Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 49(1); 2015 > Article
-
Brief Case Report
Placental Mesenchymal Dysplasia with Fetal Gastroschisis - Binnari Kim, Jiyeon Hyeon, Minju Lee, Hyewon Hwang, Yooju Shin, Suk-Joo Choi1, Jung-Sun Kim,
-
Journal of Pathology and Translational Medicine 2015;49(1):71-74.
DOI: https://doi.org/10.4132/jptm.2014.12.14
Published online: January 15, 2015
Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
1Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- Corresponding Author: Jung-Sun Kim, M.D. Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea Tel: +82-2-3410-2767, Fax: +82-2-3410-0025, E-mail: jsunkim@skku.edu
• Received: October 6, 2014 • Revised: December 8, 2014 • Accepted: December 14, 2014
© 2015 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
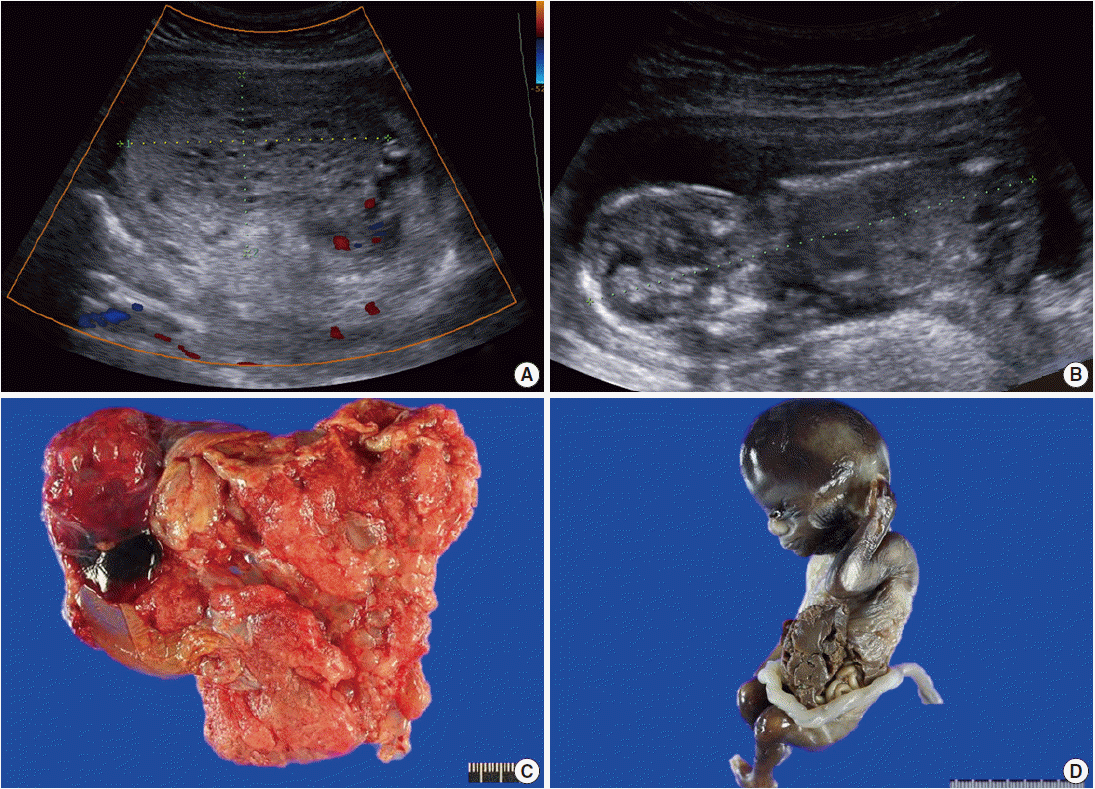
- A 29-year-old female with abnormal ultrasonographic findings was transferred to our institution. The patient had a normal sequential screening with the exception of an elevated serum β-hCG of 165,870 mIU/mL. Serum α-fetoprotein was not evaluated. Transabdominal ultrasound at 14+0 weeks of gestation revealed a thick globular placenta measuring 6.5×4.5 cm with multiple small, hypoechoic areas. Subsequent ultrasound at 14+5 weeks of gestation demonstrated a large multicystic honeycomb-like placenta measuring 8.8×5.7 cm with no sign of blood flow within the lesion (Fig. 1A). A fetus was identified with a crown-rump length of 9.6 cm (Fig. 1B). Based on the clinical setting, partial hydatidiform mole was suspected. Therapeutic termination was performed at 15+0 weeks of gestation.
