Search
- Page Path
- HOME > Search
- KRAS Mutation Test in Korean Patients with Colorectal Carcinomas: A Methodological Comparison between Sanger Sequencing and a Real-Time PCR-Based Assay
- Sung Hak Lee, Arthur Minwoo Chung, Ahwon Lee, Woo Jin Oh, Yeong Jin Choi, Youn-Soo Lee, Eun Sun Jung
- J Pathol Transl Med. 2017;51(1):24-31. Published online December 25, 2016
- DOI: https://doi.org/10.4132/jptm.2016.10.03
- 12,707 View
- 170 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Mutations in the KRAS gene have been identified in approximately 50% of colorectal cancers (CRCs). KRAS mutations are well established biomarkers in anti–epidermal growth factor receptor therapy. Therefore, assessment of KRAS mutations is needed in CRC patients to ensure appropriate treatment.
Methods
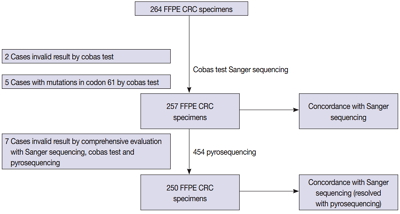
We compared the analytical performance of the cobas test to Sanger sequencing in 264 CRC cases. In addition, discordant specimens were evaluated by 454 pyrosequencing.
Results
KRAS mutations for codons 12/13 were detected in 43.2% of cases (114/264) by Sanger sequencing. Of 257 evaluable specimens for comparison, KRAS mutations were detected in 112 cases (43.6%) by Sanger sequencing and 118 cases (45.9%) by the cobas test. Concordance between the cobas test and Sanger sequencing for each lot was 93.8% positive percent agreement (PPA) and 91.0% negative percent agreement (NPA) for codons 12/13. Results from the cobas test and Sanger sequencing were discordant for 20 cases (7.8%). Twenty discrepant cases were subsequently subjected to 454 pyrosequencing. After comprehensive analysis of the results from combined Sanger sequencing–454 pyrosequencing and the cobas test, PPA was 97.5% and NPA was 100%.
Conclusions
The cobas test is an accurate and sensitive test for detecting KRAS-activating mutations and has analytical power equivalent to Sanger sequencing. Prescreening using the cobas test with subsequent application of Sanger sequencing is the best strategy for routine detection of KRAS mutations in CRC. -
Citations
Citations to this article as recorded by- Single-center study on clinicopathological and typical molecular pathologic features of metastatic brain tumor
Su Hwa Kim, Young Suk Lee, Sung Hak Lee, Yeoun Eun Sung, Ahwon Lee, Jun Kang, Jae-Sung Park, Sin Soo Jeun, Youn Soo Lee
Journal of Pathology and Translational Medicine.2023; 57(4): 217. CrossRef - Assessment of KRAS and NRAS status in metastatic colorectal cancer: Experience of the National Institute of Oncology in Rabat Morocco
Chaimaa Mounjid, Hajar El Agouri, Youssef Mahdi, Abdelilah Laraqui, En-nacer Chtati, Soumaya Ech-charif, Mouna Khmou, Youssef Bakri, Amine Souadka, Basma El Khannoussi
Annals of Cancer Research and Therapy.2022; 30(2): 80. CrossRef - The current understanding on the impact of KRAS on colorectal cancer
Mingjing Meng, Keying Zhong, Ting Jiang, Zhongqiu Liu, Hiu Yee Kwan, Tao Su
Biomedicine & Pharmacotherapy.2021; 140: 111717. CrossRef - Droplet digital PCR revealed high concordance between primary tumors and lymph node metastases in multiplex screening of KRAS mutations in colorectal cancer
Barbora Vanova, Michal Kalman, Karin Jasek, Ivana Kasubova, Tatiana Burjanivova, Anna Farkasova, Peter Kruzliak, Dietrich Busselberg, Lukas Plank, Zora Lasabova
Clinical and Experimental Medicine.2019; 19(2): 219. CrossRef - CRISPR Technology for Breast Cancer: Diagnostics, Modeling, and Therapy
Rachel L. Mintz, Madeleine A. Gao, Kahmun Lo, Yeh‐Hsing Lao, Mingqiang Li, Kam W. Leong
Advanced Biosystems.2018;[Epub] CrossRef
- Single-center study on clinicopathological and typical molecular pathologic features of metastatic brain tumor
- Differential Immunohistochemical Profiles for Distinguishing Prostate Carcinoma and Urothelial Carcinoma
- Woo Jin Oh, Arthur Minwoo Chung, Jee Soon Kim, Ji Heun Han, Sung Hoo Hong, Ji Yeol Lee, Yeong Jin Choi
- J Pathol Transl Med. 2016;50(5):345-354. Published online August 7, 2016
- DOI: https://doi.org/10.4132/jptm.2016.06.14
- 15,300 View
- 351 Download
- 32 Web of Science
- 34 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
The pathologic distinction between high-grade prostate adenocarcinoma (PAC) involving the urinary bladder and high-grade urothelial carcinoma (UC) infiltrating the prostate can be difficult. However, making this distinction is clinically important because of the different treatment modalities for these two entities.
