Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 49(6); 2015 > Article
-
Brief Case Report
Intrahepatic Cholangiocarcinoma with Ductal Plate Malformation-like Feature Associated with Bile Duct Adenoma - Ah-Young Kwon, Hye Jin Lee, Hee Jung An, Haeyoun Kang, Jin-Hyung Heo, Gwangil Kim
-
Journal of Pathology and Translational Medicine 2015;49(6):531-534.
DOI: https://doi.org/10.4132/jptm.2015.06.19
Published online: July 31, 2015
Department of Pathology, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- Corresponding Author: Gwangil Kim, MD Department of Pathology, CHA Bundang Medical Center, CHA University, 59 Yatap-ro, Bundang-gu, Seongnam 13496, Korea Tel: +82-31-780-5452, Fax: +82-31-780-5476, E-mail:blacknw@cha.ac.kr
© 2015 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- The publication of the case information and materials was approved by the Institutional Review Board of CHA Bundang Medical Center, CHA University.
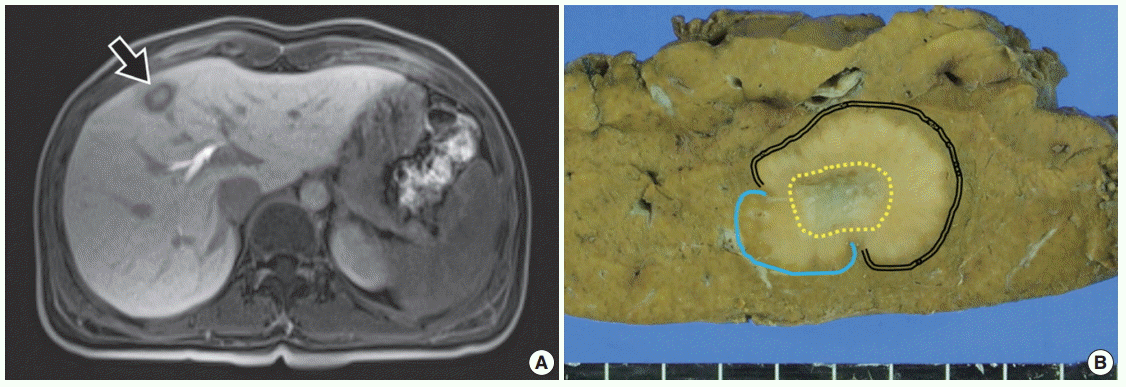
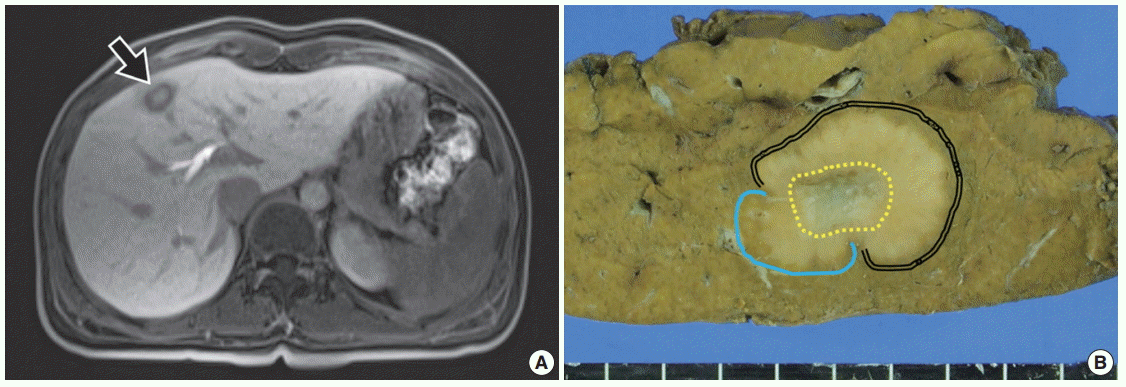
- A 34-year-old female patient was referred for further evaluation of a small hepatic nodule found on a regular health check-up. She did not have any remarkable medical history associated with liver disease. On magnetic resonance imaging, a 2-cm-sized mass was present in liver segment 4, showing high signal on T1- and low signal on T2-weighted images (Fig. 1A).
