
- 14. Choe YS, Lee KH. Targeted in vivo imaging of angiogenesis: present status and perspectives. Curr Pharm Des 2007; 13: 17-31. ArticlePubMed
- 15. Iagaru A, Gambhir SS. Imaging tumor angiogenesis: the road to clinical utility. AJR Am J Roentgenol 2013; 201: W183-91. ArticlePubMed
- 16. Jung KH, Lee KH, Paik JY, et al. Favorable biokinetic and tumor-targeting properties of 99mTc-labeled glucosamino RGD and effect of paclitaxel therapy. J Nucl Med 2006; 47: 2000-7. PubMed
- 17. Lee KH, Jung KH, Song SH, et al. Radiolabeled RGD uptake and alphav integrin expression is enhanced in ischemic murine hindlimbs. J Nucl Med 2005; 46: 472-8. PubMed
- 18. Yang TJ, Haimovitz-Friedman A, Verheij M. Anticancer therapy and apoptosis imaging. Exp Oncol 2012; 34: 269-76. PubMed
- 19. Jung KH, Lee JH, Park JW, et al. Annexin V imaging detects diabetes-accelerated apoptosis and monitors the efficacy of benfotiamine treatment in ischemic limbs of mice. Mol Imaging 2014; 13: 1-7. ArticlePDF
- 20. Kartachova M, van Zandwijk N, Burgers S, van Tinteren H, Verheij M, Valdés Olmos RA. Prognostic significance of 99mTc Hynic-rhannexin V scintigraphy during platinum-based chemotherapy in advanced lung cancer. J Clin Oncol 2007; 25: 2534-9. ArticlePubMed
- 21. Allen AM, Ben-Ami M, Reshef A, et al. Assessment of response of brain metastases to radiotherapy by PET imaging of apoptosis with 18F-ML-10. Eur J Nucl Med Mol Imaging 2012; 39: 1400-8. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Ionic Cell Microscopy: A new modality for visualizing cells using microfluidic impedance cytometry and generative artificial intelligence
Mahtab Kokabi, Gulam M. Rather, Mehdi Javanmard
Biosensors and Bioelectronics: X.2025; 24: 100619. CrossRef - Multimodal imaging in cancer detection: the role of SPIONs and USPIONs as contrast agents for MRI, SPECT, and PET
Zahra Shaghaghi, Ramin Mansouri, Sahar Nosrati, Maryam Alvandi
Future Oncology.2025; 21(18): 2367. CrossRef - Supramolecular fluorescence biosensing based on macrocycles
Jia-Hong Tian, Haiqi Xu, Xin-Yue Hu, Dong-Sheng Guo
Supramolecular Materials.2024; 3: 100063. CrossRef - A non-invasive osteopontin-targeted phase changeable fluorescent nanoprobe for molecular imaging of myocardial fibrosis
Xueli Zhao, Yuze Qin, Bo Wang, Jiao Liu, Yueyue Wang, Kun Chen, Jia Zhao, Lanlan Zhang, Yuanming Wu, Liwen Liu
Nanoscale Advances.2024; 6(14): 3590. CrossRef - The Role of Molecular Imaging in Personalized Medicine
Suliman Salih, Aisyah Elliyanti, Ajnas Alkatheeri, Fatima AlYafei, Bashayer Almarri, Hasina Khan
Journal of Personalized Medicine.2023; 13(2): 369. CrossRef - Development of a multifunctional platform for near-infrared imaging and targeted radionuclide therapy for tumors
Huihui He, Ke Li, Hang Li, Shiliang Zhu, Shuai Qin, Yong Mao, Jianguo Lin, Ling Qiu, Chunjing Yu
European Journal of Pharmaceutics and Biopharmaceutics.2023; 185: 107. CrossRef - Quantum Biotechnology
Nicolas P. Mauranyapin, Alex Terrasson, Warwick P. Bowen
Advanced Quantum Technologies.2022;[Epub] CrossRef - Preparation Fe3O4@chitosan-graphene quantum dots nanocomposites for fluorescence and magnetic resonance imaging
Kai Wang, Xiaoguang Xu, Yan Li, Mayifei Rong, Lifeng Wang, Liying Lu, Jian Wang, Fengwen Zhao, Bowen Sun, Yong Jiang
Chemical Physics Letters.2021; 783: 139060. CrossRef - Network Medicine: A Clinical Approach for Precision Medicine and Personalized Therapy in Coronary Heart Disease
Teresa Infante, Luca Del Viscovo, Maria Luisa De Rimini, Sergio Padula, Pio Caso, Claudio Napoli