- Grossly, the placenta was markedly enlarged with a diameter of 10 cm and the placental disc had multiple cystic vesicles; enlarged chorionic vessels were not found (Fig. 1C). A nonviable male fetus with a body weight of 73.57 g and with a crown-rump length of 9.8 cm, which were appropriate for gestational age, was delivered. However, he had a left lateral abdominal wall defect from which the liver and bowels protruded (Fig. 1D).
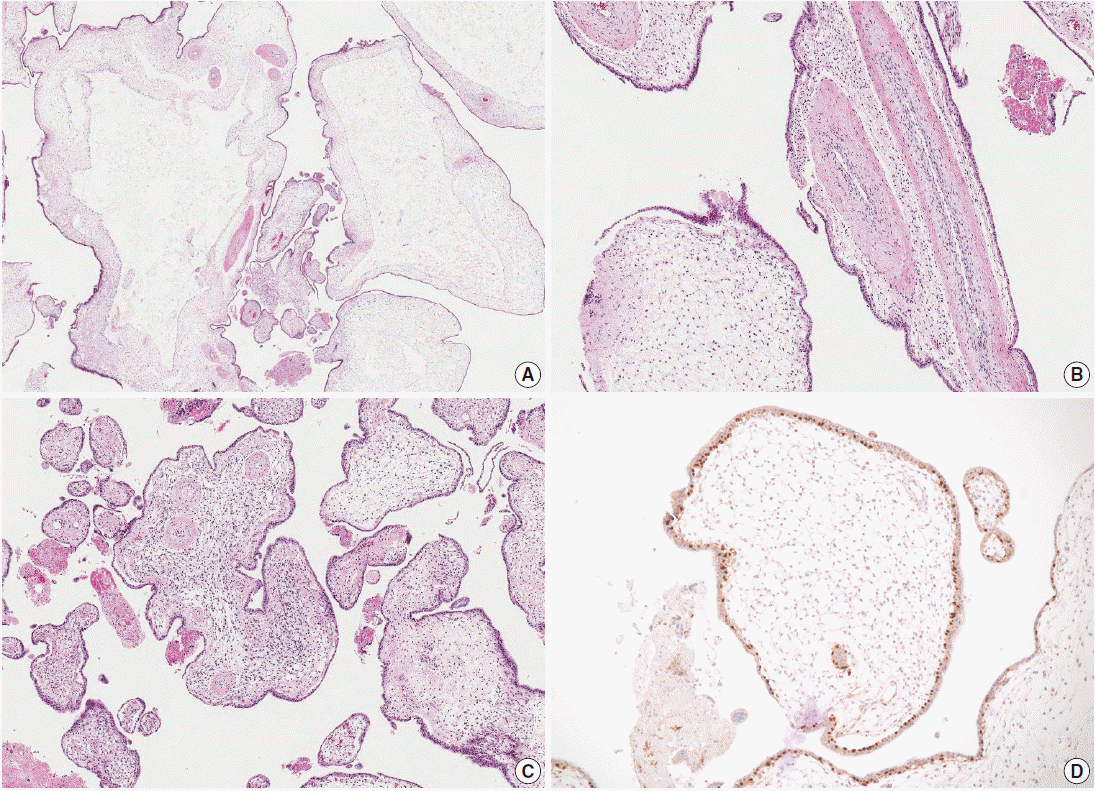
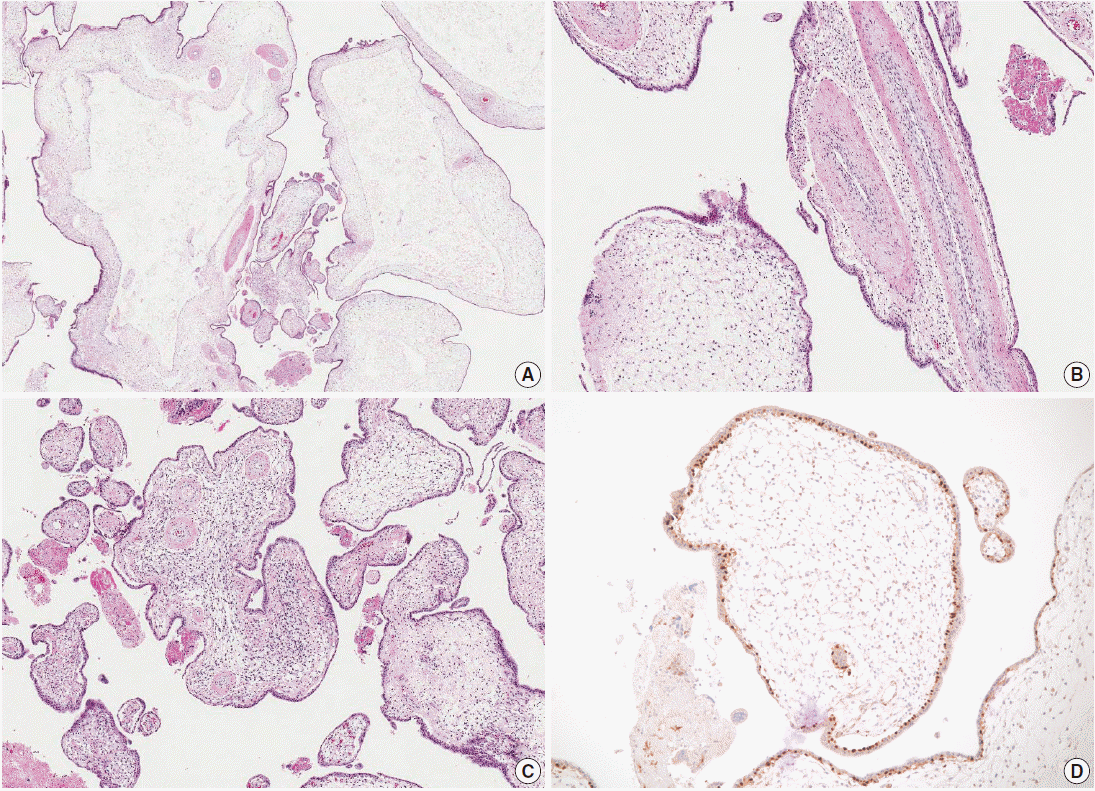
- Microscopic examination of the placenta revealed several enlarged villi with cistern formation and abnormally thick vessels scattered among normal-sized terminal villi without abnormal trophoblast proliferation. Some chorionic villi showed proliferation of primitive stromal cells (Fig. 2A-C). Cytotrophoblasts were diffusely positive for p57KIP2 on immunohistochemical staining (Fig. 2D). Macroscopic and microscopic morphology confirmed a diagnosis of PMD accompanied by gastroschisis of the fetus.
CASE REPORT
- PMD was first described as a rare, benign condition associated with an enlarged placenta in 1991 by Moscoso et al. [2]. The true incidence of PMD remains unclear, but it was reported to account for about 0.02% (7/30,758) of placentas examined at a Japanese hospital [3]. Most cases are asymptomatic and are diagnosed postpartum upon delivery of an abnormally large placenta. The typical sonographic feature of PMD is a thickened placenta with hypoechoic cystic spaces, which overlaps with that of molar pregnancy [4]. Thus, the differentiation of the