Methods
A total of 249 patient cases (PAC, 111 cases; UC, 138 cases) collected between June 1995 and July 2009 at Seoul St. Mary’s Hospital were studied. An immunohistochemical evaluation of prostatic markers (prostate-specific antigen [PSA], prostate-specific membrane antigen [PSMA], prostate acid phosphatase [PAP], P501s, NKX3.1, and α-methylacyl coenzyme A racemase [AMACR]) and urothelial markers (CK34βE12, p63, thrombomodulin, S100P, and GATA binding protein 3 [GATA3]) was performed using tissue microarrays from each tumor.
Results
The sensitivities of prostatic markers in PAC were 100% for PSA, 83.8% for PSMA, 91.9% for PAP, 93.7% for P501s, 88.3% for NKX 3.1, and 66.7% for AMACR. However, the urothelial markers CK34βE12, p63, thrombomodulin, S100P, and GATA3 were also positive in 1.8%, 0%, 0%, 3.6%, and 0% of PAC, respectively. The sensitivities of urothelial markers in UC were 75.4% for CK34βE12, 73.9% for p63, 45.7% for thrombomodulin, 22.5% for S100P, and 84.8% for GATA3. Conversely, the prostatic markers PSA, PSMA, PAP, P501s, NKX3.1, and AMACR were also positive in 9.4%, 0.7%, 18.8%, 0.7%, 0%, and 8.7% of UCs, respectively.
Conclusions
Prostatic and urothelial markers, including PSA, NKX3.1, p63, thrombomodulin, and GATA3 are very useful for differentiating PAC from UC. The optimal combination of prostatic and urothelial markers could improve the ability to differentiate PAC from UC pathologically. -
Citations
Citations to this article as recorded by- Comparative histologic survey and transcriptomic investigation into canine prostate carcinoma
Nathan K. Hoggard, Said M. Elshafae, Nigel A. Daniels, Jonathan A. Young, Chris Premanandan, John B. Echols, Darshan S. Chandrashekar, Blake E. Hildreth, Michael C. Haffner, Thomas J. Rosol
Research in Veterinary Science.2026; 198: 105981. CrossRef - Plasmacytoid Urothelial Carcinoma with Initial Presentation as a Secondary
Prostatic Tumor: Diagnostic Pitfalls
and Literature Review
丰锦 李
Advances in Clinical Medicine.2026; 16(02): 1264. CrossRef - Impact of hormone sensitivity status on aberrant expression of CK7, CK20, CDX2, GATA3 and TTF1 in prostate cancer
Qing Yin Wang, Nazim Benzerdjeb, Samuel Jaquet, Andreea Stepanov, Mame-Kany Diop, Mirela Birlea, Fred Saad, Dominique Trudel
Human Pathology.2025; 163: 105877. CrossRef - Unusual Perineal Metastasis in a Case of Prostate Cancer on 68Ga-PSMA-11 PET/CT
Ritanshu Solanki, Bhagwant Rai Mittal, Rajender Kumar, Aravindh Sekar, Narender Kumar
Clinical Nuclear Medicine.2024; 49(2): e73. CrossRef - NKX3.1 Expression in Non-Prostatic Tumors and Characterizing its Expression in Esophageal/Gastroesophageal Adenocarcinoma
Ansa Mehreen, Kiran G. Manjee, Divyangi Paralkar, Gladell P. Paner, Thanh Lan
Advances in Anatomic Pathology.2024; 31(3): 202. CrossRef - Clinical Management of Intraductal Carcinoma of the Prostate
Gabriel Wasinger, Olivier Cussenot, Eva Compérat
Cancers.2024; 16(9): 1650. CrossRef - Adenocarcinomas of the Gynecologic Tract Involving the Urinary Bladder: A Series of 16 Cases Potentially Mimicking Urothelial Malignancy
Daniel H. Russell, Jonathan I. Epstein, Oleksandr N. Kryvenko, Matthew Schlumbrecht, Merce Jorda, Andre Pinto
Archives of Pathology & Laboratory Medicine.2024; 148(6): 705. CrossRef - Assessing the diagnostic impact of P63, PSA and BCL-2 proteins in premalignant and malignant prostate tissues
