Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 49(3); 2015 > Article
-
Original Article
Proposal of an Appropriate Decalcification Method of Bone Marrow Biopsy Specimens in the Era of Expanding Genetic Molecular Study - Sung-Eun Choi, Soon Won Hong, Sun Och Yoon
-
Journal of Pathology and Translational Medicine 2015;49(3):236-242.
DOI: https://doi.org/10.4132/jptm.2015.03.16
Published online: May 15, 2015
Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
- Corresponding Author Sun Och Yoon, M.D., Ph.D. Department of Pathology, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 120-752, Korea Tel: +82-2-2228-1763 Fax: +82-2-2227-7939 E-mail: revita@naver.com
© 2015 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Germline and somatic testing for homologous repair deficiency in patients with prostate cancer (part 1 of 2)
Andrew J. Armstrong, Amy Taylor, Michael C. Haffner, Wassim Abida, Alan H. Bryce, Lawrence I. Karsh, Scott T. Tagawa, Przemyslaw Twardowski, Anthony V. Serritella, Joshua M. Lang
Prostate Cancer and Prostatic Diseases.2025; 28(3): 652. CrossRef - Spatial transcriptomic approaches for characterising the bone marrow landscape: pitfalls and potential
Rosalin A. Cooper, Emily Thomas, Anna M. Sozanska, Carlo Pescia, Daniel J. Royston
Leukemia.2025; 39(2): 291. CrossRef - Qualitative comparison of decalcifiers for mouse bone cryosections for subsequent biophotonic analysis
Shibarjun Mandal, Ramya Motganhalli Ravikumar, Astrid Tannert, Annett Urbanek, Rustam R. Guliev, Max Naumann, Sina M. Coldewey, Uta Dahmen, Lina Carvalho, Luís Bastião Silva, Ute Neugebauer
Scientific Reports.2025;[Epub] CrossRef - Spatial Platform for Periodontal Ligament Angulation and Regeneration: In Vivo Pilot Study
Min Guk Kim, Do-Yeon Kim, Hyoung-Gon Ko, Jin-Seok Byun, Joong-Hyun Kim, Chan Ho Park
Journal of Functional Biomaterials.2025; 16(3): 99. CrossRef - Spatial proteomics and transcriptomics characterization of tissue and multiple cancer types including decalcified marrow
Cecilia CS Yeung, Daniel C Jones, David W. Woolston, Brandon Seaton, Elizabeth Lawless Donato, Minggang Lin, Coral Backman, Vivian Oehler, Kristin L Robinson, Kristen Shimp, Rima Kulikauskas, Annalyssa N Long, David Sowerby, Anna E Elz, Kimberly S Smythe,
Cancer Biomarkers.2025;[Epub] CrossRef - Circulating tumor cell markers for early detection and drug resistance assessment through liquid biopsy
Priya Yadav, Saravanan Rajendrasozhan, Ramzi Hadj Lajimi, Raja Ramadevi Patel, Dominique Heymann, N. Rajendra Prasad
Frontiers in Oncology.2025;[Epub] CrossRef - Morphological Bone Score as a Predictive Tool for Molecular Profiling Success
Kirill Kriukov, Dmitry Ivchenkov, Anna Bejanyan, Aleksandr Sarachakov, Aleksandra Kviatkovskaia, Gleb Khegai, Dominique Knipper-Davis, Amber Berlinski, Tayla Soares, Jochen K. Lennerz, Vladimir Kushnarev
The Journal of Molecular Diagnostics.2025; 27(8): 747. CrossRef - Optimizing cytology and small biopsy specimen processing for ancillary studies: recommendations from the American Society of Cytopathology taskforce
Sinchita Roy-Chowdhuri, Christine N. Booth, Jonas J. Heymann, Elizabeth Jenkins, Joshua R. Menke, Sara E. Monaco, Ritu Nayar, Michiya Nishino, Roberto Ruiz-Cordero, Donna K. Russell, Anjali Saqi, Kaitlin E. Sundling, Michael J. Thrall, Vanda F. Torous, Ch
Journal of the American Society of Cytopathology.2025; 14(5): 285. CrossRef - Ethylenediaminetetraacetic Acid (EDTA)-Decalcified, Formalin-Fixed Paraffin-Embedded (FFPE) Tumor Tissue Shows Comparable Quality and Quantity of DNA to Non-Decalcified Tissue in Next-Generation Sequencing (NGS)