two conditions may be difficult without placental pathology. One of the common laboratory abnormalities observed in PMD is increased maternal serum alpha fetoprotein, which is thought to originate from the fetus [2]. Serum β-hCG is typically normal to slightly elevated; the serum β-hCG level in this case was higher than that of normal pregnancy, which was unusual.
- Grossly, the placenta is markedly enlarged for gestational age and the placental disc has multiple grape-like vesicles from 0.3 to 2.5 cm in size, which resemble those of molar pregnancy. Enlarged chorionic vessels are usually found in third-trimester PMD placentas, not seen in hydatidiform mole. However, prominent chorionic vessels may not be identified grossly during the early stages, as in this case. Histologically, enlarged villi with cistern formation and abnormally thick vessels are scattered among normal-sized terminal villi. Some chorionic villi show mesenchymal cell hyperplasia. Neither abnormal trophoblast proliferation nor scalloping of the villous surface, common histologic findings of hydatidiform mole, is present. p57KIP2 immunopositivity of the cytotrophoblasts in our case was helpful for excluding the possibility of complete hydatidiform mole [4].
- The precise etiology of PMD has not been established. One theory is that it results from a congenital malformation of the mesoderm. Hypoxia and hypoperfusion of unknown origin may be responsible for the pathologic placental changes. More recently, androgenetic/biparental mosaicism has been suggested to be the underlying cause of PMD [4].