Aderonke C. Ogunlayi, Victor O. Ekundina, Adedapo O. Kehinde, Linus A. Enye, Adegoke O. Aremu
International Journal of Scientific Reports.2024; 10(6): 188. CrossRef - Concurrent occurrence of adenocarcinoma and urothelial carcinoma of the prostate gland: A case report
Jhe Yuan Hsu, Yi Sheng Lin, Li Hua Huang, Tang Yi Tsao, Chao Yu Hsu, Yen Chuan Ou, Min Che Tung
World Journal of Clinical Cases.2024; 12(26): 5952. CrossRef - Metastatic prostate cancer presenting as a posterior mediastinal mass: A rare presentation
Muhammad Haider, Arun Umesh Mahtani, Bachar Botrus, Foma Munoh Kenne, Madiha Fatima Master
Clinical Case Reports.2023;[Epub] CrossRef - Diagnostic and Prognostic Roles of GATA3 Immunohistochemistry in Urothelial Carcinoma
Daeseon Yoo, Kyueng-Whan Min, Jung-Soo Pyo, Nae Yu Kim
Medicina.2023; 59(8): 1452. CrossRef - Primary high-grade urothelial carcinoma of prostate with prostatic hyperplasia: a rare case report and review of the literature
Liang Liu, Fu-zhen Sun, Pan-ying Zhang, Yu Xiao, Xiao Yue, Dong-Ming Wang, Qiang Wang
The Aging Male.2023;[Epub] CrossRef - Expression of Gata Binding Protein 3 as a Prognostic Factor in Urogenital Lesions and Its Association With Morphology
T Govardhan, Debahuti Mohapatra, Sujata Naik, Prateek Das, Pranita Mohanty, Ankita Pal
Cureus.2023;[Epub] CrossRef - Histological and immunohistochemical investigation of canine prostate carcinoma with identification of common intraductal carcinoma component
Simone de Brot, Jennifer Lothion‐Roy, Llorenç Grau‐Roma, Emily White, Franco Guscetti, Mark A. Rubin, Nigel P. Mongan
Veterinary and Comparative Oncology.2022; 20(1): 38. CrossRef - Urothelial Carcinoma and Prostate-specific Membrane Antigen: Cellular, Imaging, and Prognostic Implications
Arsalan Tariq, Amy E. McCart Reed, Andrew Morton, Sima Porten, Ian Vela, Elizabeth D. Williams, John W. Yaxley, Peter C. Black, Matthew J. Roberts
European Urology Focus.2022; 8(5): 1256. CrossRef - Immunohistochemical Reactivity of Prostate-Specific Membrane Antigen in Salivary Gland Tumors
Haruto Nishida, Yoshihiko Kondo, Takahiro Kusaba, Hiroko Kadowaki, Tsutomu Daa
Head and Neck Pathology.2022; 16(2): 427. CrossRef - Weak NKX3.1 expression in a urothelial carcinoma: A diagnostic pitfall
Maryam Abdo, Robert Hoyt, Ashley Highfill, Daniel Mettman
Human Pathology Reports.2022; 27: 300599. CrossRef - Gene of the month: NKX3.1
Jon Griffin, Yuqing Chen, James W F Catto, Sherif El-Khamisy
Journal of Clinical Pathology.2022; 75(6): 361. CrossRef - Diagnostic Value of GATA3 and Uroplakin 3 in Differentiating Urothelial Carcinoma from Prostatic and Colorectal Carcinoma
Maha Salama, Dina A. Khairy
Open Access Macedonian Journal of Medical Sciences.2022; 10(A): 514. CrossRef - Diagnostic challenges for the distinction of high-grade prostatic adenocarcinoma and high-grade urothelial carcinoma of simultaneous occurrences - A literature review
Shreyas Bhushan Jayade, Manana Jikurashvili
GEORGIAN SCIENTISTS.2022;[Epub] CrossRef - Cytomorphology, immunoprofile, and clinicopathologic correlation of metastatic prostatic carcinoma
Xiaoqi Lin, Qiuying Shi, Ximing J. Yang
Human Pathology.2022; 130: 36. CrossRef - Cutaneous Metastasis of Prostate Adenocarcinoma: A Rare Presentation of a Common Disease
Alexander Dills, Okechukwu Obi, Kevin Bustos, Jesse Jiang, Shweta Gupta