Francis Hong Xin Yap, Jen-Hwei Sng, Jeremy Wee Kiat Ng, Hanis Abdul Kadir, Pei Yi Chan, Timothy Kwang Yong Tay
Journal of Molecular Pathology.2025; 6(3): 21. CrossRef - Optimization of Formic Acid-Formalin-Based Decalcification Protocol for Rat Calvarial Bone Histology
S. Amitha Banu, Khan Sharun, Merlin Mamachan, Athira Subash, Vadapalli Deekshita, Kirtika Sharma, Karikalan Mathesh, Obli Rajendran Vinodh kumar, Swapan Kumar Maiti, Abhijit M. Pawde, Laith Abualigah, Kuldeep Dhama, Amarpal
Journal of Experimental Biology and Agricultural Sciences.2024; 12(2): 218. CrossRef - Effects of fixation and demineralization on histomorphology and DNA amplification of canine bone marrow
Gabriella M. L. Diamantino, Janet Beeler-Marfisi, Robert A. Foster, William Sears, Alice Defarges, William Vernau, Dorothee Bienzle
Veterinary Pathology.2024; 61(6): 943. CrossRef - In situ metabolomic analysis of osteonecrosis of the femoral head (ONFH) using MALDI MSI
Chen Li, Jikun Liu, Yiqi Sheng, Yinghao Wang, Lan Jia, Yinguang Zhang, Jiantao Li, Shuangshuang Di, Honggang Nie, Yehua Han
Analytical and Bioanalytical Chemistry.2024; 416(23): 5155. CrossRef - A set of pretreatment reagents including improved formula fixation and decalcification facilitating immunohistochemistry and DNA analyses of formalin-fixed paraffin-embedded bone marrow trephine biopsy
Ting Sun, Liming Xu, Hongtian Yao, Jing Zhao, Zhen Chen, Zexin Chen, Bo Wang, Wei Ding
Acta Histochemica.2024; 126(8): 152188. CrossRef - To Freeze or Not to Freeze? Recommendations for Intraoperative Examination and Gross Prosection of Thyroid Glands
Fouad R. Zakka, Nicole A. Cipriani
Surgical Pathology Clinics.2023; 16(1): 15. CrossRef - Effect of Surface Decalcification With Hydrochloric Acid on the Determination of Estrogen Receptor, Progesterone Receptor, Ki67, and Human Epidermal Growth Factor Receptor 2 Expressions in Invasive Breast Carcinoma Based on Immunohistochemistry and Fluore
Wu Ping, Rao Xin, Zhang Li, Chen Yupeng, Song Fangling, Ren Caihong, Hu Shun, Zhang Sheng
Applied Immunohistochemistry & Molecular Morphology.2023; 31(4): 232. CrossRef - Diagnostic value of MDM2 RNA in situ hybridization for low-grade osteosarcoma: Consistency comparison of RNA in situ hybridization, fluorescence in situ hybridization, and immunohistochemistry
Chen Chen, Xin He, Min Chen, Tianhai Du, Weiji Qin, Wenyi Jing, Hongying Zhang
Virchows Archiv.2023; 482(6): 999. CrossRef - Bone marrow fibrosis is associated with non‐response to CD19 CAR T‐cell therapy in B‐acute lymphoblastic leukemia
Joshua Anil, Ahab Alnemri, Andrew Lytle, Brian Lockhart, Ashley E. Anil, Michael Baumgartner, Kirubel Gebre, Jared McFerran, Stephan A. Grupp, Susan R. Rheingold, Vinodh Pillai
American Journal of Hematology.2023; 98(12): 1888. CrossRef - Epithelioid haemangioendothelioma of the mandible – A case report and review of the literature
Ali Rizvi, Tim K. Blackburn, Guy N. J. Betts
Oral Surgery.2022; 15(3): 387. CrossRef - Evaluation of EDTA and nitric acid solutions for decalcification of joints in AG/WT, BALB/c, C57, DBA1/J mice, and in Wistar rats
Eduarda Correa Freitas, Suelen Pizzolatto Dalmolin, Mateus Müller da Silva, Francine Hehn de Oliveira, Emily Ferreira Salles Pilar
Biotechnic & Histochemistry.2022; 97(5): 372. CrossRef - Coupling Lipid Labeling and Click Chemistry Enables Isolation of Extracellular Vesicles for Noninvasive Detection of Oncogenic Gene Alterations