- The patient underwent hepatic segmentectomy. The liver showed a relatively well-demarcated, subcapsular (5 mm from the capsule), nonencapsulated, solid, rubbery, and pale brown mass. It was multilobulated with a central fibrous scar (Fig. 1B).
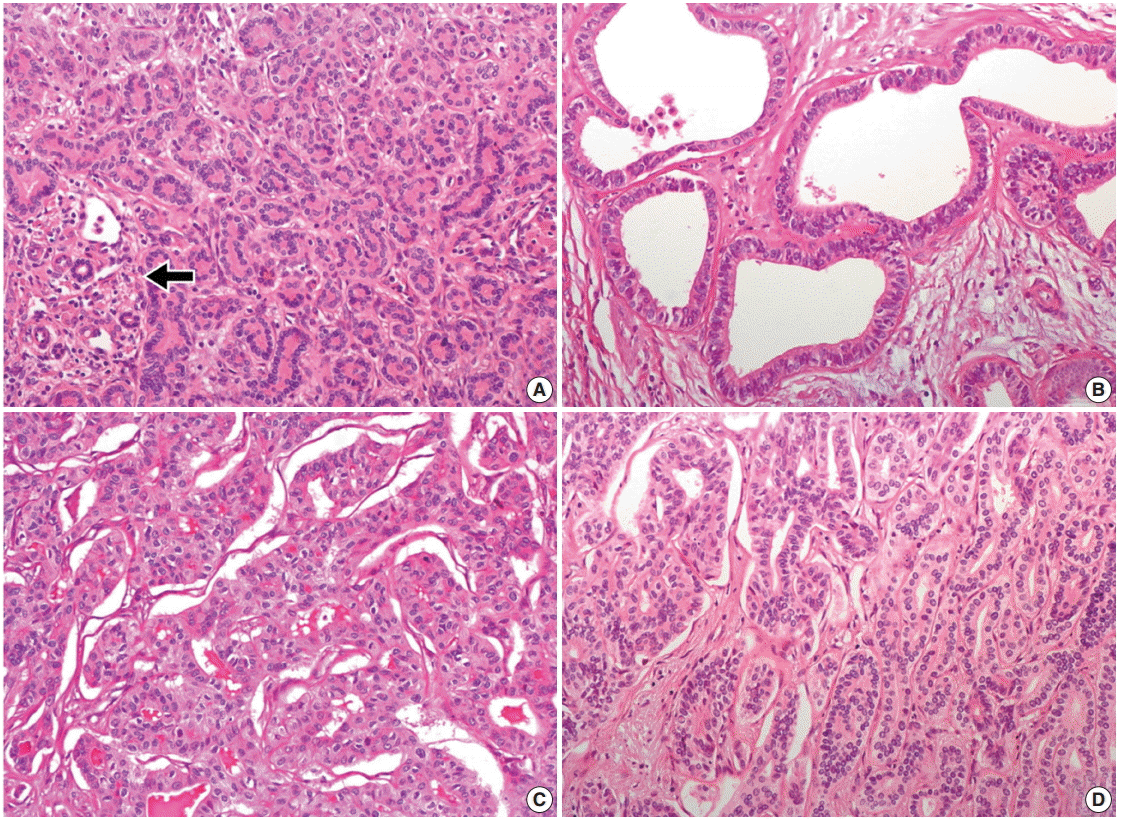
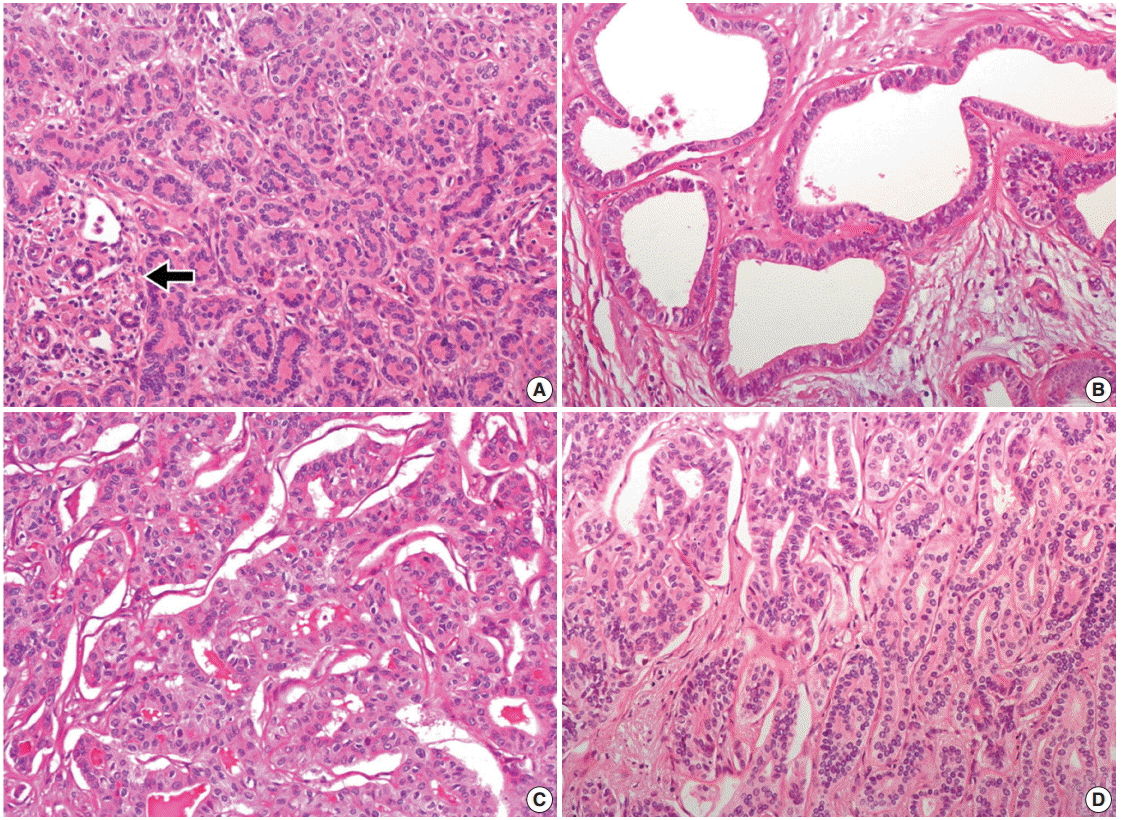
- Histologically, the nodule was composed of three distinct areas. First, many compact, small, tubular structures lined by single cuboidal to low columnar epithelial cells were present without bile or dilated ducts. Nuclei were small and uniform without any mitotic activity, which was compatible with BDA containing portal tracts (Fig. 2A). Second, the central area showed DPMlike features, having irregularly dilated ductal structures lined by low columnar neoplastic epithelial cells with mild pleomorphism within fibrous stroma (Fig. 2B). Third, the opposite side of the BDA showed ICC. Columnar to cuboidal epithelial cells forming fused glandular structures with nuclear anaplasia and frequent mitoses were present (Fig. 2C). There were transitional areas from BDA to ICC (Fig. 2D).