Journal of Investigative Medicine High Impact Case Reports.2021;[Epub] CrossRef - Mining The Cancer Genome Atlas gene expression data for lineage markers in distinguishing bladder urothelial carcinoma and prostate adenocarcinoma
Ewe Seng Ch’ng
Scientific Reports.2021;[Epub] CrossRef - Immunohistochemical analysis of thrombomodulin expression in myocardial tissue from autopsy cases of ischemic heart disease
Takeshi Kondo, Motonori Takahashi, Gentaro Yamasaki, Marie Sugimoto, Azumi Kuse, Mai Morichika, Kanako Nakagawa, Makoto Sakurada, Migiwa Asano, Yasuhiro Ueno
Legal Medicine.2021; 51: 101897. CrossRef - Application and Pitfalls of Immunohistochemistry in Diagnosis of Challenging Genitourinary Cases
Jenny Ross, Guangyuan Li, Ximing J. Yang
Archives of Pathology & Laboratory Medicine.2020; 144(3): 290. CrossRef - New Screening Test Improves Detection of Prostate Cancer Using Circulating Tumor Cells and Prostate-Specific Markers
Karin Ried, Tasnuva Tamanna, Sonja Matthews, Peter Eng, Avni Sali
Frontiers in Oncology.2020;[Epub] CrossRef - An Unlikely Culprit: Gastric Metastasis from Primary Prostatic Adenocarcinoma
Eric Omar Then, Spoorthi Nutakki, Andrew Ofosu, Saad Saleem, Vijay Gayam, Tagore Sunkara, Vinaya Gaduputi
Journal of Gastrointestinal Cancer.2020; 51(3): 1081. CrossRef - MRI of prostatic urethral mucinous urothelial carcinoma: Expanding the differential diagnosis for T2 hyperintense prostatic masses
Neel Patel, Bryan R. Foster, Elena K. Korngold, Kyle Jensen, Kevin R. Turner, Fergus V. Coakley
Clinical Imaging.2020; 68: 68. CrossRef - Morphological and Immunohistochemical Biomarkers in Distinguishing Prostate Carcinoma and Urothelial Carcinoma: A Comprehensive Review
Francesca Sanguedolce, Davide Russo, Vito Mancini, Oscar Selvaggio, Beppe Calò, Giuseppe Carrieri, Luigi Cormio
International Journal of Surgical Pathology.2019; 27(2): 120. CrossRef - A Case of Metastatic Prostate Cancer to the Urethra That Resolved After Androgen Deprivation Therapy
Darren J. Bryk, Kenneth W. Angermeier, Eric A. Klein
Urology.2019; 129: e4. CrossRef - The Homeodomain Transcription Factor NKX3.1 Modulates Bladder Outlet Obstruction Induced Fibrosis in Mice
Mehul S. Patel, Diana K. Bowen, Nicholas M. Tassone, Andrew D. Gould, Kirsten S. Kochan, Paula R. Firmiss, Natalie A. Kukulka, Megan Y. Devine, Belinda Li, Edward M. Gong, Robert W. Dettman
Frontiers in Pediatrics.2019;[Epub] CrossRef - Cancer of unknown primary: Ancillary testing of cytologic and small biopsy specimens in the era of targeted therapy
Morgan L. Cowan, Christopher J. VandenBussche
Cancer Cytopathology.2018; 126(S8): 724. CrossRef - Glandular Tumors of the Urachus and Urinary Bladder: A Practical Overview of a Broad Differential Diagnosis
Alexander S. Taylor, Rohit Mehra, Aaron M. Udager
Archives of Pathology & Laboratory Medicine.2018; 142(10): 1164. CrossRef - S100P as a Marker for Urothelial Histogenesis: A Critical Review and Comparison With Novel and Traditional Urothelial Immunohistochemical Markers
Moushumi Suryavanshi, Julian Sanz-Ortega, Deepika Sirohi, Mukul K. Divatia, Chisato Ohe, Claudia Zampini, Daniel Luthringer, Steven C. Smith, Mahul B. Amin
Advances in Anatomic Pathology.2017; 24(3): 151. CrossRef
- Comparative histologic survey and transcriptomic investigation into canine prostate carcinoma
- Classic Papillary Thyroid Carcinoma with Tall Cell Features and Tall Cell Variant Have Similar Clinicopathologic Features