Na Sun, Benjamin V. Tran, Zishan Peng, Jing Wang, Ceng Zhang, Peng Yang, Tiffany X. Zhang, Josephine Widjaja, Ryan Y. Zhang, Wenxi Xia, Alexandra Keir, Jia‐Wei She, Hsiao‐hua Yu, Jing‐Jong Shyue, Hongguang Zhu, Vatche G. Agopian, Renjun Pei, James S. Toml
Advanced Science.2022;[Epub] CrossRef - The Expressions of CD30 and CD123 of Mastocytosis in Taiwan
Ching-Fen Yang, Chih-Yi Hsu
Applied Immunohistochemistry & Molecular Morphology.2022; 30(4): 278. CrossRef - Unusual Patterns of HER2 Expression in Breast Cancer: Insights and Perspectives
Dora Grassini, Eliano Cascardi, Ivana Sarotto, Laura Annaratone, Anna Sapino, Enrico Berrino, Caterina Marchiò
Pathobiology.2022; 89(5): 278. CrossRef - Expert opinion on NSCLC small specimen biomarker testing — Part 1: Tissue collection and management
Frédérique Penault-Llorca, Keith M. Kerr, Pilar Garrido, Erik Thunnissen, Elisabeth Dequeker, Nicola Normanno, Simon J. Patton, Jenni Fairley, Joshua Kapp, Daniëlle de Ridder, Aleš Ryška, Holger Moch
Virchows Archiv.2022; 481(3): 335. CrossRef - Comparison of bone demineralisation procedures for DNA recovery from burned remains
Meghan Mckinnon, Denice Higgins
Forensic Science International: Genetics.2021; 51: 102448. CrossRef - A review of the current understanding of burned bone as a source of DNA for human identification
Meghan Mckinnon, Maciej Henneberg, Denice Higgins
Science & Justice.2021; 61(4): 332. CrossRef - Time is bone — Quantitative comparison of decalcification solvents in human femur samples using dual-X-ray-absorptiometry and computed tomography
Joshua Gawlitza, Jakob Steinhäuser, Arno Bücker, Gabriela Krasteva-Christ, Thomas Tschernig
Annals of Anatomy - Anatomischer Anzeiger.2021; 235: 151696. CrossRef - Molecular biomarker testing for non–small cell lung cancer: consensus statement of the Korean Cardiopulmonary Pathology Study Group
Sunhee Chang, Hyo Sup Shim, Tae Jung Kim, Yoon-La Choi, Wan Seop Kim, Dong Hoon Shin, Lucia Kim, Heae Surng Park, Geon Kook Lee, Chang Hun Lee
Journal of Pathology and Translational Medicine.2021; 55(3): 181. CrossRef - Effect of EDTA decalcification on estrogen receptor and progesterone receptor immunohistochemistry and HER2/neu fluorescence in situ hybridization in breast carcinoma
Erik Washburn, Xiaoyu Tang, Carla Caruso, Michelle Walls, Bing Han
Human Pathology.2021; 117: 108. CrossRef - Performances of single tube nested polymerase chain reaction and GeneXpert ultra on Formalin fixed paraffin embedded tissues in the diagnosis of tuberculous spondylodiscitis
Emna Romdhane, Soumaya Rammeh, Chelli Mouna Bouaziz, Hend Riahi, Meriam Rekaya Ben, Meriam Ksentini, Yosra Chebbi, Wafa Achour, Asma Ferjani, Ben Boubaker Ilhem Boutiba, Leila Slim-Saidi, Mohamed Fethi Ladeb
Clinical Rheumatology.2021; 40(10): 4317. CrossRef - Molecular Characterization of Prostate Cancers in the Precision Medicine Era
Emilio Francesco Giunta, Laura Annaratone, Enrico Bollito, Francesco Porpiglia, Matteo Cereda, Giuseppe Luigi Banna, Alessandra Mosca, Caterina Marchiò, Pasquale Rescigno
Cancers.2021; 13(19): 4771. CrossRef - Increased NF-κB Activity in Osteoprogenitor-Lineage Cells Impairs the Balance of Bone Versus Fat in the Marrow of Skeletally Mature Mice
Tzuhua Lin, Jukka Pajarinen, Yusuke Kohno, Akira Nabeshima, Laura Lu, Karthik Nathan, Zhenyu Yao, Joy Y. Wu, Stuart Goodman
Regenerative Engineering and Translational Medicine.2020; 6(1): 69. CrossRef - Percutaneous CT-guided biopsy of lytic bone lesions in patients clinically suspected of lung cancer: Diagnostic performances for pathological diagnosis and molecular testing
