Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 50(5); 2016 > Article
-
Original Article
Differential Immunohistochemical Profiles for Distinguishing Prostate Carcinoma and Urothelial Carcinoma - Woo Jin Oh*,, Arthur Minwoo Chung*,, Jee Soon Kim, Ji Heun Han, Sung Hoo Hong1, Ji Yeol Lee1, Yeong Jin Choi
-
Journal of Pathology and Translational Medicine 2016;50(5):345-354.
DOI: https://doi.org/10.4132/jptm.2016.06.14
Published online: August 7, 2016
Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
1Department of Urology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- Corresponding Author Yeong Jin Choi, MD, PhD Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 222 Banpo-daero, Seocho-gu, Seoul 06591, Korea Tel: +82-2-2258-1616 Fax: +82-2-2258-1627 E-mail: mdyjchoi@catholic.ac.kr
- *Woo Jin Oh and Arthur Minwoo Chung contributed equally to this work.
© 2016 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- The pathologic distinction between high-grade prostate adenocarcinoma (PAC) involving the urinary bladder and high-grade urothelial carcinoma (UC) infiltrating the prostate can be difficult. However, making this distinction is clinically important because of the different treatment modalities for these two entities.
-
Methods
- A total of 249 patient cases (PAC, 111 cases; UC, 138 cases) collected between June 1995 and July 2009 at Seoul St. Mary’s Hospital were studied. An immunohistochemical evaluation of prostatic markers (prostate-specific antigen [PSA], prostate-specific membrane antigen [PSMA], prostate acid phosphatase [PAP], P501s, NKX3.1, and α-methylacyl coenzyme A racemase [AMACR]) and urothelial markers (CK34βE12, p63, thrombomodulin, S100P, and GATA binding protein 3 [GATA3]) was performed using tissue microarrays from each tumor.
-
Results
- The sensitivities of prostatic markers in PAC were 100% for PSA, 83.8% for PSMA, 91.9% for PAP, 93.7% for P501s, 88.3% for NKX 3.1, and 66.7% for AMACR. However, the urothelial markers CK34βE12, p63, thrombomodulin, S100P, and GATA3 were also positive in 1.8%, 0%, 0%, 3.6%, and 0% of PAC, respectively. The sensitivities of urothelial markers in UC were 75.4% for CK34βE12, 73.9% for p63, 45.7% for thrombomodulin, 22.5% for S100P, and 84.8% for GATA3. Conversely, the prostatic markers PSA, PSMA, PAP, P501s, NKX3.1, and AMACR were also positive in 9.4%, 0.7%, 18.8%, 0.7%, 0%, and 8.7% of UCs, respectively.
-
Conclusions
- Prostatic and urothelial markers, including PSA, NKX3.1, p63, thrombomodulin, and GATA3 are very useful for differentiating PAC from UC. The optimal combination of prostatic and urothelial markers could improve the ability to differentiate PAC from UC pathologically.
- Patients and materials
- We performed a retrospective analysis of a prospectively maintained database of patients approved by the Institutional Review Board of Seoul St. Mary’s Hospital, the Catholic University of Korea (KC13SISI0909). We enrolled a total of 111 patients with PAC and 138 patients with UC who were treated at Seoul St. Mary’s Hospital between June 1995 and July 2009.
- Cases of PAC were divided according to low (Gleason score <7) and high grade (Gleason score ≥7). Of the 111 PACs from radical prostatectomy specimens, 64 cases (57.6%) were low grade and 47 (42.3%) were high grade. Cases of UC, whether noninvasive papillary or infiltrating, were divided into low and high grade according to the World Health Organization classification. Of the 138 UCs from cystectomy or tranurethral resection of bladder specimens, 28 cases (20.3%) were noninvasive papillary low grade, and 110 (79.7%) were noninvasive papillary or infiltrating cases with high-grade morphology. The male: female ratio was 7:1. None of the patients received neoadjuvant chemotherapy, hormone, or radiation therapy.
- Immunohistochemistry
- We analyzed and evaluated the immunoprofile of urothelial and prostatic markers using tissue microarrays (TMAs). Needle punches (0.5-mm diameter) of paraffin-embedded tissue blocks were transferred and arrayed in the recipient block. Tissue punches yielding equivocal results were excluded from data analysis. Only interpretable positive or negative staining was accepted as a positive or negative result. Five-micrometer sections from the TMA blocks were prepared for immunohistochemical staining. All tissues were fixed in neutral buffered formalin, paraffin embedded, and processed in a standard tissue processor.
- The immunohistochemical stains were performed on an automated immunostainer (LV-1 Autostainer, Lab Vision, Fremont, CA, USA) using the standard avidin-biotin peroxidase method after antigen retrieval according to the manufacturer’s instructions. The immunohistochemical stains for this study included PSA, PSMA, PAP, P501s, NKX3.1, α-methylacyl coenzyme A racemase (AMACR), CK34βE12, p63, thrombomodulin, S100P, and GATA3. Primary antibodies used in this study were as follows: PSA (polyclonal, Dako, Carpinteria, CA, USA), PSMA (monoclonal, Novocastra Lab, Ltd., Newcastle upon Tyne, UK), PAP (monoclonal, Dako), P501s (monoclonal, Dako), NKX3.1 (monoclonal, Athena ES, Baltimore, MD, USA), AMACR (monoclonal, Cell Marque, Rocklin, CA, USA), CK34βE12 (monoclonal, Dako), p63 (monoclonal, Lab Vision), thrombomodulin (monoclonal, Dako), S100P (monoclonal, Dako), and GATA3 (monoclonal, prediluted, Cell Marque). Detailed information about the antibodies used in this study is included in Table 1. The staining patterns for PSA, PSMA, AMACR, and thrombomodulin were cytoplasmic. The P501s staining was in a perinuclear cytoplasmic location and had a speckled pattern. CK34βE12 showed membranous and/or cytoplasmic staining. Only nuclear stains with or without cytoplasmic staining were accepted as positive results for the p63, NKX3.1, S100P, and GATA3 stains. All immunohistochemical staining reactions were reviewed by experienced genitourinary pathologists. The patterns of all immunostaining markers in this study were diffuse and homogeneous. The immunohistochemical staining results were recorded as positive stains when ≥10% of cells showed positive reaction regardless of the intensity of the staining. SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistic analysis. We used chi-square tests to analyse the reactivity of all antibody panels. p-values ≤.05 were considered statistically significant.
MATERIALS AND METHODS
- The results for PSA, PSMA, PAP, P501s, NKX3.1, AMACR, CK34βE12, p63, thrombomodulin, S100P, and GATA3 were summarized (Table 2).