- Woo Jin Oh, Young Sub Lee, Uiju Cho, Ja Seong Bae, Sohee Lee, Min Hee Kim, Dong Jun Lim, Gyeong Sin Park, Youn Soo Lee, Chan Kwon Jung
- Korean J Pathol. 2014;48(3):201-208. Published online June 26, 2014
- DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.3.201
- 21,393 View
- 175 Download
- 40 Crossref
-
 Abstract
Abstract
 PDF
PDF Background The tall cell variant of papillary thyroid carcinoma (TCVPTC) is more aggressive than classic papillary thyroid carcinoma (PTC), but the percentage of tall cells needed to diagnose TCVPTC remains controversial. In addition, little is known about the clinicopathologic features of classic PTC with tall cell features (TCF).
Methods We retrospectively selected and reviewed the clinicopathologic features and presence of the
BRAF mutation in 203 cases of classic PTC, 149 cases of classic PTC with TCF, and 95 cases of TCVPTCs, which were defined as PTCs having <10%, 10-50%, and ≥50% tall cells, respectively.Results TCVPTCs and classic PTCs with TCF did not vary significantly in clinicopathologic characteristics such as pathologic (p) T stage, extrathyroidal extension, pN stage, lateral lymph node metastasis, or
BRAF mutations; however, these features differed significantly in TCVPTCs and classic PTCs with TCF in comparison to classic PTCs. Similar results were obtained in a subanalysis of patients with microcarcinomas (≤1.0 cm in size).Conclusions Classic PTCs with TCF showed a similar
BRAF mutation rate and clinicopathologic features to TCVPTCs, but more aggressive characteristics than classic PTCs.-
Citations
Citations to this article as recorded by- Clinicopathologic characteristics of papillary thyroid carcinoma, tall cell subtype and subtype with tall cell features, an institutional experience
Xueting Jin, Shunsuke Koga, Xiao Zhou, Niaz Z. Khan, Zubair W. Baloch
Human Pathology.2025; 161: 105867. CrossRef - Association Between BRAF V600E Allele Frequency and Aggressive Behavior in Papillary Thyroid Microcarcinoma
Luiza Tatar, Saruchi Bandargal, Marc P. Pusztaszeri, Véronique-Isabelle Forest, Michael P. Hier, Jasmine Kouz, Raisa Chowdhury, Richard J. Payne
Cancers.2025; 17(15): 2553. CrossRef - Papillary Thyroid Carcinoma and Body Mass Index: The Role of Immune System in Tumor Microenvironment
Rebecca Sparavelli, Riccardo Giannini, Francesca Signorini, Gabriele Materazzi, Alessio Basolo, Ferruccio Santini, Clara Ugolini
International Journal of Molecular Sciences.2025; 26(17): 8290. CrossRef - External validation of a deep learning-based algorithm for detection of tall cells in papillary thyroid carcinoma: A multicenter study
Sebastian Stenman, Sylvain Bétrisey, Paula Vainio, Jutta Huvila, Mikael Lundin, Nina Linder, Anja Schmitt, Aurel Perren, Matthias S. Dettmer, Caj Haglund, Johanna Arola, Johan Lundin
Journal of Pathology Informatics.2024; 15: 100366. CrossRef - Focal Tall Cell Change in Papillary Thyroid Carcinoma: Lessons Learned from Practices Adopting Rigid Criteria (Height to Width Ratio of 3)
Esther Diana Rossi, Liron Pantanowitz
Endocrine Pathology.2024; 35(1): 80. CrossRef - Predicting tall-cell subtype of papillary thyroid carcinomas independently with preoperative multimodal ultrasound
Bei-Bei Ye, Yun-Yun Liu, Ying Zhang, Bo-Ji Liu, Le-Hang Guo, Qing Wei, Yi-Feng Zhang, Hui-Xiong Xu