Anne-Claire Toffart, Stéphane Asfari, Anne Mc Leer, Emilie Reymond, Adrien Jankowski, Denis Moro-Sibilot, Olivier Stephanov, Julien Ghelfi, Sylvie Lantuejoul, Gilbert R. Ferretti
Lung Cancer.2020; 140: 93. CrossRef - Effect of decalcification protocols on immunohistochemistry and molecular analyses of bone samples
Elodie Miquelestorena-Standley, Marie-Lise Jourdan, Christine Collin, Corinne Bouvier, Frédérique Larousserie, Sébastien Aubert, Anne Gomez-Brouchet, Jean-Marc Guinebretière, Matthias Tallegas, Bénédicte Brulin, Louis-Romée Le Nail, Anne Tallet, François
Modern Pathology.2020; 33(8): 1505. CrossRef - Identifying Opportunities and Challenges for Patients With Sarcoma as a Result of Comprehensive Genomic Profiling of Sarcoma Specimens
Margaret A. Hay, Eric A. Severson, Vincent A. Miller, David A. Liebner, Jo-Anne Vergilio, Sherri Z. Millis, James L. Chen
JCO Precision Oncology.2020; (4): 176. CrossRef - Comparison of Methods for the Histological Evaluation of Odontocete Spiral Ganglion Cells
Tania Ramírez, Simona Sacchini, Yania Paz, Rubén S. Rosales, Nakita Câmara, Marisa Andrada, Manuel Arbelo, Antonio Fernández
Animals.2020; 10(4): 683. CrossRef - Molecular Pathology of Primary Non-small Cell Lung Cancer
David Ilan Suster, Mari Mino-Kenudson
Archives of Medical Research.2020; 51(8): 784. CrossRef - Comparison of ethylenediaminetetraacetic acid and rapid decalcificier solution for studying human temporal bones by immunofluorescence
Sumana Ghosh, Mark B. Lewis, Bradley J. Walters
Laryngoscope Investigative Otolaryngology.2020; 5(5): 919. CrossRef - A novel cryo-embedding method for in-depth analysis of craniofacial mini pig bone specimens
Pavla Ticha, Igor Pilawski, Xue Yuan, Jie Pan, Ustun S. Tulu, Benjamin R. Coyac, Waldemar Hoffmann, Jill A. Helms
Scientific Reports.2020;[Epub] CrossRef - Accelerating precision medicine in metastatic prostate cancer
Joaquin Mateo, Rana McKay, Wassim Abida, Rahul Aggarwal, Joshi Alumkal, Ajjai Alva, Felix Feng, Xin Gao, Julie Graff, Maha Hussain, Fatima Karzai, Bruce Montgomery, William Oh, Vaibhav Patel, Dana Rathkopf, Matthew Rettig, Nikolaus Schultz, Matthew Smith,
Nature Cancer.2020; 1(11): 1041. CrossRef - Tissue Morphology and Antigenicity in Mouse and Rat Tibia: Comparing 12 Different Decalcification Conditions
Kristofor Bogoevski, Anna Woloszyk, Keith Blackwood, Maria A. Woodruff, Vaida Glatt
Journal of Histochemistry & Cytochemistry.2019; 67(8): 545. CrossRef - Cellular and collagen reference values of gingival and periodontal ligament tissues in rats: a pilot study
Antoine Alves, Nina Attik, Carine Wirth, Yves Bayon, Alexis Piat, Brigitte Grosgogeat, Kerstin Gritsch
Histochemistry and Cell Biology.2019; 152(2): 145. CrossRef - Implementing Precision Medicine Programs and Clinical Trials in the Community-Based Oncology Practice: Barriers and Best Practices
Jennifer L. Ersek, Lora J. Black, Michael A. Thompson, Edward S. Kim
American Society of Clinical Oncology Educational Book.2018; (38): 188. CrossRef - Integration of next-generation sequencing in clinical diagnostic molecular pathology laboratories for analysis of solid tumours; an expert opinion on behalf of IQN Path ASBL
Zandra C Deans, Jose Luis Costa, Ian Cree, Els Dequeker, Anders Edsjö, Shirley Henderson, Michael Hummel, Marjolijn JL Ligtenberg, Marco Loddo, Jose Carlos Machado, Antonio Marchetti, Katherine Marquis, Joanne Mason, Nicola Normanno, Etienne Rouleau, Ed S