- Prostatic markers in the PAC group
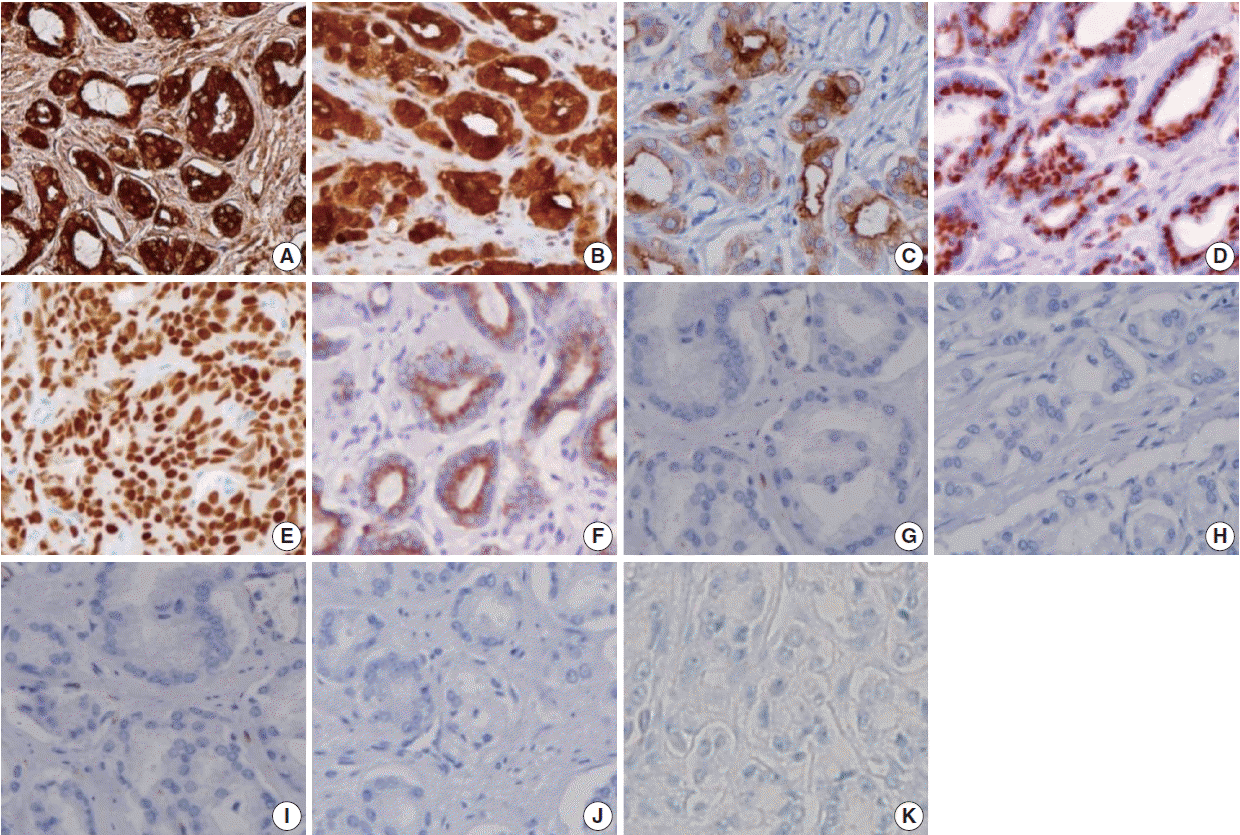
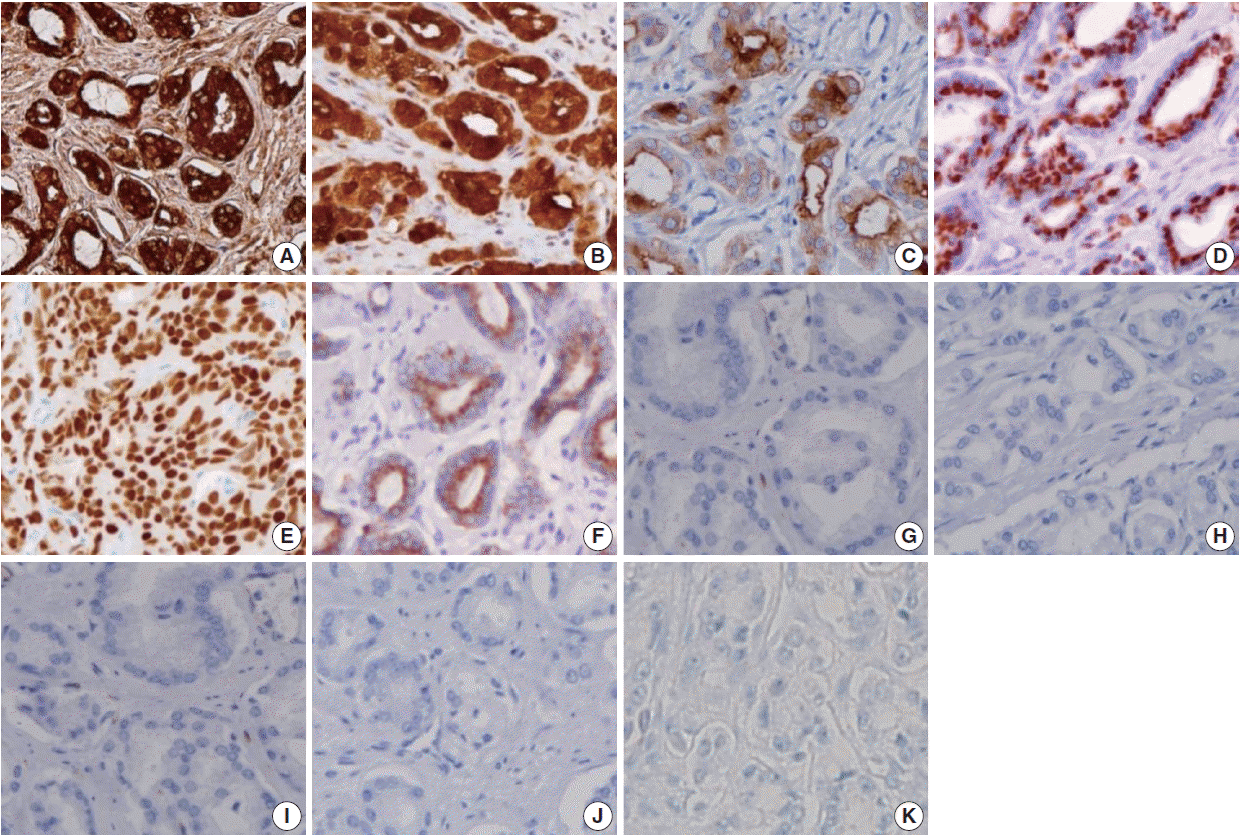
- The sensitivities of prostatic markers in PAC were as follows: PSA (100%), PSMA (83.8%), PAP (91.9%), P501s (93.7%), NKX3.1 (88.3%), and AMACR (66.7%). The specificities of prostatic markers in PAC were as follows: PSA (90.6%), PSMA (99.3%), PAP (81.2%), P501s (99.3%), NKX3.1 (100%), and AMACR (91.3%). The positive predictive values (PPVs) of prostatic markers in PAC were as follows: PSA (89.5%), PSMA (98.9%), PAP (79.7%), P501s (99.1%), NKX3.1 (100%), and AMACR (86.1%). The negative predictive values (NPVs) of prostatic markers in PAC were as follows: PSA (100%), PSMA (88.4%), PAP (92.6%), P501s (95.1%), NKX3.1 (91.4%), and AMACR (77.3%) (Fig. 1).
- Urothelial markers in the PAC group
- Only a small number of PAC was positive for urothelial markers. CK34βE12, p63, thrombomodulin, S100P, and GATA3 immunostains were positive in 1.8%, 0%, 0%, 3.6%, and 0% of PAC, respectively. The specificities of urothelial markers in PAC were as follows: CK34βE12 (24.6%), p63 (26.1%), thrombomodulin (54.3%), S100P (77.5%), and GATA3 (15.2%). The PPVs of urothelial markers in PAC were as follows: CK34βE12 (1.9%), p63 (0%), thrombomodulin (0%), S100P (11.4%), and GATA3 (0%). The NPVs of urothelial markers in PAC were as follows: CK34βE12 (23.8%), p63 (24.5%), thrombomodulin (40.3%), S100P (50.0%), and GATA3 (15.9%) (Fig. 1).