British Journal of Radiology.2024; 97(1159): 1311. CrossRef - TERT mutations and aggressive histopathologic characteristics of radioiodine-refractory papillary thyroid cancer
Ju Yeon Pyo, Yoon Jin Cha, SoonWon Hong
Journal of Pathology and Translational Medicine.2024; 58(6): 310. CrossRef - Papillary Thyroid Carcinomas with Tall Cell Features: An Intermediate Entity Between Classic and Tall Cell Subtypes
Athanasios Bikas, Kristine Wong, Theodora Pappa, Sara Ahmadi, Craig B. Wakefield, Ellen Marqusee, Pingping Xiang, Benjamin Altshuler, Jacob Haase, Justine A. Barletta, Iñigo Landa, Erik K. Alexander
Thyroid.2023; 33(6): 697. CrossRef - A novel nomogram for identifying high-risk patients among active surveillance candidates with papillary thyroid microcarcinoma
Li Zhang, Peisong Wang, Kaixuan Li, Shuai Xue
Frontiers in Endocrinology.2023;[Epub] CrossRef - The Impact of BRAF V600E Mutation Allele Frequency on the Histopathological Characteristics of Thyroid Cancer
Mawaddah Abdulhaleem, Saruchi Bandargal, Marc Philippe Pusztaszeri, Mohannad Rajab, Hannah Greenspoon, Joshua Ross Krasner, Sabrina Daniela Da Silva, Véronique-Isabelle Forest, Richard J. Payne
Cancers.2023; 16(1): 113. CrossRef - Protruding Huge Thyroid Mass Concurrent Hemorrhage and Skin Necrosis: A Case Report
Solji An, Joonseon Park, Kwangsoon Kim, Ja Seong Bae, Jeong Soo Kim
Journal of Endocrine Surgery.2023; 23(4): 143. CrossRef - CD56 Expression in Papillary Thyroid Carcinoma Is Highly Dependent on the Histologic Subtype: A Potential Diagnostic Pitfall
Uiju Cho, Yourha Kim, Sora Jeon, Chan Kwon Jung
Applied Immunohistochemistry & Molecular Morphology.2022; 30(5): 389. CrossRef - Aggressive histopathological variants of papillary thyroid carcinoma, diagnostic challenge, and clinical significance—A case series
PK Pravanya, KR Anila, Shaji Thomas, A Sreekumar, K Jayasree
Medical Journal of Dr. D.Y. Patil Vidyapeeth.2022; 15(6): 922. CrossRef - Tall cell variant papillary thyroid carcinoma impacts disease-free survival at the 10 % cut-point on multivariate analysis
Shabnam Samankan, Leah Militello, Gabriella Seo, Sedef Everest, Quinn O'Malley, Sarah L. Spaulding, Monica Xing, Ammar Matloob, John Beute, Raymond Chai, Scott Doyle, Mark L. Urken, Margaret Brandwein-Weber
Pathology - Research and Practice.2022; 236: 154012. CrossRef - A population-based study of the three major variants of papillary thyroid carcinoma
Junming Xu, Yingying Zhang, Jun Liu, Shenglong Qiu, Min Wang
Journal of International Medical Research.2021;[Epub] CrossRef - Tall cell percentage alone in PTC without aggressive features should not guide patients’ clinical management
Anello Marcello Poma, David Viola, Elisabetta Macerola, Agnese Proietti, Eleonora Molinaro, Dario De Vietro, Rossella Elisei, Gabriele Materazzi, Paolo Miccoli, Fulvio Basolo, Clara Ugolini
The Journal of Clinical Endocrinology & Metabolism.2021; 106(10): e4109. CrossRef - Molecular Pathology of Non-familial Follicular Epithelial–Derived Thyroid Cancer in Adults: From RAS/BRAF-like Tumor Designations to Molecular Risk Stratification
Paula Soares, Antónia Afonso Póvoa, Miguel Melo, João Vinagre, Valdemar Máximo, Catarina Eloy, José Manuel Cameselle-Teijeiro, Manuel Sobrinho-Simões
Endocrine Pathology.2021; 32(1): 44. CrossRef - Deep Neck Infection: Atypical Presentation of Papillary Thyroid Cancer
Apichana Mahattanapreut, Rangsima Aroonroch, Chalermchai Chintrakarn, Chutintorn Sriphrapradang, Dinesh K. Chhetri