Virchows Archiv.2017; 470(1): 5. CrossRef - Protocolo para el estudio de muestras y estandarización del informe patológico de tumores óseos
Isidro Machado, José Juan Pozo, David Marcilla, Julia Cruz, Juan C. Tardío, Aurora Astudillo, Sílvia Bagué
Revista Española de Patología.2017; 50(1): 34. CrossRef - Extremely Well-Differentiated Papillary Thyroid Carcinoma Resembling Adenomatous Hyperplasia Can Metastasize to the Skull: A Case Report
Ju Yeon Pyo, Jisup Kim, Sung-eun Choi, Eunah Shin, Seok-Woo Yang, Cheong Soo Park, Seok-Mo Kim, SoonWon Hong
Yonsei Medical Journal.2017; 58(1): 255. CrossRef - Treatment of steroid-induced osteonecrosis of the femoral head using porous Se@SiO2 nanocomposites to suppress reactive oxygen species
Guoying Deng, Kerun Niu, Feng Zhou, Buxiao Li, Yingjie Kang, Xijian Liu, Junqing Hu, Bo Li, Qiugen Wang, Chengqing Yi, Qian Wang
Scientific Reports.2017;[Epub] CrossRef - Precision Medicine Starts With Preanalytics: Real-Time Assessment of Tissue Fixation Quality by Ultrasound Time-of-Flight Analysis
Melissa L. Lerch, Daniel R. Bauer, David Chafin, Abbey Theiss, Michael Otter, Geoffrey S. Baird
Applied Immunohistochemistry & Molecular Morphology.2017; 25(3): 160. CrossRef - Good Laboratory Standards for Clinical Next-Generation Sequencing Cancer Panel Tests
Jihun Kim, Woong-Yang Park, Nayoung K. D. Kim, Se Jin Jang, Sung-Min Chun, Chang-Ohk Sung, Jene Choi, Young-Hyeh Ko, Yoon-La Choi, Hyo Sup Shim, Jae-Kyung Won
Journal of Pathology and Translational Medicine.2017; 51(3): 191. CrossRef - An international survey about nail histology processing techniques
Christina Wlodek, Pauline Lecerf, Josette Andre, Beth S. Ruben, David de Berker
Journal of Cutaneous Pathology.2017; 44(9): 749. CrossRef - pSTAT5 and ERK exhibit different expression in myeloproliferative neoplasms
Ewa Wiśniewska-Chudy, Łukasz Szylberg, Grzegorz Dworacki, Ewa Mizera-Nyczak, Andrzej Marszałek
Oncology Reports.2017; 37(4): 2295. CrossRef - How we do: optimizing bone marrow biopsy logistics for sign-out within 2 days
I. de Laak–de Vries, A. G. Siebers, L. Burgers, C. Diepenbroek, M. Link, P. Groenen, J. H. J. M. van Krieken, K. M. Hebeda
Journal of Hematopathology.2016; 9(2): 67. CrossRef - Do More With Less: Tips and Techniques for Maximizing Small Biopsy and Cytology Specimens for Molecular and Ancillary Testing: The University of Colorado Experience
Dara L. Aisner, Mathew D. Rumery, Daniel T. Merrick, Kimi L. Kondo, Hala Nijmeh, Derek J. Linderman, Robert C. Doebele, Natalie Thomas, Patrick C. Chesnut, Marileila Varella-Garcia, Wilbur A. Franklin, D. Ross Camidge
Archives of Pathology & Laboratory Medicine.2016; 140(11): 1206. CrossRef - Analysis of the Effects of Bone Marrow Biopsy Decalcification Methods on Histopathological Examination
Ji Young Park, Kyung Hee Han
The Korean Journal of Clinical Laboratory Science.2016; 48(4): 371. CrossRef - Distinguishing between Microbial Habitats Unravels Ecological Complexity in Coral Microbiomes
Amy Apprill, Laura G. Weber, Alyson E. Santoro, Nicole S. Webster
mSystems.2016;[Epub] CrossRef - Optimal Fixation and Decalcification Methods for Bone Marrow Biopsy
Myung-Sub Choi, Hyunsup Lee, Hyuk-Chul Kwon, Moon-Hwan Bae, Young-Hye Ko, Hee-Jin Kim, Beom-Se Lee, Bon-Kyung Koo
Korean Journal of Clinical Laboratory Science.2015; 47(4): 243. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure





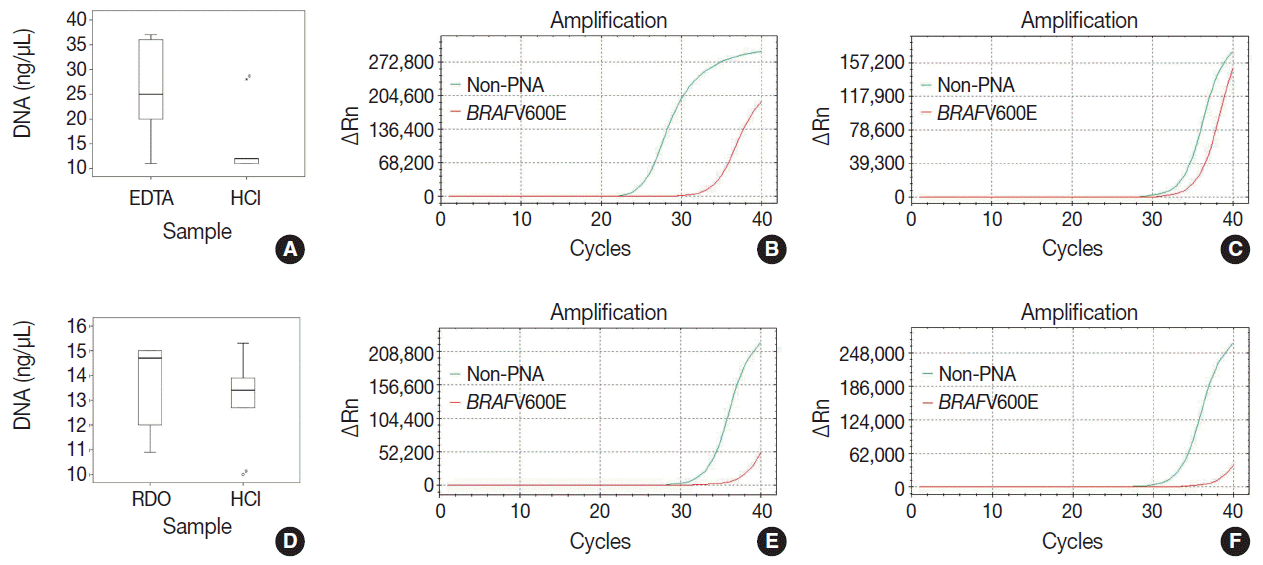
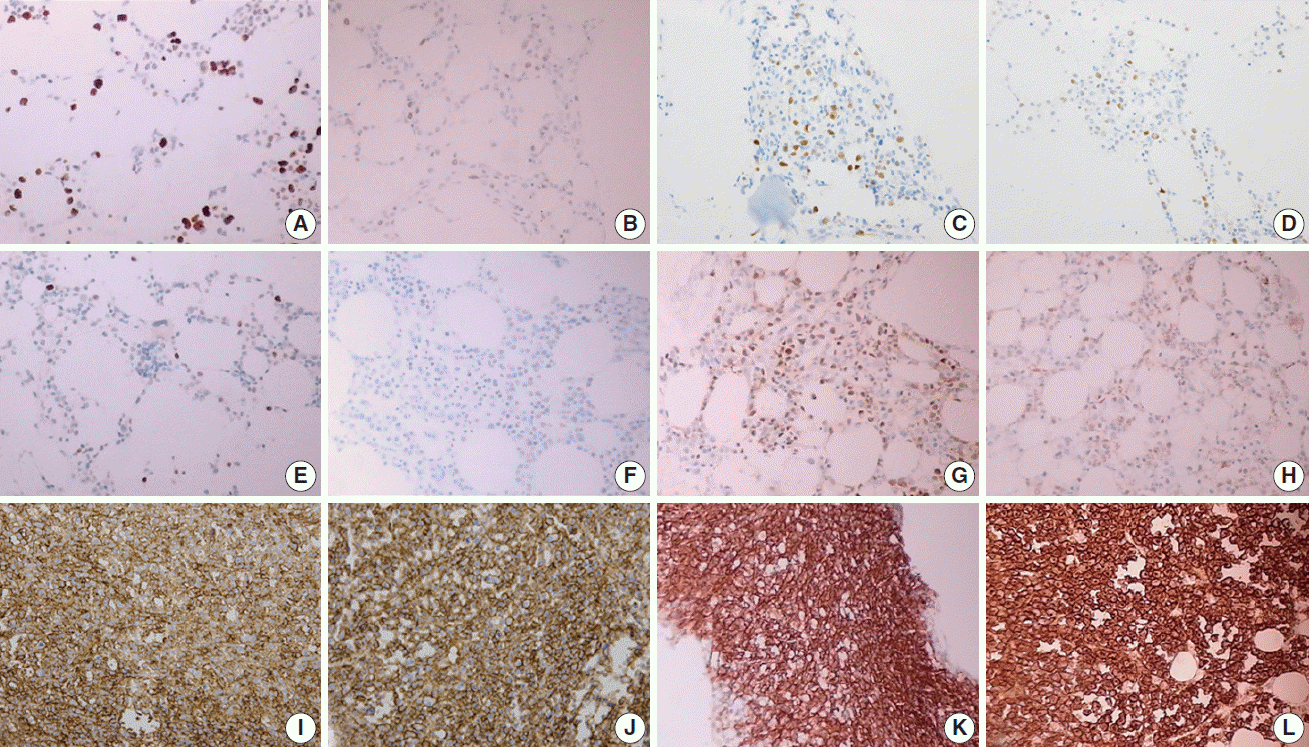
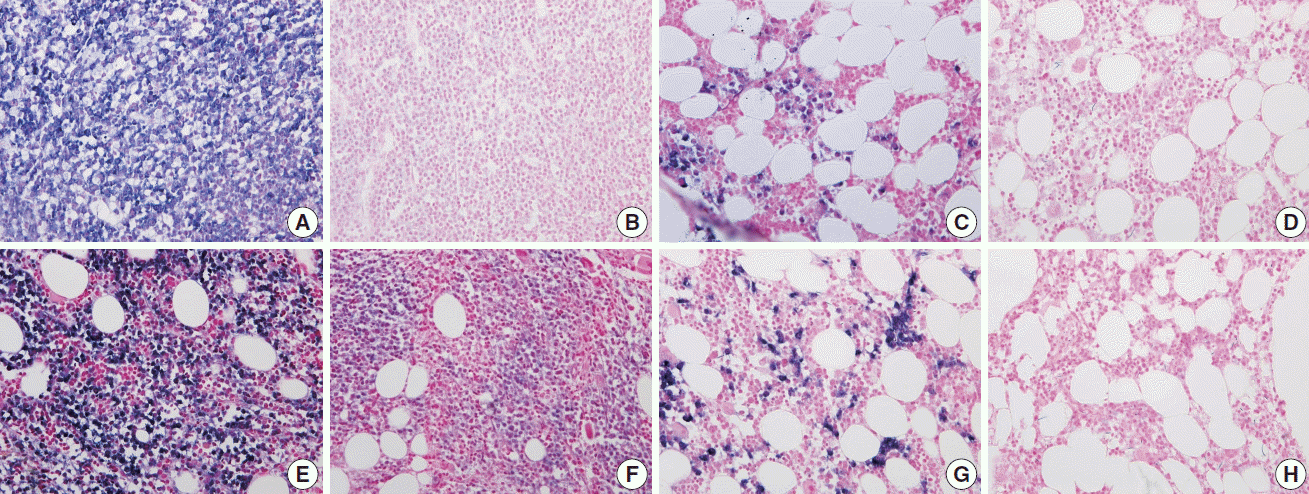
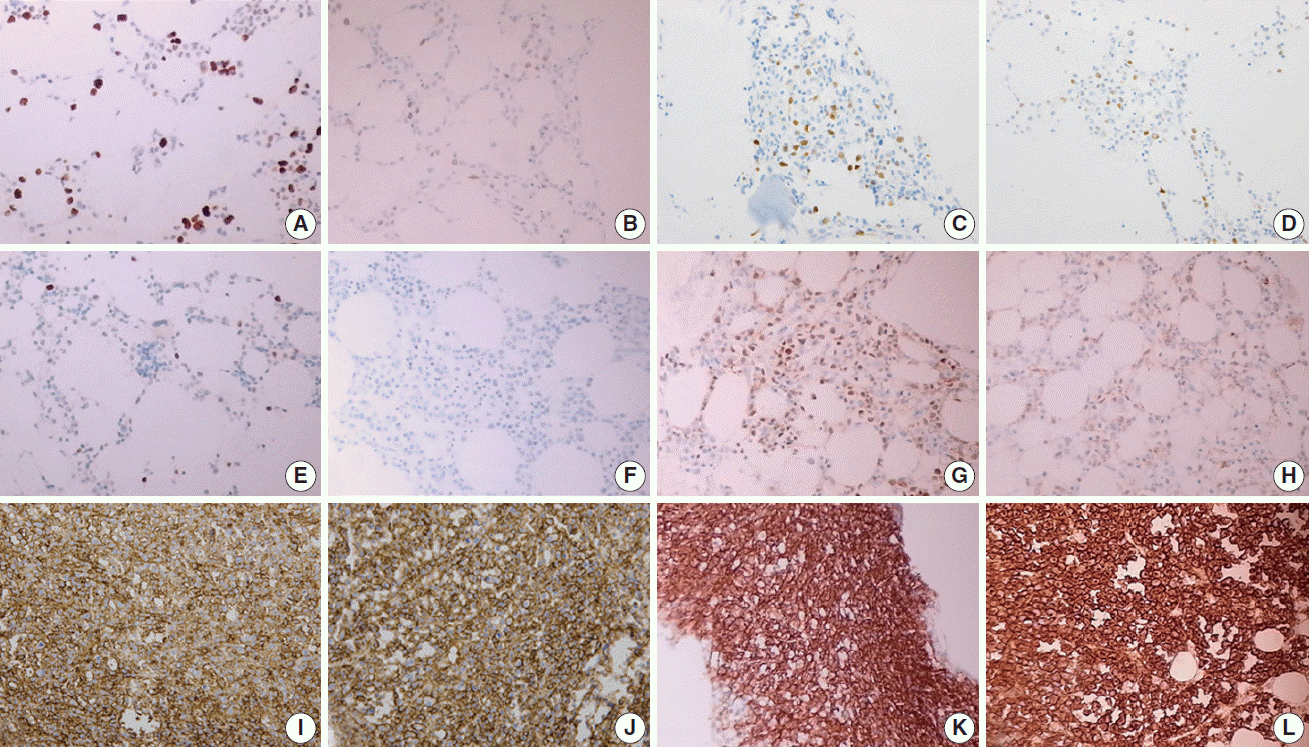
Fig. 1.