- Urothelial markers in the UC group
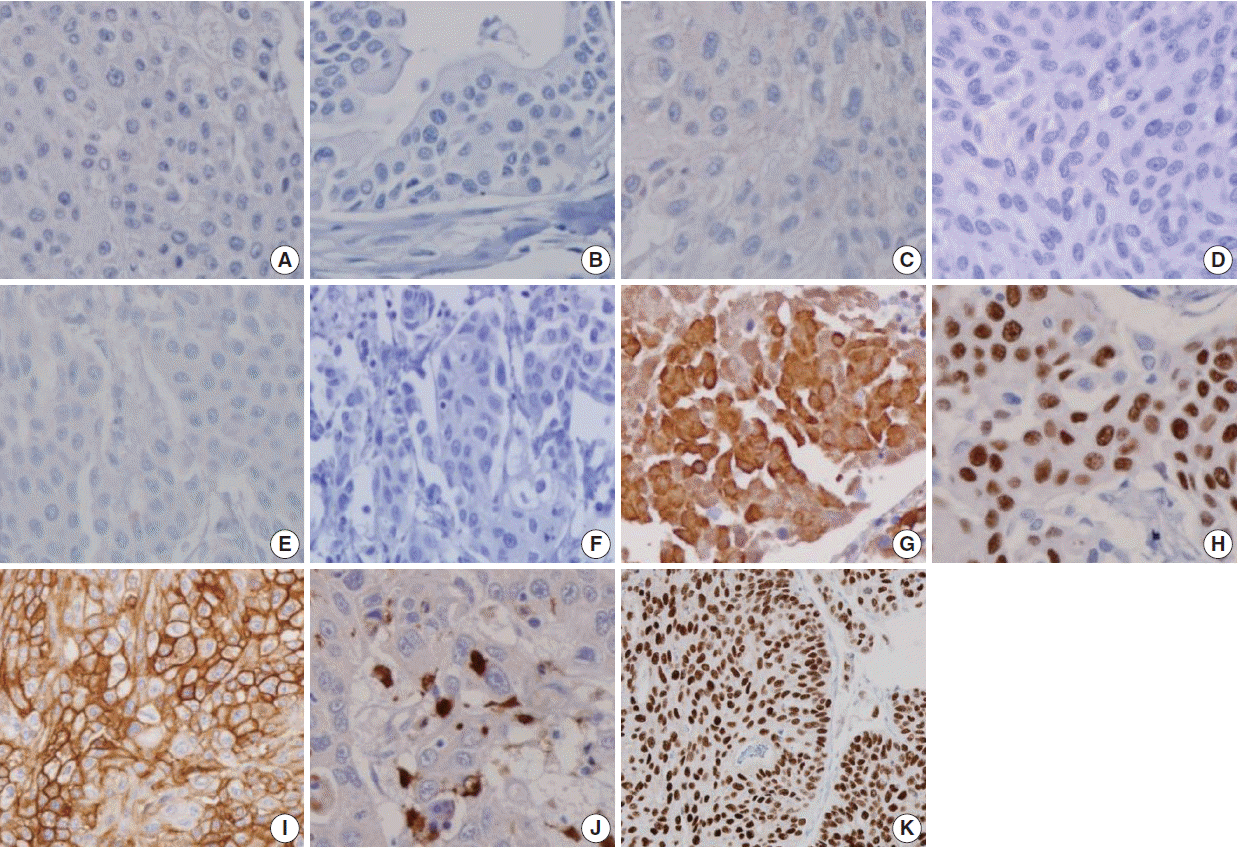
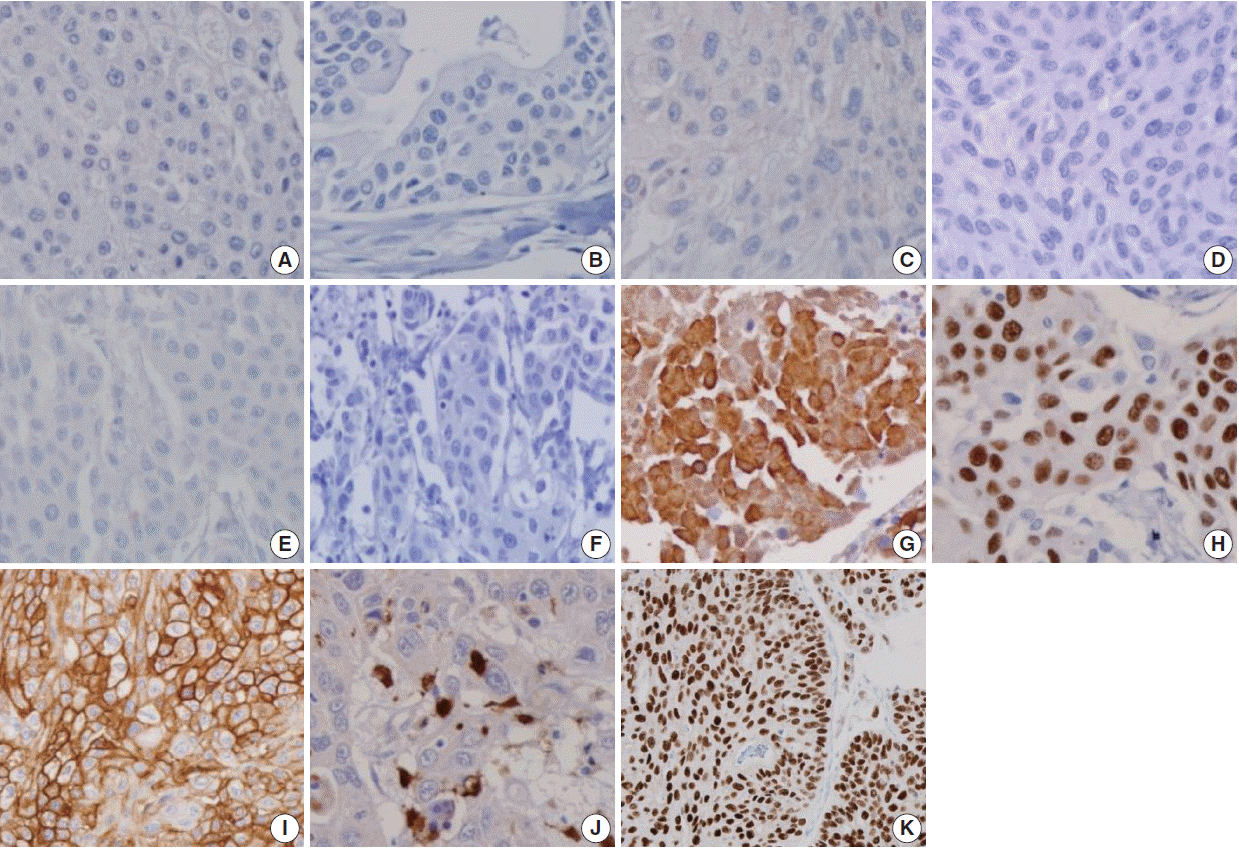
- The sensitivities of urothelial markers in the UC group were as follows: CK34βE12 (75.4%), p63 (73.9%), thrombomodulin (45.7%), S100P (22.5%), and GATA3 (84.8%). The specificities of urothelial markers in UC were as follows: CK34βE12 (98.2%), p63 (100%), thrombomodulin (100%), S100P (96.4%), and GATA3 (100%). The PPVs of urothelial markers in UC were as follows: CK34βE12 (98.1%), p63 (100%), thrombomodulin (100%), S100P (88.6%), and GATA3 (100%). The NPVs of urothelial markers in UC were as follows: CK34βE12 (76.2%), p63 (75.5%), thrombomodulin (59.7%), S100P (50.0%), and GATA3 (84.1%) (Figs. 2, 3).
- Prostatic markers in the UC group
- The prostatic markers PSA, PSMA, PAP, P501s, NKX3.1, and AMACR were also positive in 9.4%, 0.7%, 18.8%, 0.7%, 0%, and 8.7% of UC, respectively. The specificities of prostatic markers in UC were as follows: PSA (0%), PSMA (16.2%), PAP (8.1%), P501s (6.3%), and NKX3.1 (11.7%), and AMACR (33.3%). The PPVs of prostatic markers in the UC group were as follows: PSA (10.5%), PSMA (1.1%), PAP (20.3%), P501s (0.9%), NKX3.1 (0%), and AMACR (13.9%). The NPVs of prostatic markers in UC were as follows: PSA (0%), PSMA (11.6%), PAP (42.9%), P501s (4.9%), NKX3.1 (8.6%), and AMACR (22.7%) (Figs. 2, 3).
- Subanalysis of high-grade PAC and high-grade UC
- We only included the patients with high-grade PAC (47 cases) and high-grade UC (110 cases) and analyzed the sensitivities and specificities of prostatic and urothelial markers. The results for high-grade PAC and high-grade UC were in the same range. No significant differences in the sensitivities and specificities were observed between the entire tumor groups and the high-grade groups (Table 3).
RESULTS
- Although the pathologic identification of PAC and UC using hematoxylin and eosin staining is not difficult in most cases, some cases may present a challenging diagnosis because the histologic appearance of poorly differentiated PAC can be very similar to that of high-grade UC [3].