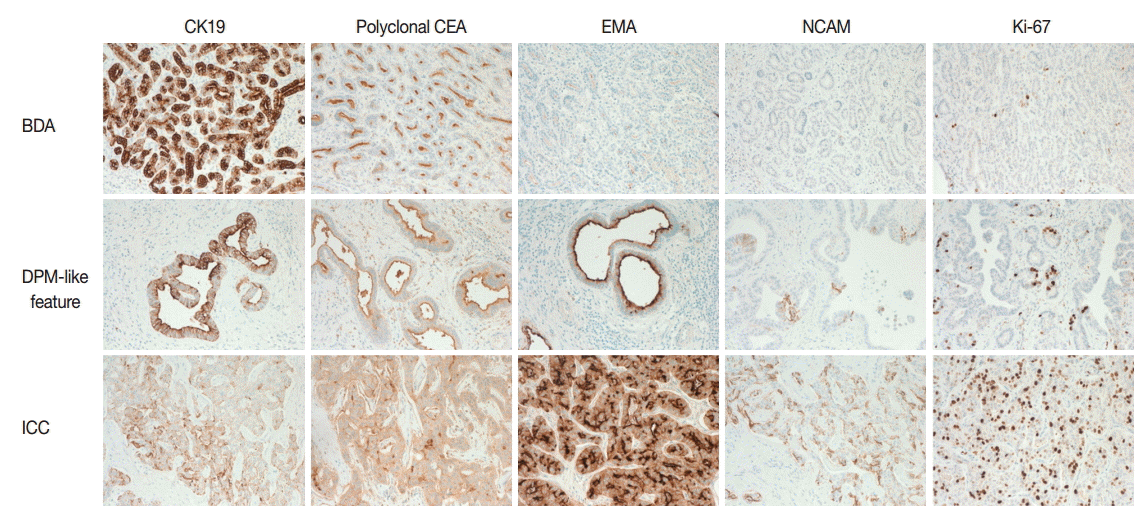
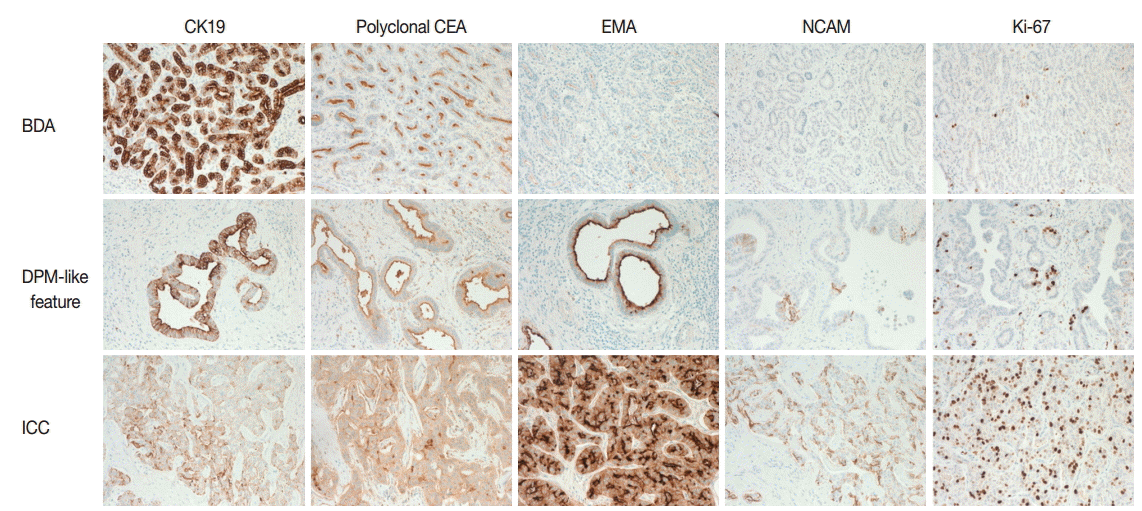
- On immunohistochemistry, cytokeratin (CK) 7, CK19, and epithelial cellular adhesion molecule (EpCAM) were positive, and monoclonal carcinoembryonic antigen (CEA), CD117, p53, and hepatocyte antigen were negative in all three areas. The ICC area showed diffuse positivity for polyclonal CEA; in contrast, the BDA and DPM-like areas showed apical reactivity only. Epithelial membrane antigen was negative in the BDA area, apically reactive in the DPM-like area, and strongly reactive in the ICC area. NCAM was positive in the ICC area, focally positive in the DPM area, but negative in the BDA area. The Ki-67 labeling index was variable, with values of 1%–2% in the BDA area, 10%–20% in the DPM-like area, and 40%–50% in the ICC area (Table 1, Fig. 3).
- The remaining parenchyme did not show VMC or DPM features. No recurrence or metastasis was observed at a 28-month follow-up.
CASE REPORT
- Some benign hepatic biliary lesions, such as VMC or bile duct adenofibroma, are known candidate precursors of ICC [4]. VMC is a congenital anomaly of biliary cells forming a hepatic tumorlike lesion [5]. Intrahepatic cholangiocarcinoma arising in VMC has been observed since 1961 [2,6]. According to the ductal plate hypothesis proposed in 2011 [7], VMC is implicated in DPM as a developmental anomaly of fetal biliary cells (ductal plate). Recently, cases of ICC with VMC features in a large proportion of the tumor are reported as ICC with predominant DPM pattern (ICC-DPM), a new subtype of ICC [3].