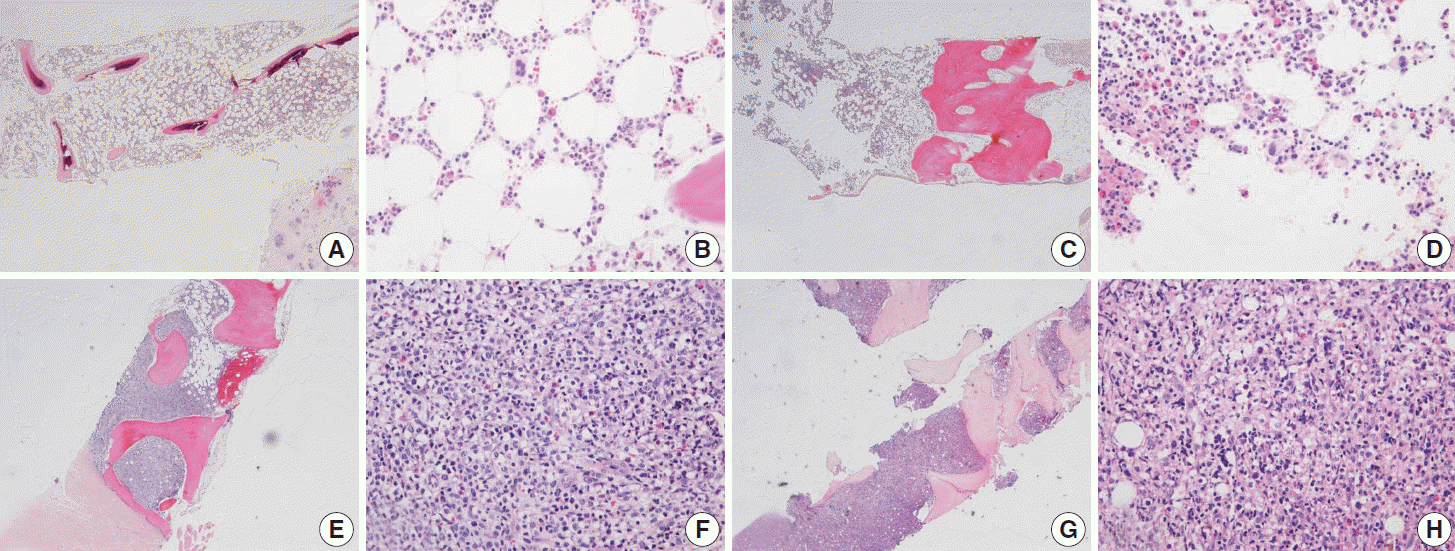
Fig. 2.
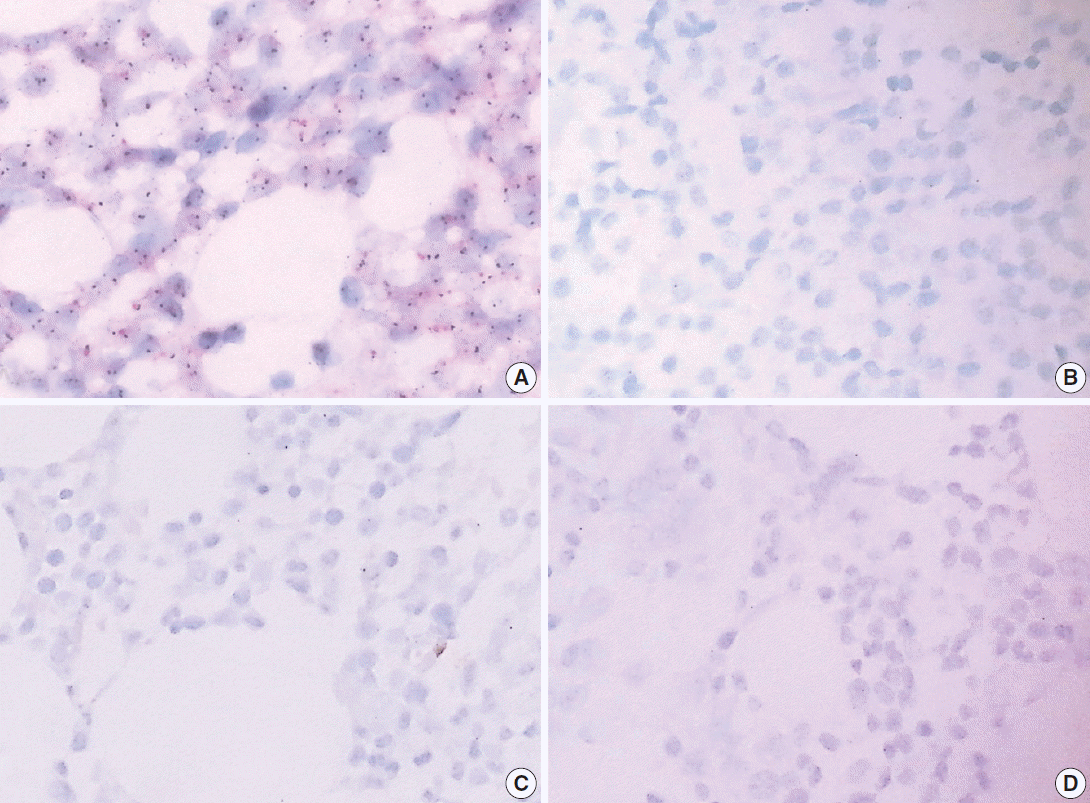
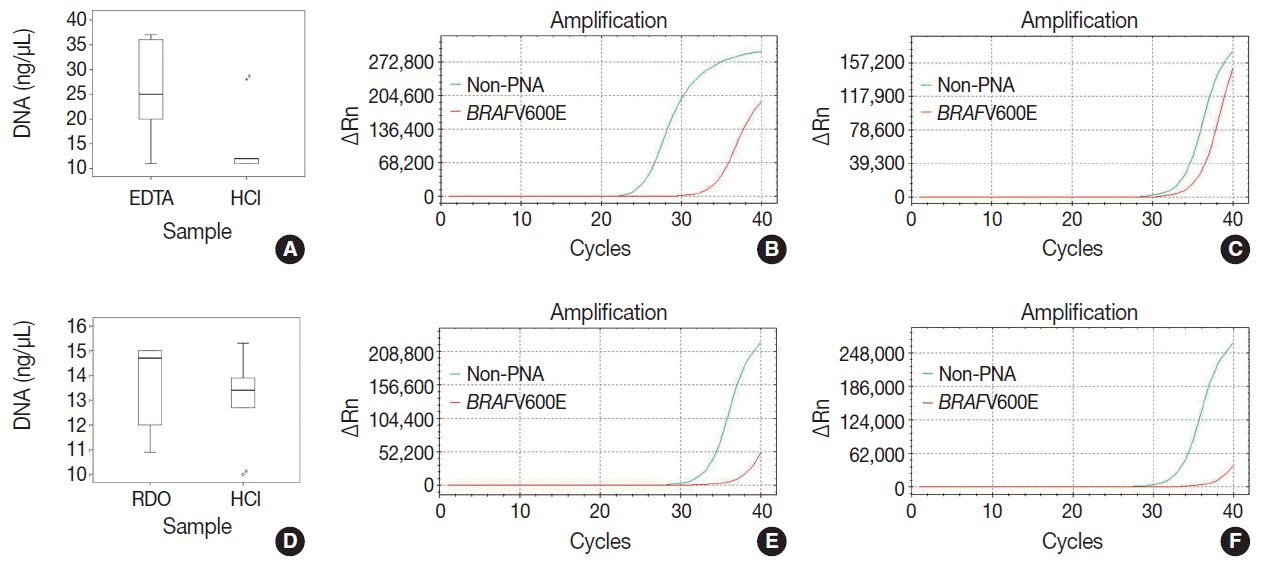
Fig. 3.