Case Reports in Otolaryngology.2021; 2021: 1. CrossRef - The evolving concept of aggressive histological variants of differentiated thyroid cancer
Juan C. Hernandez-Prera
Seminars in Diagnostic Pathology.2020; 37(5): 228. CrossRef - Papillary Thyroid Cancer—Aggressive Variants and Impact on Management: A Narrative Review
Andrés Coca-Pelaz, Jatin P. Shah, Juan C. Hernandez-Prera, Ronald A. Ghossein, Juan P. Rodrigo, Dana M. Hartl, Kerry D. Olsen, Ashok R. Shaha, Mark Zafereo, Carlos Suarez, Iain J. Nixon, Gregory W. Randolph, Antti A. Mäkitie, Luiz P. Kowalski, Vincent Van
Advances in Therapy.2020; 37(7): 3112. CrossRef - Contemporary evaluation and management of tall cell variant of papillary thyroid carcinoma
Sara Cartwright, Abbey Fingeret
Current Opinion in Endocrinology, Diabetes & Obesity.2020; 27(5): 351. CrossRef - Le carcinome papillaire de la thyroïde avec contingent à cellules hautes : facteurs pronostiques
I. Riahi, H. Jaafoura, H. Saibi, E. Chebil, I. Ben Nacef, M. Ksentini, T. Ben Ghachem, R. Lahiani, M. Ben Salah
Annales d'Endocrinologie.2020; 81(4): 345. CrossRef - Updates in the Pathologic Classification of Thyroid Neoplasms: A Review of the World Health Organization Classification
Yanhua Bai, Kennichi Kakudo, Chan Kwon Jung
Endocrinology and Metabolism.2020; 35(4): 696. CrossRef - Tall Cell Variant of Papillary Thyroid Carcinoma: Impact of Change in WHO Definition and Molecular Analysis
Kristine S. Wong, Sara E. Higgins, Ellen Marqusee, Matthew A. Nehs, Trevor Angell, Justine A. Barletta
Endocrine Pathology.2019; 30(1): 43. CrossRef - Histopatological and molecular genetic characteristics of clinically aggressive variants of papillary thyroid carcinoma
A. V. Bogolyubova, A. Yu. Abrosimov, L. S. Selivanova, P. V. Belousov
Arkhiv patologii.2019; 81(1): 46. CrossRef - Papillary Thyroid Cancers with Focal Tall Cell Change are as Aggressive as Tall Cell Variants and Should Not be Considered as Low-Risk Disease
Pim J. Bongers, Wouter P. Kluijfhout, Raoul Verzijl, Mattan Lustgarten, Marloes Vermeer, David P. Goldstein, Karen Devon, Lorne E. Rotstein, Sylvia L. Asa, James D. Brierley, Richard W. Tsang, Shereen Ezzat, Menno R. Vriens, Ozgur Mete, Jesse D. Pasternak
Annals of Surgical Oncology.2019; 26(8): 2533. CrossRef - A case-based approach to aggressive variants of papillary thyroid carcinoma with literature review
JosephAntoine Flordelis Chatto, AnnetteLaurente Salillas
Thyroid Research and Practice.2019; 16(3): 128. CrossRef - Clinically Relevant Prognostic Parameters in Differentiated Thyroid Carcinoma
Tyler Janovitz, Justine A. Barletta
Endocrine Pathology.2018; 29(4): 357. CrossRef - Prediction of novel target genes and pathways involved in tall cell variant papillary thyroid carcinoma
Fada Xia, Bo Jiang, Yong Chen, Xin Du, Yao Peng, Wenlong Wang, Zhuolu Wang, Xinying Li
Medicine.2018; 97(51): e13802. CrossRef - Papillary thyroid carcinoma with tall cell features is as aggressive as tall cell variant: a meta-analysis
Huy Gia Vuong, Nguyen Phuoc Long, Nguyen Hoang Anh, Tran Diem Nghi, Mai Van Hieu, Le Phi Hung, Tadao Nakazawa, Ryohei Katoh, Tetsuo Kondo
Endocrine Connections.2018; 7(12): R286. CrossRef - TERT Promoter Mutation in an Aggressive Cribriform Morular Variant of Papillary Thyroid Carcinoma
Eun Ji Oh, Sohee Lee, Ja Seong Bae, Yourha Kim, Sora Jeon, Chan Kwon Jung
