Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 49(4); 2015 > Article
-
Brief Case Report
Salivary Gland Hyalinizing Clear Cell Carcinoma - Jung-Chia Lin1, Jia-Bin Liao1,2, Hsiao-Ting Fu1, Ting-Shou Chang2,3,4, Jyh-Seng Wang,1,2,5
-
Journal of Pathology and Translational Medicine 2015;49(4):351-353.
DOI: https://doi.org/10.4132/jptm.2015.05.06
Published online: June 16, 2015
1Department of Pathology and Lab Medicine, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan
2National Defense Medical Center, Taipei, Taiwan
3Department of Otolaryngology, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan
4Institute of Public Health, College of Medicine, National Cheng Kung University, Tainan, Taiwan
5Department of Medicine, National Yang-Ming University School of Medicine, Taipei, Taiwan
- Corresponding Author Jyh-Seng Wang, MD, PhD Department of Pathology and Lab Medicine, Kaohsiung Veterans General Hospital, 386 Ta-Chung 1st Rd., Kaohsiung 813, Taiwan Tel: +886-7-3422121 (ext 6318), Fax: +886-3422288, E-mail: jswang@vghks.gov.tw
© 2015 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- A rare case of hyalinizing clear cell carcinoma of the tongue root: A case report and literature review
Langqing Liu, Xue Cao, Yingjie Xu, Jie Wang, Xiao Tian, Yulian Fu, Bin Ling
Oncology Letters.2025;[Epub] CrossRef - Clear cell carcinoma: a comprehensive literature review of 254 cases
A. Desai, W.C. Faquin, A.J. Iafrate, M.N. Rivera, A. Jaquinet, M.J. Troulis
International Journal of Oral and Maxillofacial Surgery.2022; 51(6): 705. CrossRef - A contemporary update on hyalinizing clear cell carcinoma: compilation of all in-house cases at our institution and a literature review spanning 2015–2020
Maria A. Gubbiotti, Kathleen Montone, Paul Zhang, Virginia Livolsi, Zubair Baloch
Human Pathology.2021; 111: 45. CrossRef - Hyalinizing clear cell carcinoma-a rare entity in the oral cavity: A case report
Alejandro Donohue-Cornejo, Oslei Paes de Almeida, Celeste Sánchez-Romero, León Francisco Espinosa-Cristóbal, Simón Yobanny Reyes-López, Juan Carlos Cuevas-González
World Journal of Clinical Cases.2020; 8(1): 133. CrossRef - Clear cell carcinoma of the hard palate: A case report
Tsukasa Tsuji, Hideaki Kitada, Shinnosuke Abe, Michiko Okita, Noriyuki Otsuka, Eiji Nakayama
Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology.2019; 31(2): 113. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
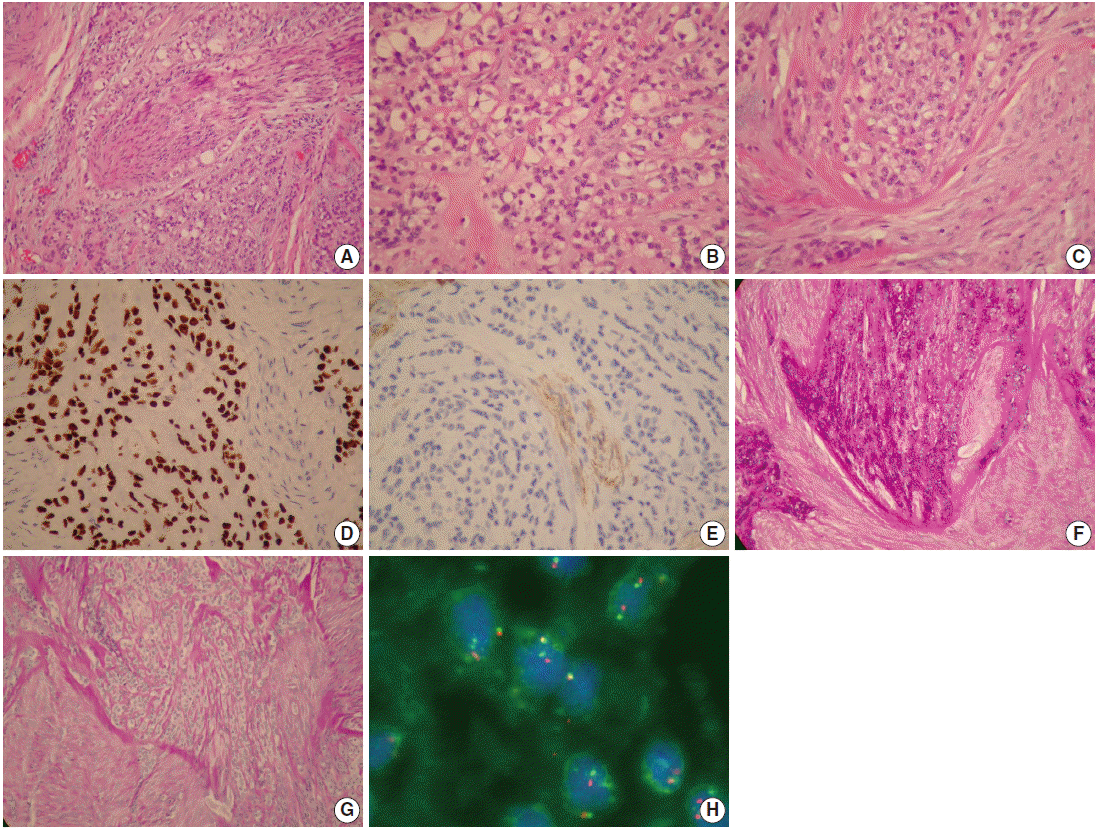
- Figure


 E-submission
E-submission