Journal of Atherosclerosis and Thrombosis.2020; 27(4): 279. CrossRef - Nanodrug Delivery Systems for the Treatment of Ovarian Cancer
Jonathan M. Pantshwa, Pierre P. D. Kondiah, Yahya E. Choonara, Thashree Marimuthu, Viness Pillay
Cancers.2020; 12(1): 213. CrossRef - Molecular imaging of the urokinase plasminogen activator receptor: opportunities beyond cancer
V. M. Baart, R. D. Houvast, L. F. de Geus-Oei, P. H. A. Quax, P. J. K. Kuppen, A. L. Vahrmeijer, C. F. M. Sier
EJNMMI Research.2020;[Epub] CrossRef - In vivo SPECT imaging of an 131I-labeled PM 2.5 mimic substitute
Dong-Hui Pan, Jie Sheng, Xin-Yu Wang, Qian-Huan Huang, Jun-Jie Yan, Li-Zhen Wang, Run-Ling Yang, Dong-Jian Shi, Yu-Ping Xu, Ming-Qing Chen
Nuclear Science and Techniques.2020;[Epub] CrossRef - Optofluidics in bio-imaging applications
Sihui Chen, Rui Hao, Yi Zhang, Hui Yang
Photonics Research.2019; 7(5): 532. CrossRef - Nitrogen-vacancy centers in diamond for nanoscale magnetic resonance imaging applications
Alberto Boretti, Lorenzo Rosa, Jonathan Blackledge, Stefania Castelletto
Beilstein Journal of Nanotechnology.2019; 10: 2128. CrossRef - Online molecular image repository and analysis system: A multicenter collaborative open-source infrastructure for molecular imaging research and application
Mahabubur Rahman, Hiroshi Watabe
Computers in Biology and Medicine.2018; 96: 233. CrossRef - Nε-Acryloyllysine Piperazides as Irreversible Inhibitors of Transglutaminase 2: Synthesis, Structure–Activity Relationships, and Pharmacokinetic Profiling
Robert Wodtke, Christoph Hauser, Gloria Ruiz-Gómez, Elisabeth Jäckel, David Bauer, Martin Lohse, Alan Wong, Johanna Pufe, Friedrich-Alexander Ludwig, Steffen Fischer, Sandra Hauser, Dieter Greif, M. Teresa Pisabarro, Jens Pietzsch, Markus Pietsch, Reik Lö
Journal of Medicinal Chemistry.2018; 61(10): 4528. CrossRef - Genomic Interventions in Medicine
Oluwadurotimi S Aworunse, Oluwatomiwa Adeniji, Olusola L Oyesola, Itunuoluwa Isewon, Jelili Oyelade, Olawole O Obembe
Bioinformatics and Biology Insights.2018;[Epub] CrossRef - Restriction spectrum imaging: An evolving imaging biomarker in prostate MRI
Ryan L. Brunsing, Natalie M. Schenker-Ahmed, Nathan S. White, J. Kellogg Parsons, Christopher Kane, Joshua Kuperman, Hauke Bartsch, Andrew Karim Kader, Rebecca Rakow-Penner, Tyler M. Seibert, Daniel Margolis, Steven S. Raman, Carrie R. McDonald, Nikdokht
Journal of Magnetic Resonance Imaging.2017; 45(2): 323. CrossRef - Personalized medicine: a new option for nuclear medicine and molecular imaging in the third millennium
Orazio Schillaci, Nicoletta Urbano
European Journal of Nuclear Medicine and Molecular Imaging.2017; 44(4): 563. CrossRef - Nano-Magnetic Resonance Imaging (Nano-MRI) Gives Personalized Medicine a New Perspective
Lorenzo Rosa, Jonathan Blackledge, Albert Boretti
Biomedicines.2017; 5(1): 7. CrossRef - Optical nanoprobes for biomedical applications: shining a light on upconverting and near-infrared emitting nanoparticles for imaging, thermal sensing, and photodynamic therapy
E. Hemmer, P. Acosta-Mora, J. Méndez-Ramos, S. Fischer
Journal of Materials Chemistry B.2017; 5(23): 4365. CrossRef - Drug Discovery by Molecular Imaging and Monitoring Therapy Response in Lymphoma
Senthilkumar Kalimuthu, Ju Hye Jeong, Ji Min Oh, Byeong-Cheol Ahn
International Journal of Molecular Sciences.2017; 18(8): 1639. CrossRef - Chemistry and engineering of cyclodextrins for molecular imaging
Wing-Fu Lai, Andrey L. Rogach, Wing-Tak Wong