Endocrine Pathology.2017; 28(1): 49. CrossRef - Update on the cytologic features of papillary thyroid carcinoma variants
Marc Pusztaszeri, Manon Auger
Diagnostic Cytopathology.2017; 45(8): 714. CrossRef - Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive f
Uiju Cho, Ozgur Mete, Min-Hee Kim, Ja Seong Bae, Chan Kwon Jung
Modern Pathology.2017; 30(6): 810. CrossRef - BRAF-positive paucicellular variant of anaplastic carcinoma in the presence of tall cell variant papillary thyroid cancer
O. V. Dolzhansky, E. M. Paltseva, D. N. Khmelkova, F. A. Konovalov, I. V. Kanivets, A. V. Lavrov, D. V. Pyankov, S. A. Korostelev, O. A. Levendyuk, V. M. Pominalnaya, D. N. Fedorov
Arkhiv patologii.2017; 79(3): 27. CrossRef - A comparison of the clinicopathological features and prognoses of the classical and the tall cell variant of papillary thyroid cancer: a meta-analysis
Zeming Liu, Wen Zeng, Tianwen Chen, Yawen Guo, Chao Zhang, Chunping Liu, Tao Huang
Oncotarget.2017; 8(4): 6222. CrossRef - Clinical utility of TERT promoter mutations and ALK rearrangement in thyroid cancer patients with a high prevalence of the BRAF V600E mutation
Ja Seong Bae, Yourha Kim, Sora Jeon, Se Hee Kim, Tae Jung Kim, Sohee Lee, Min-Hee Kim, Dong Jun Lim, Youn Soo Lee, Chan Kwon Jung
Diagnostic Pathology.2016;[Epub] CrossRef - Tall cell variant of papillary thyroid carcinoma: current evidence on clinicopathologic features and molecular biology
Xiaofei Wang, Wenli Cheng, Chongqing Liu, Jingdong Li
Oncotarget.2016; 7(26): 40792. CrossRef - The Warthin-Like Variant of Papillary Thyroid Carcinoma: A Comparison with Classic Type in the Patients with Coexisting Hashimoto’s Thyroiditis
Min-kyung Yeo, Ja Seong Bae, Sohee Lee, Min-Hee Kim, Dong-Jun Lim, Youn Soo Lee, Chan Kwon Jung
International Journal of Endocrinology.2015; 2015: 1. CrossRef - BRAF Immunohistochemistry Using Clone VE1 is Strongly Concordant with BRAFV600E Mutation Test in Papillary Thyroid Carcinoma
Jung-Soo Pyo, Jin Hee Sohn, Guhyun Kang
Endocrine Pathology.2015; 26(3): 211. CrossRef - Pathologie de la thyroïde. Cas no 3. Carcinome papillaire de la thyroïde, variante à cellules hautes
Emmanuelle Leteurtre
Annales de Pathologie.2015; 35(5): 402. CrossRef
- Clinicopathologic characteristics of papillary thyroid carcinoma, tall cell subtype and subtype with tall cell features, an institutional experience
- Esophageal Squamous Cell Carcinoma
In Situ Overlying Leiomyoma Mimicking Invasive Cancer: A Brief Case Report - Woo Jin Oh, Eun Jung Lee, Youn Soo Lee, Tae-Jung Kim
- Korean J Pathol. 2014;48(2):162-163. Published online April 28, 2014
- DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.2.162
- 7,585 View
- 43 Download
- 2 Crossref
-
 PDF
PDF -
Citations
Citations to this article as recorded by- Esophageal squamous cell carcinoma or high-grade dysplasia overlying leiomyoma, rare but not to be neglected
Changyuan Guo, Dan Liu, Yong Liu, Lei Guo, Lulu Rong, Guiqi Wang, Ning Lu, Liyan Xue
Esophagus.2021; 18(1): 125. CrossRef - Esophageal leiomyoma and simultaneous overlying squamous cell carcinoma: a case report and review of the literature
Saadat Mehrabi, Mohammad Javad Yavari Barhaghtalab, Safoora Hejazinia, Hossein Saedi
BMC Surgery.2021;[Epub] CrossRef
- Esophageal squamous cell carcinoma or high-grade dysplasia overlying leiomyoma, rare but not to be neglected

 E-submission
E-submission

 First
First Prev
Prev