- High-grade PAC may have enlarged nuclei and prominent nucleoli similar to UC, but little variability in the nuclear size or shape is generally observed in PAC compared with UC [2,3]. Additionally, even in high-grade PAC, there are few mitosis and pleomorphism compared with high-grade UC [3]. Although high-grade UC commonly exhibits more pronounced pleomorphism compared with PAC [2,3], there have been cases of high-grade UC that were indistinguishable from high-grade PAC in terms of pleomorphism and cytologic atypia [3]. UC tends to grow in nests and often shows conspicuous squamous differentiation and glassy eosinophilic cytoplasm. In contrast, the cytoplasm of PAC is generally pale and foamy [3]. Additionally, the findings of focal cribriform glandular differentiation or infiltrating cords of cells are more typical features of PAC than UC [2,3]. As the findings with routine hematoxylin and eosin staining may overlap, immunohistochemical staining may help solve the diagnostic dilemma [3]. Particularly, in poorly differentiated carcinomas involving both the prostate and bladder without any glandular differentiation, the pathology of the case should be evaluated immunohistochemically (Table 4).
- PSA, a serine protease member of the human glandular kallikrein family, is almost exclusively synthesized in the prostate ductal and acinar epithelium, making it a highly specific marker for the prostatic lineage [2]. However, PSA has also been reported to be present in some non-prostatic tissue, such as the urethral, periurethral, and perianal glands [4]. Extraprostatic neoplasms that frequently express PSA include urethral and periurethral adenocarcinoma, cloacogenic carcinoma, salivary gland pleomorphic adenoma, salivary duct carcinoma, and rare breast carcinomas [8]. PSA has been shown to be a highly specific marker, but some authors suggest that there is an inverse correlation between the Gleason score and PSA staining intensity [9]. Previous studies have reported that high-grade PAC that was completely negative for PSA stain ranged from 3% to 27% [3,5,6,9,10]. However, in our study, no PAC specimens were devoid of PSA expression, including high-grade PAC, with 100% sensitivity. Therefore, PSA expression is very useful and valuable for clarifying the prostatic origin of tumors.
- PSMA, a 750 amino acid type II membrane glycoprotein, is expressed by benign and malignant prostatic epithelial cells, with stronger staining observed in the latter [11]. Although PSMA is a very specific marker of prostatic lineage, it is also expressed in non-prostatic tissues, such as the duodenal mucosa, neuroendocrine cells of colonic crypts, endothelial cells of some neoplasms, and proximal renal tubules [12,13]. Some studies have reported an inverse correlation between PSMA staining and the Gleason score [11,12]. The sensitivities of PSMA for PAC ranged from 86.8% to 100% in various studies [3,12-15]. In our study, the sensitivity of PSMA in PAC (83.8%) was lower than PSA, but its specificity (99.3%) was higher than PSA. PSMA has been reported to stain 11% of urinary bladder adenocarcinomas, a fact worth noting [16]. We detected scattered patterns of positive PSMA staining in only one from 138 cases (0.7%) of UC.
- PAP is an early prostatic marker used to confirm the diagnosis of PAC5 [15], and remains a specific marker for prostate tissue. Mhawech et al. [5] reported that 87% of high-grade PAC showed immunopositivity for PAP and observed an inverse correlation between the Gleason score and PAP staining. In this study, PAP was stained in 91.9% of PAC and showed a relative lack of specificity compared with PSMA (81.2% vs 99.3%), with a more variable staining pattern. Monoclonal antibodies to PAP have been reported to have lower sensitivities than their polyclonal counterparts but be more specific [2]. PAP staining has been known to be consistently negative in UC [5,10,17], but a recent study reported immunopositivity in 11.1% of UC [6]. Unexpectedly, we also detected PAP staining with a scattered pattern in 26 of 138 cases of UC (18.8%).
- P501s, a 553-amino acid protein located in the Golgi complex, is a newer prostate-specific protein identified by a combination of high-throughput microarray screening with cDNA subtraction [18]. P501s is expressed by benign and malignant prostatic epithelium and has not been detected in the urothelium or non-prostatic tissue [19]. P501s was reported to be expressed in 94% of a total of observed 113 PAC cases, independent of the metastatic status and Gleason score [19]. Chuang et al. [3] reported that P501s was expressed in all 38 high-grade PAC cases. In the current study, P501s showed high sensitivity (93.7%) and specificity (99.3%) for PAC, and only one of 138 cases of UC (0.7%) was positive for that marker. To date, P501s expression has not been shown in tumors except PAC, making it of great utility in differentiating poorly differentiated PAC from high-grade UC [3,16].
- NKX3.1, a prostate specific androgen regulated homeobox gene [20], is expressed in the prostatic epithelium, rare ureteral and urothelial cells, normal testis, lobular carcinoma of the breast, and bronchial mucous glands [21,22]. Gelmann et al. [22] reported that all 40 observed cases of UC were negative for NKX3.1. In the current study, none of the 138 cases of UC was positive for NKX3.1. The sensitivities of NKX3.1 for PAC reported in previous studies were 92.1%, 89.5%, 87.4%, and 69.2% [3,21-23]. This study also showed a comparable result, with 88.3% sensitivity of NKX3.1 for PAC.
- The AMACR, localized predominantly in peroxisomal structures, plays a critical role in peroxisomal beta oxidation of branched chain fatty acid. Jiang et al. [24] demonstrated that both PAC and high-grade prostate intraepithelial neoplasia (HG-PIN) consistently revealed a significantly higher expression than normal epithelium. However, AMACR expression has repeatedly been demonstrated in HG-PIN and some benign mimickers of PAC. Moreover, Kunju et al. [6] reported that AMACR is expressed in 36% of UC cases. In our study, AMACR was expressed in 66.7% of 111 cases of PAC and 8.7% of 138 cases of UC. AMACR is less sensitive than other prostate markers for PAC and is of limited utility in resolving the difficult problems involving both the prostate and urinary bladder.
- Although PSA, PSMA, PAP, P501s, and NKX3.1 are sensitive and specific markers for evaluating the prostatic origin of tumors, lack of staining was also detected for most markers, except PSA, in this study, at 16.2% for PSMA, 8.1% for PAP, 6.3% for P501s, and 11.7% for NKX3.1 of 111 PAC cases. Therefore, the lack of immunoreactivity of prostate markers in a poorly differentiated carcinoma does not exclude the possibility of a prostatic origin. In addition, false-positives were detected in UC in five of six established prostate markers in this study, ranging from 0.7% to 18.8%, suggesting that the immunohistochemical panel is necessary and useful to discriminate poorly differentiated high-grade carcinomas involving both the prostate and bladder.
- Many immunohistochemical stains have been investigated for UC, but no single marker has been found to be unequivocally diagnostic of urothelial origin. Thus, investigators have recommended a panel of markers to demonstrate the urothelial origin of tumor, such as CK34βE12, p63, thrombomodulin, S100P, and GATA3.
- The monoclonal antibody CK34βE12, which reactive specifically against high-molecular-weight cytokeratins (CKs), including CK1, CK5, CK14, and CK20 [2], is an extremely sensitive marker of urothelial lineage. It is reported to match the sensitivity of p63 and surpass that of uroplakin III and thrombomodulin [3,25]. Compared with previous studies showing sensitivities of 97.2%, 91.4%, and 65.2% for CK34βE12 in UC [3,6,10], our study found 75.4% sensitivity in UC. It is worth noting that CK34βE12 can label squamous epithelia, including areas of squamous differentiation in recurrent PAC after therapy. Thus, Parwani et al. [26] argued that immunopositivity for CK34βE12 restricted to areas of squamous differentiation does not exclude the possibility of PAC.
- p63, a homologue of the p53 tumor suppressor gene, encodes at least six different proteins with a wide range of biologic functions, including a role in urothelial differentiation [2]. Immunostaining for p63 is typically present in more than 90% of the nuclei of the normal urothelia [2]. Many UCs retain a pattern of p63 expression, but p63 expression may be partially lost in high-grade UC [3,27]. Although p63 sensitivity for UC in our study (73.9%) was lower than that of previous studies (82.9%–91.7%) [3,6], its specificity was 100% for UC.