- In the present case, the tumor showed three histologically distinct areas of BDA, DPM, and ICC, and their proportions were 30%, 20%, and 50%, respectively.
- BDA is a rare solitary intrahepatic lesion that consists of many small, uniform ducts with benign cuboidal cells and a narrow lumen. The BDA area in the present case was typical and localized to one side. Although BDA can be confused with bile ductular carcinoma foci of ICC-DPM, the latter show malignant epithelium and similar immunoreactivity to ICC-DPM. In contrast to VMC, BDA is not regarded as a precursor of ICC because ICC with BDA has been reported in only three cases (Table 2) [8-10].
- DPM-like areas in our case revealed irregularly dilated glands within fibrous stroma, resembling VMC. The neoplastic columnar cells were different from typical VMC. This DPM-like feature might be a part of ICC-DPM or represent a transitional area between BDA and ICC. There were several unique points in the present DPM-like features that differ from the previously reported ICC-DPM. First, the typical irregular protrusions and bridging structures were not prominent in the DPM-like area in the present case. Second, there was no obvious stromal invasion in this area. Third, ICC and BDA in this case were distinguishable from the DPM-like area grossly, histologically, and immunohistochemically (especially with respect to CEA, EpCAM, NCAM, and Ki-67) [3].
- The results of immunohistochemical staining of each area corresponded to the histological diagnosis. Intriguingly, NCAM was expressed in ICC and focally in the DPM-like area. This result supports the previous suggestion that ICC with DPM features is a subtype of hepatocellular-cholangiocarcinoma with stem cell features [5].
- In summary, we present a case of ICC with DPM-like features associated with BDA. Although the etiologic relationship between ICC and BDA or DPM needs further study, the possibility of BDA as a precursor of ICC is presented. Such a situation should be considered when BDA is found on a needle biopsy.
DISCUSSION
| Reference | Year | Sex/Age (yr) | Location | Size (cm) | Operation | Histology | Associated liver disease |
|---|---|---|---|---|---|---|---|
| Hasebe et al. [8] | 1995 | M/59 | S4 | 2.2 | Partial resection | ICC with BDA and VMC | No |
| Takahashi et al. [10] | 2010 | M/76 | S6 | 3 | Resection | ICC with BDA and VMC | No |
| Pinho et al. [9] | 2012 | F/60 | S5 | 3.83 | Liver biopsy (at the age of 58) | BDA | No |
| Right hepatectomy | ICC | ||||||
| Present case | 2015 | F/36 | S4 | 2 | Segmentectomy | ICC with DPM pattern associated with BDA | No |
- 1. Jain D, Nayak NC, Saigal S. Hepatocellular carcinoma arising in association with von-Meyenburg’s complexes: an incidental finding or precursor lesions? A clinicopatholigic study of 4 cases. Ann Diagn Pathol 2010; 14: 317-20. ArticlePubMed
- 2. Xu AM, Xian ZH, Zhang SH, Chen XF. Intrahepatic cholangiocarcinoma arising in multiple bile duct hamartomas: report of two cases and review of the literature. Eur J Gastroenterol Hepatol 2009; 21: 580-4. ArticlePubMed
- 3. Nakanuma Y, Sato Y, Ikeda H, et al. Intrahepatic cholangiocarcinoma with predominant “ductal plate malformation” pattern: a new subtype. Am J Surg Pathol 2012; 36: 1629-35. PubMed
- 4. Nakanuma Y, Tsutsui A, Ren XS, Harada K, Sato Y, Sasaki M. What are the precursor and early lesions of peripheral intrahepatic cholangiocarcinoma? Int J Hepatol 2014; 2014: 805973. ArticlePubMedPMCPDF