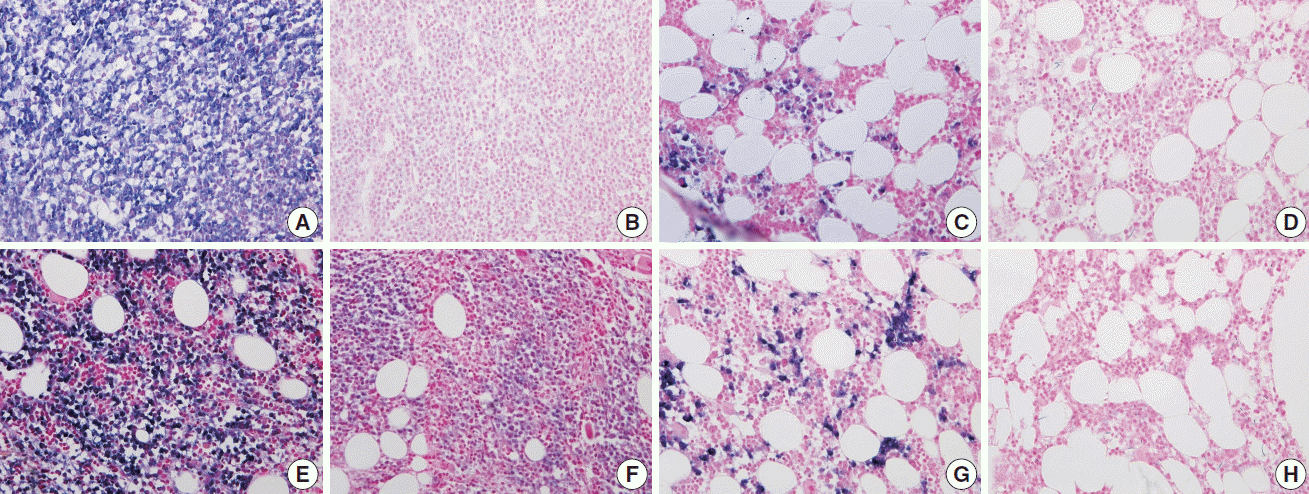
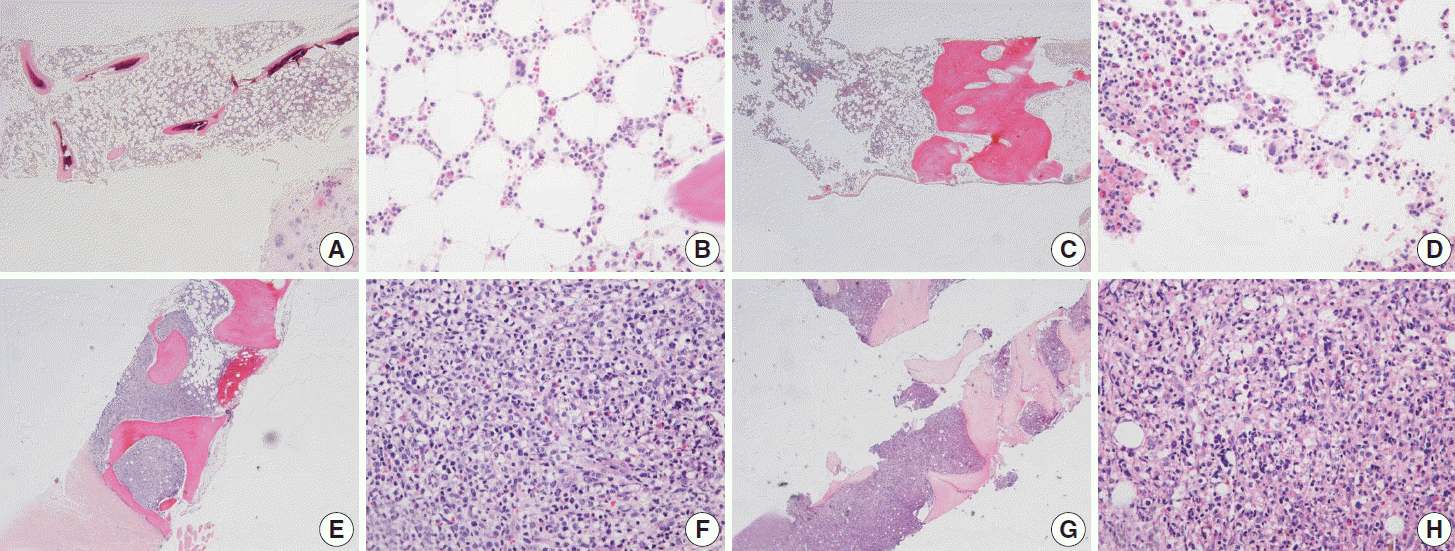
Fig. 4.