- Thrombomodulin, also designated CD141, is an endothelial cell associated cofactor for the thrombin-mediated activator of protein C [2]. Previous studies have shown that thrombomodulin was immunostained in 48.8%–68.6% of UC [3,5], but our study found a slightly lower expression at 45.7%.
- S100P is highly expressed in the urothelial epithelium [28]. Higgins et al. [28] reported that the polyclonal antibody against S100P labeled 85% of UC and 3% of PAC, whereas the monoclonal antibody against S100P detected 77% of UC and 2% of PAC. Chuang et al. [3] also reported that the monoclonal S100P detected 51.4% of UC and 7.9% of PAC. In our study with a monoclonal antibody against S100P, 22.5% of UC and 3.6% of PAC were stained, which was less than in previous studies [3,28].
- Although CK34βE12 and p63 have been reported to intermittently label PAC in a non-basal cell distribution, thrombomodulin has not been reported to show cross reactivity [5,6,10]. We found that CK34βE12 and p63 immunostains were superior to thrombomodulin or S100P as differential markers of urothelial origin. Only a few scattered cells of PAC were labeled with CK34βE12 (1.8%) and S100P (3.6%), but no PAC was immunopositive for thrombomodulin or p63 in our study.
- GATA3 is a member of a zinc finger transcription factor family that plays an important role in promoting and directing cell proliferation, differentiation, and development [2]. GATA3 is a very sensitive marker for UC, and it is also highly specific in excluding high-grade PAC [29]. Chang et al. [29] reported that none of the 38 high-grade PACs was positive for GATA3. In this study, the sensitivity of GATA3 was 0% in PAC and 84.8% in UC. Uroplakin III is considered the most specific marker for urothelial differentiation, but it has not received popularity due to the lack of uniform expression in UCs [29]. Our study has some limitations because we did not include studies of Uroplakin III.
- In conclusion, prostatic markers, including PSA, PSMA, PAP, and P501s, are very useful for distinguishing PAC from UC. Urothelial markers are less sensitive in identifying UC but rarely stain PAC. In the current study, we found that PSA is most sensitive prostatic marker for distinguishing PAC from UC cases with high sensitivity and negative predictive value. In addition, NKX3.1 is the most specific prostatic marker for distinguishing PAC from UC cases with high specificity and positive predictive value. p63 and thrombomodulin are the most specific urothelial markers for distinguishing UC from PAC cases with high specificities. GATA3 was positive in 117 of 137 cases of UCs and none of the 111 PACs was positive for GATA3. We found that the best combination of immunohistochemical markers for distinguishing PAC from UC is panels consisting of PSA, NKX3.1, p63, thrombomodulin, and GATA3. The optimal combination of immunohistochemical panels of prostatic and urothelial markers could improve the ability to establish the pathologic diagnosis of poorly differentiated high-grade carcinomas involving either the prostate or urinary bladder.
DISCUSSION

Values are presented as number (%).
PAC, prostatic adenocarcinoma; UC, urothelial carcinoma; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; PAP, prostate acid phosphatase; AMACR, α-methylacyl coenzyme A racemase; TM, thrombomodulin; GATA3, GATA binding protein 3; PPV, positive predictive value; NPV, negative predictive value.
| Immunohistochemical marker |
Current study (2016) |
Chuang et al. (2007) [3] |
Kunju et al. (2006) [6] |
Mhawech et al. (2002) [5] |
Genega et al. (2000) [10] |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| PAC | UC | PAC | UC | PAC | UC | PAC | UC | PAC | UC | |
| PSA | 111/111 (100) | 16/138 (11.6) | 37/38 (97.4) | 0/35 (0) | 40/42 (95.2) | 0/36 (0) | 34/40 (85.0) | 0/45 (0) | 32/34 (94.1) | 0/46 (0) |
| PSMA | 93/111 (83.8) | 1/138 (0.7) | 35/38 (92.1) | 0/35 (0) | - | - | - | - | - | - |
| PAP | 102/111 (91.9) | 26/138 (18.8) | - | - | 40/42 (95.2) | 4/36 (11.1) | 38/40 (95.0) | 0/45 (0) | 32/34 (94.1) | 0/46 (0) |
| P501s | 104/111 (93.7) | 1/138 (0.7) | 38/38 (100) | 2/35 (5.7) | - | - | - | - | - | - |
| NKX3.1 | 98/111 (88.3) | 2/138 (1.4) | 36/38 (94.7) | 0/35 (0) | - | - | - | - | - | |
| AMACR | 74/111 (66.7) | 12/138 (8.7) | - | - | 37/42 (88.1) | 13/36 (36.1) | - | - | - | - |
| CK34βE12 | 2/111 (1.8) | 104/138 (75.4) | 3/38 (7.9) | 32/35 (91.4) | 1/42 (2.4) | 35/36 (97.2) | - | - | 2/34 (5.9) | 30/46 (65.2) |
| p63 | 0/111 (0) | 102/138 (73.9) | 0/38 (0) | 29/35 (82.9) | 0/42 (0) | 33/36 (91.7) | - | - | - | - |
| TM | 0/111 (0) | 63/138 (45.7) | 2/38 (5.3) | 24/35 (68.6) | - | - | 0/40 (0) | 22/45 (48.8) | - | - |
| S100P | 4/111 (3.6) | 31/138 (22.5) | 3/38 (7.9) | 25/35 (71.4) | - | - | - | - | - | - |
| GATA3 | 0/111 (0) | 117/138 (95.9) | - | - | - | - | - | - | - | - |
| CK7 | - | - | - | - | 4/42 (9.5) | 34/36 (94.4) | 11/40 (27.5) | 39/45 (86.6) | 4/34 (11.8) | 38/46 (82.6) |
| CK20 | - | - | - | - | 2/42 (4.8) | 19/36 (52.8) | 4/40 (10.0) | 30/45 (66.6) | 8/34 (23.5) | 10/46 (21.7) |
| Uroplakin III | - | - | - | - | - | - | 0/40 (0) | 27/45 (60) | - | - |
Values are presented as number (%).
PAC, prostatic adenocarcinoma; UC, urothelial carcinoma; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; PAP, prostate acid phosphatase; AMACR, α-methylacyl coenzyme A racemase; TM, thrombomodulin; GATA3, GATA binding protein 3; CK, cytokeratin.
- 1. Bates AW, Baithun SI. Secondary neoplasms of the bladder are histological mimics of nontransitional cell primary tumours: clinicopathological and histological features of 282 cases. Histopathology 2000; 36: 32-40. ArticlePubMedPDF
- 2. Dabbs DJ. Diagnostic imunohistochemistry. 3rd ed. Philadelphia: Saunders-Elsevier, 2010; 621-5.