Chemical Society Reviews.2017; 46(20): 6379. CrossRef - Prototypes of Lanthanide(III) Agents Responsive to Enzymatic Activities in Three Complementary Imaging Modalities: Visible/Near-Infrared Luminescence, PARACEST-, and T1-MRI
Jiefang He, Célia S. Bonnet, Svetlana V. Eliseeva, Sara Lacerda, Thomas Chauvin, Pascal Retailleau, Frederic Szeremeta, Bernard Badet, Stéphane Petoud, Éva Tóth, Philippe Durand
Journal of the American Chemical Society.2016; 138(9): 2913. CrossRef - Nanoparticles in practice for molecular-imaging applications: An overview
Parasuraman Padmanabhan, Ajay Kumar, Sundramurthy Kumar, Ravi Kumar Chaudhary, Balázs Gulyás
Acta Biomaterialia.2016; 41: 1. CrossRef - A new neuroinformatics approach to personalized medicine in neurology: The Virtual Brain
Maria I. Falcon, Viktor Jirsa, Ana Solodkin
Current Opinion in Neurology.2016; 29(4): 429. CrossRef - Targeted multimodal nano-reporters for pre-procedural MRI and intra-operative image-guidance
Joonseok Lee, Andrew C. Gordon, Hacksung Kim, Wooram Park, Soojeong Cho, Byeongdu Lee, Andrew C. Larson, Elena A. Rozhkova, Dong-Hyun Kim
Biomaterials.2016; 109: 69. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Molecular Imaging in the Era of Personalized Medicine






Fig. 1. Similarity of molecular imaging and pathology in utilizing probes to contribute to personalized medicine.
Fig. 2. Basic principles of molecular imaging. (A) Molecular imaging probes containing targeting components that interact with molecules-of-interest and signaling components that allow detection from outside of the body. (B) Representative small animal-dedicated molecular imaging devices that visualize signals emitted from probes within the living bodies of animal models. PET-CT, positron emission tomography–computed tomography; MRI, magnetic resonance imaging.
Fig. 3. The overall process of molecular imaging research, which is similar to that of new drug development.
Fig. 4. Example of tumor receptor imaging. (A) Quantum dot (QDot) probe surface-conjugated with epidermal growth factor (EGF) for targeting and radioisotopes for signaling. (B) A human breast cancer xenograft shows high probe uptake at baseline that is blocked by cetuximab therapy (Tx). (C) Tumor tissue sections show co-localization of fluorescent signals from the QDot with that from fluorescent-conjugated antibodies against EGF receptors (EGFR), indicating receptor-specific probe targeting.
Fig. 5. Example of angiogenesis imaging. (A) Cyclic Arg-Gly-Asp (RGD) peptide probes can interrogate αvβ3 integrin receptors overexpressed on activated endothelial cells. (B–D) Examples of a xenografted tumor-bearing mouse model (B), a hindlimb ischemia mouse model (C), and a myocardial infarction rat model (D) showing increased uptake of radiolabeled RGD probes in lesions with angiogenesis.
Fig. 6. Example of apoptosis imaging. (A) Annexin-V (AnxV) based probes target phosphatidyl serine exposed to the surface of cells that are undergoing apoptotic death. Here, AnxV is bound to streptavidin conjugated with PECy5.5 for fluorescent signaling. (B, C) In vivo fluorescent images of hindlimbs of living mice (supine with torso covered to block background signals; B) and ex vivo images of extracted tissue (C). Probe uptake is significantly increased in the ischemic hindlimb of the diabetic mouse due to apoptosis.
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Molecular Imaging in the Era of Personalized Medicine

 E-submission
E-submission