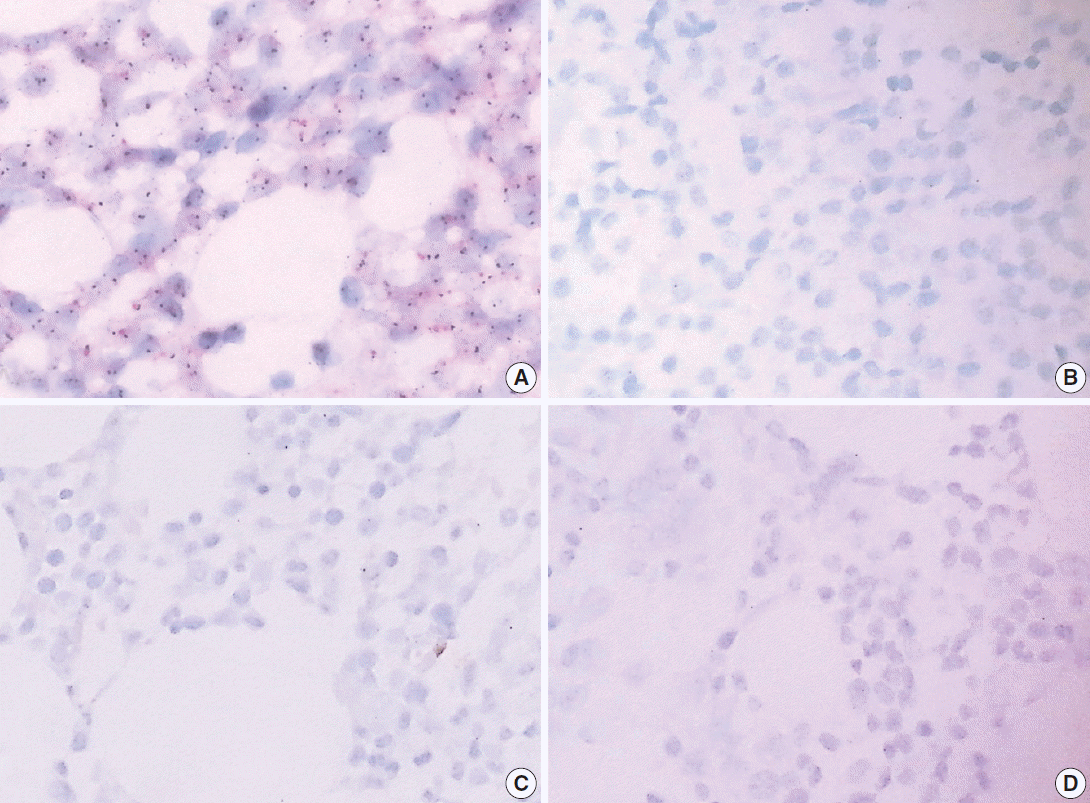
Fig. 5.
| Solution | Processing time (hr) | Processing temperature |
|---|---|---|
| HCl (100%) | 3 | Room temperature |
| EDTA (12.5%) | 3 or 24 |
Room temperature |
| RDO (100%) | 0.5–1 | Room temperature |
| Product name | Dilution | Clonality | Clone | Company |
|---|---|---|---|---|
| Cyclin D1 (SP4) | 1:50 | Monoclonal | SP4 | LabVision |
| Ki67 | 1:1,000 | Monoclonal | MIB-1 | DAKO |
| Bcl2 Bond-III | 1:50 | Monoclonal | bcl2/100/D5 | Novocastra |
| Bcl6 | Prediluent | Monoclonal | LN22 | Novocastra |
| TdT | 1:100 | Polyclonal | - | Cell Marque |
| CD138 | Prediluent | Monoclonal | ML15 | DAKO |
| CD20 | 1:400 | Monoclonal | L26 | Novocastra |
| CD79a (B cell) | 1:100 | Monoclonal | JCB117 | DAKO |
| CD3 | 1:200 | Monoclonal | SP7 | LabVision |
| CD5 | 1:100 | Monoclonal | 4C7 | Novocastra |
| CD23 | 1:100 | Monoclonal | SP23 | LabVision |
| CD10 | 1:75 | Monoclonal | 56C6 | Novocastra |
| CD30 | 1:50 | Monoclonal | Ber-H2 | DAKO |
| Myeloperoxidase | 1:2,000 | Polyclonal | - | DAKO |
| DNA yield (median, range) | p-value | Ct value (median, range) | p-value | ||
|---|---|---|---|---|---|
| EDTA vs HCl | EDTA | 25 (11.0–37.0) | .168 | 25.0 (24.6–27.2) | <.001 |
| HCl | 12 (11.0–28.0) | 32.7 (28.9–33.3) | |||
| RDO vs HCl | RDO | 14.7 (10.9–15.0) | .753 | 33.6 (33.0–34.1) | .754 |
| HCl | 13.4 (10.0–15.3) | 33.5 (33.2–35.2) |
| Item | EDTA vs HCl |
RDO vs HCl |
||
|---|---|---|---|---|
| EDTA | HCl | RDO | HCl | |
| HER2/CEP17 SISH | 5/5 (100) |
0/5 (0) | 0/5 (0) | 0/5 (0) |
| Kappa ISH | 7/7 (100) | 4/7 (57.1) | 2/3 (66.7) | 2/3 (66.7) |
| Lambda ISH | 5/5 (100) | 1/5 (20) | 2/2 (100) | 0/2 (0) |
| CyclinD1 | 9/9 (100) | 2/9 (22.2) | 3/3 (100) | 1/3 (33.3) |
| Ki67 | 9/9 (100) | 5/9 (55.6) | 10/10 (100) | 6/10 (45.5) |
| Bcl2 | 1/1 (100) | 1/1 (100) | 4/4 (100) | 4/4 (100) |
| Bcl6 | No data | No data | 1/1 (100) | 1/1 (100) |
| TdT | No data | No data | 1/1 (100) | 0/1 (0) |
| CD138 | 7/7 (100) | 7/7 (100) | 3/3 (100) | 3/3 (100) |
| CD20 | 9/9 (100) | 9/9 (100) | 9/9 (100) | 9/9 (100) |
| CD79a (B cell) | No data | No data | 2/2 (100) | 2/2 (100) |
| CD3 | 5/5 (100) | 5/5 (100) | 4/4 (100) | 4/4 (100) |
| CD5 | No data | No data | 3/3 (100) | 3/3 (100) |
| CD23 | No data | No data | 1/1 (100) | 1/1 (100) |
| CD10 | 1/1 (100) | 1/1 (100) | 1/1 (100) | 1/1 (100) |
| CD30 | 1/1 (100) | 1/1 (100) | No data | No data |
| Myeloperoxidase | 1/1 (100) | 1/1 (100) | 2/2 (100) | 2/2 (100) |
HCl, hydrochloric acid; EDTA, ethylenediaminetetraacetic acid disodium salt dehydrate; RDO, RDO GOLD. The processing time of EDTA method was mostly 3 hours. It was 24 hours for few cases that contained more cortical bone due to the oblique direction when inserting biopsy-needle.
Lab vision, Waltham, MA; DAKO, Carpinteria, CA; Novocastra, Buffalo Grave, IL; Cell Marque, Rocklin, CA.
Mann-Whitney U test was used to compare the median of each variables. HCl, hydrochloric acid; EDTA, ethylenediaminetetraacetic acid disodium salt dehydrate; RDO, RDO GOLD.
HCl, hydrochloric acid; EDTA, ethylenediaminetetraacetic acid disodium salt dehydrate; RDO, RDO GOLD; SISH, silver Case number showing intact stain result among overall case number stained with each item (%). Positive expression in the indicated tumor cells or internal controls was considered as intact stain. For example, positive expression of cyclin D1 in mantle cell lymphoma cells or endothelial cells was interpreted as intact stain result.

 E-submission
E-submission