- 3. Chuang AY, DeMarzo AM, Veltri RW, Sharma RB, Bieberich CJ, Epstein JI. Immunohistochemical differentiation of high-grade prostate carcinoma from urothelial carcinoma. Am J Surg Pathol 2007; 31: 1246-55. ArticlePubMed
- 4. Varma M, Jasani B. Diagnostic utility of immunohistochemistry in morphologically difficult prostate cancer: review of current literature. Histopathology 2005; 47: 1-16. ArticlePubMed
- 5. Mhawech P, Uchida T, Pelte MF. Immunohistochemical profile of high-grade urothelial bladder carcinoma and prostate adenocarcinoma. Hum Pathol 2002; 33: 1136-40. ArticlePubMed
- 6. Kunju LP, Mehra R, Snyder M, Shah RB. Prostate-specific antigen, high-molecular-weight cytokeratin (clone 34betaE12), and/or p63: an optimal immunohistochemical panel to distinguish poorly differentiated prostate adenocarcinoma from urothelial carcinoma. Am J Clin Pathol 2006; 125: 675-81. ArticlePubMed
- 7. Chibber PJ, McIntyre MA, Hindmarsh JR, Hargreave TB, Newsam JE, Chisholm GD. Transitional cell carcinoma involving the prostate. Br J Urol 1981; 53: 605-9. ArticlePubMed
- 8. Kamoshida S, Tsutsumi Y. Extraprostatic localization of prostatic acid phosphatase and prostate-specific antigen: distribution in cloacogenic glandular epithelium and sex-dependent expression in human anal gland. Hum Pathol 1990; 21: 1108-11. PubMed
- 9. Goldstein NS. Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores. Am J Clin Pathol 2002; 117: 471-7. ArticlePubMed
- 10. Genega EM, Hutchinson B, Reuter VE, Gaudin PB. Immunophenotype of high-grade prostatic adenocarcinoma and urothelial carcinoma. Mod Pathol 2000; 13: 1186-91. ArticlePubMedPDF
- 11. Marchal C, Redondo M, Padilla M, et al. Expression of prostate specific membrane antigen (PSMA) in prostatic adenocarcinoma and prostatic intraepithelial neoplasia. Histol Histopathol 2004; 19: 715-8. PubMed
- 12. Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer 1998; 82: 2256-61. ArticlePubMed
- 13. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 1997; 3: 81-5. PubMed
- 14. Chang SS, Reuter VE, Heston WD, Gaudin PB. Comparison of anti-prostate-specific membrane antigen antibodies and other immunomarkers in metastatic prostate carcinoma. Urology 2001; 57: 1179-83. ArticlePubMed
- 15. Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998; 52: 637-40. ArticlePubMed
- 16. Lane Z, Hansel DE, Epstein JI. Immunohistochemical expression of prostatic antigens in adenocarcinoma and villous adenoma of the urinary bladder. Am J Surg Pathol 2008; 32: 1322-6. ArticlePubMed
- 17. Bassily NH, Vallorosi CJ, Akdas G, Montie JE, Rubin MA. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol 2000; 113: 383-8. ArticlePubMed
- 18. Xu J, Kalos M, Stolk JA, et al. Identification and characterization of prostein, a novel prostate-specific protein. Cancer Res 2001; 61: 1563-8. PubMed
- 19. Kalos M, Askaa J, Hylander BL, et al. Prostein expression is highly restricted to normal and malignant prostate tissues. Prostate 2004; 60: 246-56. ArticlePubMed
- 20. He WW, Sciavolino PJ, Wing J, et al. A novel human prostate-specific, androgen-regulated homeobox gene (NKX3.1) that maps to 8p21, a region frequently deleted in prostate cancer. Genomics 1997; 43: 69-77. ArticlePubMed
- 21. Bowen C, Bubendorf L, Voeller HJ, et al. Loss of NKX3.1 expression in human prostate cancers correlates with tumor progression. Cancer Res 2000; 60: 6111-5. PubMed
- 22. Gelmann EP, Bowen C, Bubendorf L. Expression of NKX3.1 in normal and malignant tissues. Prostate 2003; 55: 111-7. ArticlePubMed
- 23. Aslan G, Irer B, Tuna B, Yorukoglu K, Saatcioglu F, Celebi I. Analysis of NKX3.1 expression in prostate cancer tissues and correlation with clinicopathologic features. Pathol Res Pract 2006; 202: 93-8. ArticlePubMed
- 24. Jiang Z, Li C, Fischer A, Dresser K, Woda BA. Using an AMACR (P504S)/34betaE12/p63 cocktail for the detection of small focal prostate carcinoma in needle biopsy specimens. Am J Clin Pathol 2005; 123: 231-6. ArticlePubMed
- 25. Parker DC, Folpe AL, Bell J, et al. Potential utility of uroplakin III, thrombomodulin, high molecular weight cytokeratin, and cytokeratin 20 in noninvasive, invasive, and metastatic urothelial (transitional cell) carcinomas. Am J Surg Pathol 2003; 27: 1-10. ArticlePubMed
- 26. Parwani AV, Kronz JD, Genega EM, Gaudin P, Chang S, Epstein JI. Prostate carcinoma with squamous differentiation: an analysis of 33 cases. Am J Surg Pathol 2004; 28: 651-7. PubMed
- 27. Comperat E, Camparo P, Haus R, et al. Immunohistochemical expression of p63, p53 and MIB-1 in urinary bladder carcinoma: a tissue microarray study of 158 cases. Virchows Arch 2006; 448: 319-24. ArticlePubMedPDF
- 28. Higgins JP, Kaygusuz G, Wang L, et al. Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol 2007; 31: 673-80. ArticlePubMed
- 29. Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol 2012; 36: 1472-6. ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Comparative histologic survey and transcriptomic investigation into canine prostate carcinoma
Nathan K. Hoggard, Said M. Elshafae, Nigel A. Daniels, Jonathan A. Young, Chris Premanandan, John B. Echols, Darshan S. Chandrashekar, Blake E. Hildreth, Michael C. Haffner, Thomas J. Rosol
Research in Veterinary Science.2026; 198: 105981. CrossRef - Impact of hormone sensitivity status on aberrant expression of CK7, CK20, CDX2, GATA3 and TTF1 in prostate cancer
Qing Yin Wang, Nazim Benzerdjeb, Samuel Jaquet, Andreea Stepanov, Mame-Kany Diop, Mirela Birlea, Fred Saad, Dominique Trudel
Human Pathology.2025; 163: 105877. CrossRef - Unusual Perineal Metastasis in a Case of Prostate Cancer on 68Ga-PSMA-11 PET/CT
Ritanshu Solanki, Bhagwant Rai Mittal, Rajender Kumar, Aravindh Sekar, Narender Kumar
Clinical Nuclear Medicine.2024; 49(2): e73. CrossRef - NKX3.1 Expression in Non-Prostatic Tumors and Characterizing its Expression in Esophageal/Gastroesophageal Adenocarcinoma
Ansa Mehreen, Kiran G. Manjee, Divyangi Paralkar, Gladell P. Paner, Thanh Lan
Advances in Anatomic Pathology.2024; 31(3): 202. CrossRef - Clinical Management of Intraductal Carcinoma of the Prostate
Gabriel Wasinger, Olivier Cussenot, Eva Compérat
Cancers.2024; 16(9): 1650. CrossRef - Adenocarcinomas of the Gynecologic Tract Involving the Urinary Bladder: A Series of 16 Cases Potentially Mimicking Urothelial Malignancy
Daniel H. Russell, Jonathan I. Epstein, Oleksandr N. Kryvenko, Matthew Schlumbrecht, Merce Jorda, Andre Pinto
Archives of Pathology & Laboratory Medicine.2024; 148(6): 705. CrossRef - Assessing the diagnostic impact of P63, PSA and BCL-2 proteins in premalignant and malignant prostate tissues