- Unlike molar pregnancy, PMD is not associated with gestational trophoblastic diseases, but does increase the risk of intrauterine fetal growth restriction and intrauterine fetal death. Approximately 15% to 25% of PMD cases are associated with Beck-with-Wiedemann syndrome [5-7]. PMD and Beckwith-Wiedemann syndrome represent a broad spectrum of phenotypes with common etiology [4,8]. In our case, PMD was associated with fetal gastroschisis, which has not been reported previously [6]. A recently-favored hypothesis for the pathogenesis of gastroschisis is folding failure of the embryonic lateral body due to defective mesoderm [9]. Mesodermal abnormality is suggested to be the cause of the association between PMD and gastroschisis in this case.
- In summary, PMD is a rare but clinically important placental abnormality that can be confused with partial hydatidiform mole and can be associated with abnormal fetal conditions. Therefore, PMD should be considered in the differential diagnosis of multicystic placenta. Awareness of this entity based on its sonographic similarity to molar pregnancy, unique pathologic features, and association with fetal complications needs to be emphasized in prenatal monitoring for the avoidance of unnecessary terminations.
DISCUSSION
Fig. 1.Radiologic and gross findings. (A) Transabdominal ultrasound at 14+5 weeks of gestation shows a large multicystic honeycomb-like placenta measuring 8.8×5.7 cm with no sign of blood flow within the lesion. (B) The fetus has a crown-rump length of 9.6 cm. (C) The placenta is markedly enlarged with multiple vesicles. (D) The fetus exhibits gastroschisis.
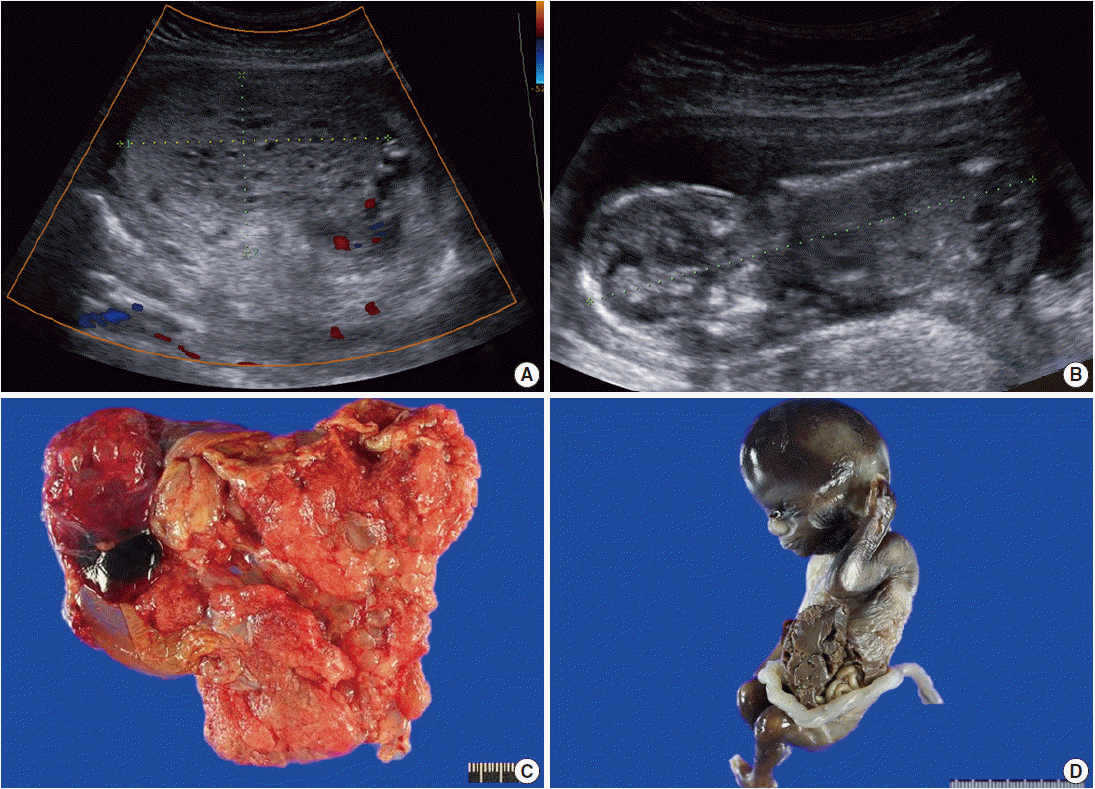

Fig. 2.Microscopic findings of the placenta. (A) Cystic vesicles are surrounded by stromal tissue and trophoblasts, indicating cysts in enlarged stem villi. No abnormal trophoblast proliferation is observed. (B) The majority of the enlarged stem villi have abnormal vessels with thickened muscular walls. (C) Some of the villi show mesenchymal cell proliferation. (D) Cytotrophoblasts are diffusely immunopositive for p57KIP2.