- 5. Terada T. Combined hepatocellular-cholangiocarcinoma with stem cell features, ductal plate malformation subtype: a case report and proposal of a new subtype. Int J Clin Exp Pathol 2013; 6: 737-48. PubMedPMC
- 6. Lindgren AG, Hansson G, Nilsson LA. Primary carcinoma arising in congenital liver in conjunction with miliary cholangiomatosis: report of case. Acta Pathol Microbiol Scand 1961; 52: 343-8. PubMed
- 7. Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. III. Implications for liver pathology. Virchows Arch 2011; 458: 271-9. ArticlePubMedPDF
- 8. Hasebe T, Sakamoto M, Mukai K, et al. Cholangiocarcinoma arising in bile duct adenoma with focal area of bile duct hamartoma. Virchows Arch 1995; 426: 209-13. ArticlePubMedPDF
- 9. Pinho AC, Melo RB, Oliveira M, et al. Adenoma-carcinoma sequence in intrahepatic cholangiocarcinoma. Int J Surg Case Rep 2012; 3: 131-3. ArticlePubMedPMC
- 10. Takahashi S, Takada K, Kawano Y, et al. Cholangiocarcinoma with bile duct adenoma and hamartoma-like lesion in the bile duct. Nihon Shokakibyo Gakkai Zasshi 2010; 107: 461-9. PubMed
REFERENCES
Figure & Data
References
Citations

- Spectrum of Biliary Lesions/Neoplasms in Hepatic Parenchyma with Reference to a Precursor of Small Duct-Type Intrahepatic Cholangiocarcinoma: Comprehensive Categorization into Three Groups
Yasuni Nakanuma, Motoko Sasaki, Yuko Kakuda, Takuma Oishi
Cancers.2026; 18(2): 328. CrossRef - Histopathological evidence of intrahepatic cholangiocarcinoma occurring in ductal plate malformation: A clinicopathologic study of 5 cases
Qian Wang, Yi Xu, Shou-Mei Wang, Ai-Yan Hu, Yun-Cui Pan, Shu-Hui Zhang
Annals of Diagnostic Pathology.2021; 55: 151828. CrossRef - Cholangiolocellular Carcinoma Arising in a Normal Liver
Chie Kitami, Yasuyuki Kawachi, Toshihiko Igarashi, Shigeto Makino, Atsushi Nishimura, Mikako Kawahara, Keiya Niikuni, Kenichi Harada
The Japanese Journal of Gastroenterological Surgery.2016; 49(10): 1006. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1.
Fig. 2.
Fig. 3.
| Antigen | Source, clone | BDA area | DPM area | ICC area |
|---|---|---|---|---|
| CK7 | Neomarker, OV-TL 12/30 | P | P | P |
| CK19 | Neomarker, A53-B/A2.26 | P | P | P |
| Polyclonal CEA | Neomarker, CEA Ab-2 | P (apical) | P (apical) | P (membranous) |
| Monoclonal CEA | Neomarker, COL1 | N | N | N |
| EpCAM | Novocastra, VU-1D9 | P | P | P |
| EMA | Cell MARQUE, E29 | N | P (apical) | P (membranous) |
| NCAM (CD56) | Roche, 123C3 | N | P (focal) | P |
| CD117 (c-Kit) | DAKO, rabbit polyclonal | N | N | N |
| p53 | DAKO, DO-7 | N | N | N |
| Hepatocyte | DAKO, OCH1E5 | N | N | N |
| Ki-67 (%) | Neomarker, SP6 | 1–2 | 10–20 | 40–50 |
| Reference | Year | Sex/Age (yr) | Location | Size (cm) | Operation | Histology | Associated liver disease |
|---|---|---|---|---|---|---|---|
| Hasebe et al. [8] | 1995 | M/59 | S4 | 2.2 | Partial resection | ICC with BDA and VMC | No |
| Takahashi et al. [10] | 2010 | M/76 | S6 | 3 | Resection | ICC with BDA and VMC | No |
| Pinho et al. [9] | 2012 | F/60 | S5 | 3.83 | Liver biopsy (at the age of 58) | BDA | No |
| Right hepatectomy | ICC | ||||||
| Present case | 2015 | F/36 | S4 | 2 | Segmentectomy | ICC with DPM pattern associated with BDA | No |
BDA, bile duct adenoma; DPM, ductal plate malfromation; ICC, intrahepatic cholangiocarcinoma; CK, cytokeratin; P, positive; CEA, carcinoembryonic antigen; N, negative; EMA, epithelial membrane antigen.
ICC, intrahepatic cholangiocarcinoma; BDA, bile duct adenoma; VMC, von Meyenburg complex; DPM, ductal plate malformation.

 E-submission
E-submission