Aderonke C. Ogunlayi, Victor O. Ekundina, Adedapo O. Kehinde, Linus A. Enye, Adegoke O. Aremu
International Journal of Scientific Reports.2024; 10(6): 188. CrossRef - Concurrent occurrence of adenocarcinoma and urothelial carcinoma of the prostate gland: A case report
Jhe Yuan Hsu, Yi Sheng Lin, Li Hua Huang, Tang Yi Tsao, Chao Yu Hsu, Yen Chuan Ou, Min Che Tung
World Journal of Clinical Cases.2024; 12(26): 5952. CrossRef - Metastatic prostate cancer presenting as a posterior mediastinal mass: A rare presentation
Muhammad Haider, Arun Umesh Mahtani, Bachar Botrus, Foma Munoh Kenne, Madiha Fatima Master
Clinical Case Reports.2023;[Epub] CrossRef - Diagnostic and Prognostic Roles of GATA3 Immunohistochemistry in Urothelial Carcinoma
Daeseon Yoo, Kyueng-Whan Min, Jung-Soo Pyo, Nae Yu Kim
Medicina.2023; 59(8): 1452. CrossRef - Primary high-grade urothelial carcinoma of prostate with prostatic hyperplasia: a rare case report and review of the literature
Liang Liu, Fu-zhen Sun, Pan-ying Zhang, Yu Xiao, Xiao Yue, Dong-Ming Wang, Qiang Wang
The Aging Male.2023;[Epub] CrossRef - Expression of Gata Binding Protein 3 as a Prognostic Factor in Urogenital Lesions and Its Association With Morphology
T Govardhan, Debahuti Mohapatra, Sujata Naik, Prateek Das, Pranita Mohanty, Ankita Pal
Cureus.2023;[Epub] CrossRef - Histological and immunohistochemical investigation of canine prostate carcinoma with identification of common intraductal carcinoma component
Simone de Brot, Jennifer Lothion‐Roy, Llorenç Grau‐Roma, Emily White, Franco Guscetti, Mark A. Rubin, Nigel P. Mongan
Veterinary and Comparative Oncology.2022; 20(1): 38. CrossRef - Urothelial Carcinoma and Prostate-specific Membrane Antigen: Cellular, Imaging, and Prognostic Implications
Arsalan Tariq, Amy E. McCart Reed, Andrew Morton, Sima Porten, Ian Vela, Elizabeth D. Williams, John W. Yaxley, Peter C. Black, Matthew J. Roberts
European Urology Focus.2022; 8(5): 1256. CrossRef - Immunohistochemical Reactivity of Prostate-Specific Membrane Antigen in Salivary Gland Tumors
Haruto Nishida, Yoshihiko Kondo, Takahiro Kusaba, Hiroko Kadowaki, Tsutomu Daa
Head and Neck Pathology.2022; 16(2): 427. CrossRef - Weak NKX3.1 expression in a urothelial carcinoma: A diagnostic pitfall
Maryam Abdo, Robert Hoyt, Ashley Highfill, Daniel Mettman
Human Pathology Reports.2022; 27: 300599. CrossRef - Gene of the month: NKX3.1
Jon Griffin, Yuqing Chen, James W F Catto, Sherif El-Khamisy
Journal of Clinical Pathology.2022; 75(6): 361. CrossRef - Diagnostic Value of GATA3 and Uroplakin 3 in Differentiating Urothelial Carcinoma from Prostatic and Colorectal Carcinoma
Maha Salama, Dina A. Khairy
Open Access Macedonian Journal of Medical Sciences.2022; 10(A): 514. CrossRef - Diagnostic challenges for the distinction of high-grade prostatic adenocarcinoma and high-grade urothelial carcinoma of simultaneous occurrences - A literature review
Shreyas Bhushan Jayade, Manana Jikurashvili
GEORGIAN SCIENTISTS.2022;[Epub] CrossRef - Cytomorphology, immunoprofile, and clinicopathologic correlation of metastatic prostatic carcinoma
Xiaoqi Lin, Qiuying Shi, Ximing J. Yang
Human Pathology.2022; 130: 36. CrossRef - Cutaneous Metastasis of Prostate Adenocarcinoma: A Rare Presentation of a Common Disease
Alexander Dills, Okechukwu Obi, Kevin Bustos, Jesse Jiang, Shweta Gupta
Journal of Investigative Medicine High Impact Case Reports.2021;[Epub] CrossRef - Mining The Cancer Genome Atlas gene expression data for lineage markers in distinguishing bladder urothelial carcinoma and prostate adenocarcinoma
Ewe Seng Ch’ng
Scientific Reports.2021;[Epub] CrossRef - Immunohistochemical analysis of thrombomodulin expression in myocardial tissue from autopsy cases of ischemic heart disease
Takeshi Kondo, Motonori Takahashi, Gentaro Yamasaki, Marie Sugimoto, Azumi Kuse, Mai Morichika, Kanako Nakagawa, Makoto Sakurada, Migiwa Asano, Yasuhiro Ueno
Legal Medicine.2021; 51: 101897. CrossRef - Application and Pitfalls of Immunohistochemistry in Diagnosis of Challenging Genitourinary Cases
Jenny Ross, Guangyuan Li, Ximing J. Yang
Archives of Pathology & Laboratory Medicine.2020; 144(3): 290. CrossRef - New Screening Test Improves Detection of Prostate Cancer Using Circulating Tumor Cells and Prostate-Specific Markers
Karin Ried, Tasnuva Tamanna, Sonja Matthews, Peter Eng, Avni Sali
Frontiers in Oncology.2020;[Epub] CrossRef - An Unlikely Culprit: Gastric Metastasis from Primary Prostatic Adenocarcinoma
Eric Omar Then, Spoorthi Nutakki, Andrew Ofosu, Saad Saleem, Vijay Gayam, Tagore Sunkara, Vinaya Gaduputi
Journal of Gastrointestinal Cancer.2020; 51(3): 1081. CrossRef - MRI of prostatic urethral mucinous urothelial carcinoma: Expanding the differential diagnosis for T2 hyperintense prostatic masses
Neel Patel, Bryan R. Foster, Elena K. Korngold, Kyle Jensen, Kevin R. Turner, Fergus V. Coakley
Clinical Imaging.2020; 68: 68. CrossRef - Morphological and Immunohistochemical Biomarkers in Distinguishing Prostate Carcinoma and Urothelial Carcinoma: A Comprehensive Review
Francesca Sanguedolce, Davide Russo, Vito Mancini, Oscar Selvaggio, Beppe Calò, Giuseppe Carrieri, Luigi Cormio
International Journal of Surgical Pathology.2019; 27(2): 120. CrossRef - A Case of Metastatic Prostate Cancer to the Urethra That Resolved After Androgen Deprivation Therapy
Darren J. Bryk, Kenneth W. Angermeier, Eric A. Klein
Urology.2019; 129: e4. CrossRef - The Homeodomain Transcription Factor NKX3.1 Modulates Bladder Outlet Obstruction Induced Fibrosis in Mice
Mehul S. Patel, Diana K. Bowen, Nicholas M. Tassone, Andrew D. Gould, Kirsten S. Kochan, Paula R. Firmiss, Natalie A. Kukulka, Megan Y. Devine, Belinda Li, Edward M. Gong, Robert W. Dettman
Frontiers in Pediatrics.2019;[Epub] CrossRef - Cancer of unknown primary: Ancillary testing of cytologic and small biopsy specimens in the era of targeted therapy
Morgan L. Cowan, Christopher J. VandenBussche
Cancer Cytopathology.2018; 126(S8): 724. CrossRef - Glandular Tumors of the Urachus and Urinary Bladder: A Practical Overview of a Broad Differential Diagnosis
Alexander S. Taylor, Rohit Mehra, Aaron M. Udager
Archives of Pathology & Laboratory Medicine.2018; 142(10): 1164. CrossRef - S100P as a Marker for Urothelial Histogenesis: A Critical Review and Comparison With Novel and Traditional Urothelial Immunohistochemical Markers
Moushumi Suryavanshi, Julian Sanz-Ortega, Deepika Sirohi, Mukul K. Divatia, Chisato Ohe, Claudia Zampini, Daniel Luthringer, Steven C. Smith, Mahul B. Amin
Advances in Anatomic Pathology.2017; 24(3): 151. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1.
Fig. 2.
Fig. 3.