- 1. Vaisbuch E, Romero R, Kusanovic JP, et al. Three-dimensional sonography of placental mesenchymal dysplasia and its differential diagnosis. J Ultrasound Med 2009; 28: 359-68. ArticlePubMedPMCPDF
- 2. Moscoso G, Jauniaux E, Hustin J. Placental vascular anomaly with diffuse mesenchymal stem villous hyperplasia: a new clinico-pathological entity? Pathol Res Pract 1991; 187: 324-8. PubMed
- 3. Arizawa M, Nakayama M. Suspected involvement of the X chromosome in placental mesenchymal dysplasia. Congenit Anom (Kyoto) 2002; 42: 309-17. ArticlePubMed
- 4. Parveen Z, Tongson-Ignacio JE, Fraser CR, Killeen JL, Thompson KS. Placental mesenchymal dysplasia. Arch Pathol Lab Med 2007; 131: 131-7. ArticlePubMedPDF
- 5. Pham T, Steele J, Stayboldt C, Chan L, Benirschke K. Placental mesenchymal dysplasia is associated with high rates of intrauterine growth restriction and fetal demise: a report of 11 new cases and a review of the literature. Am J Clin Pathol 2006; 126: 67-78. ArticlePubMed
- 6. Heazell AE, Sahasrabudhe N, Grossmith AK, Martindale EA, Bhatia K. A case of intrauterine growth restriction in association with placental mesenchymal dysplasia with abnormal placental lymphatic development. Placenta 2009; 30: 654-7. ArticlePubMed
- 7. Woo GW, Rocha FG, Gaspar-Oishi M, Bartholomew ML, Thompson KS. Placental mesenchymal dysplasia. Am J Obstet Gynecol 2011; 205: e3-5. Article
- 8. Ulker V, Aslan H, Gedikbasi A, Yararbas K, Yildirim G, Yavuz E. Placental mesenchymal dysplasia: a rare clinicopathologic entity confused with molar pregnancy. J Obstet Gynaecol 2013; 33: 246-9. ArticlePubMed
- 9. Feldkamp ML, Carey JC, Sadler TW. Development of gastroschisis: review of hypotheses, a novel hypothesis, and implications for research. Am J Med Genet A 2007; 143A: 639-52. Article
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Diagnostic Accuracy of the Partograph Alert and Action Lines to Predict Adverse Birth Outcomes: A Systematic Review
M. Bonet, O. T. Oladapo, J. P. Souza, A. M. Gülmezoglu
Obstetrical & Gynecological Survey.2020; 75(5): 269. CrossRef - Placental Mesenchymal Dysplasia
Linda M. Ernst
Journal of Fetal Medicine.2015; 02(03): 127. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Placental Mesenchymal Dysplasia with Fetal Gastroschisis


Fig. 1. Radiologic and gross findings. (A) Transabdominal ultrasound at 14+5 weeks of gestation shows a large multicystic honeycomb-like placenta measuring 8.8×5.7 cm with no sign of blood flow within the lesion. (B) The fetus has a crown-rump length of 9.6 cm. (C) The placenta is markedly enlarged with multiple vesicles. (D) The fetus exhibits gastroschisis.
Fig. 2. Microscopic findings of the placenta. (A) Cystic vesicles are surrounded by stromal tissue and trophoblasts, indicating cysts in enlarged stem villi. No abnormal trophoblast proliferation is observed. (B) The majority of the enlarged stem villi have abnormal vessels with thickened muscular walls. (C) Some of the villi show mesenchymal cell proliferation. (D) Cytotrophoblasts are diffusely immunopositive for p57KIP2.
Fig. 1.
Fig. 2.
Placental Mesenchymal Dysplasia with Fetal Gastroschisis

 E-submission
E-submission