| Antibody | Clone | Dilution | Vendor |
|---|---|---|---|
| PSA | Polyclonal | 1:1,000 | Dako |
| PSMA | Monoclonal | 1:100 | Novocastra |
| PAP | Monoclonal | Prediluted | Dako |
| P501s | Monoclonal | 1:200 | Dako |
| NKX3.1 | Monoclonal | 1:500 | Athena ES |
| AMACR | Monoclonal | 1:200 | Cell Marque |
| CK34βE12 | Monoclonal | 1:50 | Dako |
| p63 | Monoclonal | 1:800 | Lab Vision |
| TM | Monoclonal | 1:1,000 | Dako |
| S100P | Monoclonal | 1:800 | Dako |
| GATA3 | Monoclonal | Prediluted | Cell Marque |
| Variable | PSA | PSMA | PAP | P501s | NKX3.1 | AMACR | CK34βE12 | p63 | TM | S100P | GATA3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PAC | |||||||||||
| Sensitivity | 111/111 (100) | 93/111 (83.8) | 102/111 (91.9) | 104/111 (93.7) | 98/111 (88.3) | 74/111 (66.7) | 2/111 (1.8) | 0/111 (0) | 0/111 (0) | 4/111 (3.6) | 0/111 (0) |
| Specificity | 125/138 (90.6) | 137/138 (99.3) | 112/138 (81.2) | 137/138 (99.3) | 138/138 (100) | 126/138 (91.3) | 34/138 (24.6) | 36/138 (26.1) | 75/138 (54.3) | 107/138 (77.5) | 21/138 (15.2) |
| PPV | 111/124 (89.5) | 93/94 (98.9) | 102/128 (79.7) | 104/105 (99.1) | 98/98 (100) | 74/86 (86.1) | 2/106 (1.9) | 0/102 (0) | 0/63 (0) | 4/35 (11.4) | 0/117 (0) |
| NPV | 125/125 (100) | 137/155 (88.4) | 112/121 (92.6) | 137/144 (95.1) | 138/151 (91.4) | 126/163 (77.3) | 34/143 (23.8) | 36/147 (24.5) | 75/186 (40.3) | 107/214 (50) | 21/132 (15.9) |
| UC | |||||||||||
| Sensitivity | 13/138 (9.4) | 1/138 (0.7) | 26/138 (18.8) | 1/138 (0.7) | 0/138 (0) | 12/138 (8.7) | 104/138 (75.4) | 102/138 (73.9) | 63/138 (45.7) | 31/138 (22.5) | 117/138 (84.8) |
| Specificity | 0/111 (0) | 18/111 (16.2) | 9/111 (8.1) | 7/111 (6.3) | 13/111 (11.7) | 37/111 (33.3) | 109/111 (98.2) | 111/111 (100) | 111/111 (100) | 107/111 (96.4) | 111/111 (100) |
| PPV | 13/124 (10.5) | 1/94 (1.1) | 26/128 (20.3) | 1/105 (0.9) | 0/87 (0) | 12/86 (13.9) | 104/106 (98.1) | 102/102 (100) | 63/63 (100) | 31/35 (88.6) | 117/117 (100) |
| NPV | 0/25 (0) | 18/155 (11.6) | 9/21 (42.9) | 7/144 (4.9) | 13/151 (8.6) | 37/163 (22.7) | 109/143 (76.2) | 111/147 (75.5) | 111/186 (59.7) | 107/214 (50) | 106/127 (84.1) |
| Variable | PSA | PSMA | PAP | P501s | CK34βE12 | p63 | S100P | GATA3 |
|---|---|---|---|---|---|---|---|---|
| PAC | ||||||||
| Sensitivity | 47/47 (100) | 39/47 (83) | 43/47 (91.5) | 44/47 (93.6) | 0/47 (0) | 1/47 (2.1) | 2/47 (4.3) | 0/47 (0) |
| Specificity | 95/110 (86.4) | 109/110 (99.1) | 85/110 (77.3) | 109/110 (99.1) | 23/110 (20.9) | 27/110 (24.6) | 84/110 (76.4) | 17/110 (15.5) |
| PPV | 47/62 (75.8) | 39/40 (97.5) | 43/68 (63.2) | 44/45 (98.8) | 0/87 (0) | 1/84 (1.2) | 2/28 (7.1) | 0/93 (0) |
| NPV | 95/95 (100) | 109/117 (93.2) | 85/90 (95.5) | 109/112 (97.3) | 23/70 (32.9) | 27/73 (37) | 84/129 (65.1) | 17/64 (26.6) |
| UC | ||||||||
| Sensitivity | 15/110 (13.6) | 1/110 (0.9) | 25/110 (22.7) | 1/110 (0.9) | 87/110 (79.1) | 83/110 (75.5) | 26/110 (23.6) | 93/110 (84.5) |
| Specificity | 0/47 (0) | 8/47 (17.0) | 4/47 (8.5) | 3/47 (6.4) | 47/47 (100) | 46/47 (97.9) | 45/47 (95.7) | 47/47 (100) |
| PPV | 15/62 (24.2) | 1/40 (2.5) | 25/68 (36.8) | 1/45 (2.2) | 87/87 (100) | 83/84 (98.8) | 26/28 (92.9) | 93/93 (100) |
| NPV | 0/95 (0) | 8/117 (6.8) | 4/89 (4.5) | 3/112 (2.7) | 47/70 (67.1) | 46/73 (63) | 45/129 (34.9) | 46/63 (74.6) |
| Immunohistochemical marker | Current study (2016) |
Chuang et al. (2007) [3] |
Kunju et al. (2006) [6] |
Mhawech et al. (2002) [5] |
Genega et al. (2000) [10] |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| PAC | UC | PAC | UC | PAC | UC | PAC | UC | PAC | UC | |
| PSA | 111/111 (100) | 16/138 (11.6) | 37/38 (97.4) | 0/35 (0) | 40/42 (95.2) | 0/36 (0) | 34/40 (85.0) | 0/45 (0) | 32/34 (94.1) | 0/46 (0) |
| PSMA | 93/111 (83.8) | 1/138 (0.7) | 35/38 (92.1) | 0/35 (0) | - | - | - | - | - | - |
| PAP | 102/111 (91.9) | 26/138 (18.8) | - | - | 40/42 (95.2) | 4/36 (11.1) | 38/40 (95.0) | 0/45 (0) | 32/34 (94.1) | 0/46 (0) |
| P501s | 104/111 (93.7) | 1/138 (0.7) | 38/38 (100) | 2/35 (5.7) | - | - | - | - | - | - |
| NKX3.1 | 98/111 (88.3) | 2/138 (1.4) | 36/38 (94.7) | 0/35 (0) | - | - | - | - | - | |
| AMACR | 74/111 (66.7) | 12/138 (8.7) | - | - | 37/42 (88.1) | 13/36 (36.1) | - | - | - | - |
| CK34βE12 | 2/111 (1.8) | 104/138 (75.4) | 3/38 (7.9) | 32/35 (91.4) | 1/42 (2.4) | 35/36 (97.2) | - | - | 2/34 (5.9) | 30/46 (65.2) |
| p63 | 0/111 (0) | 102/138 (73.9) | 0/38 (0) | 29/35 (82.9) | 0/42 (0) | 33/36 (91.7) | - | - | - | - |
| TM | 0/111 (0) | 63/138 (45.7) | 2/38 (5.3) | 24/35 (68.6) | - | - | 0/40 (0) | 22/45 (48.8) | - | - |
| S100P | 4/111 (3.6) | 31/138 (22.5) | 3/38 (7.9) | 25/35 (71.4) | - | - | - | - | - | - |
| GATA3 | 0/111 (0) | 117/138 (95.9) | - | - | - | - | - | - | - | - |
| CK7 | - | - | - | - | 4/42 (9.5) | 34/36 (94.4) | 11/40 (27.5) | 39/45 (86.6) | 4/34 (11.8) | 38/46 (82.6) |
| CK20 | - | - | - | - | 2/42 (4.8) | 19/36 (52.8) | 4/40 (10.0) | 30/45 (66.6) | 8/34 (23.5) | 10/46 (21.7) |
| Uroplakin III | - | - | - | - | - | - | 0/40 (0) | 27/45 (60) | - | - |
PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; PAP, prostate acid phosphatase; AMACR, α-methylacyl coenzyme A racemase; TM, thrombomodulin; GATA3, GATA binding protein 3.
Values are presented as number (%). PAC, prostatic adenocarcinoma; UC, urothelial carcinoma; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; PAP, prostate acid phosphatase; AMACR, α-methylacyl coenzyme A racemase; TM, thrombomodulin; GATA3, GATA binding protein 3; PPV, positive predictive value; NPV, negative predictive value.
Values are presented as number (%). PAC, prostatic adenocarcinoma; UC, urothelial carcinoma; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; PAP, prostate acid phosphatase; GATA3, GATA binding protein 3; PPV, positive predictive value; NPV, negative predictive value.
Values are presented as number (%). PAC, prostatic adenocarcinoma; UC, urothelial carcinoma; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; PAP, prostate acid phosphatase; AMACR, α-methylacyl coenzyme A racemase; TM, thrombomodulin; GATA3, GATA binding protein 3; CK, cytokeratin.

 E-submission
E-submission