Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 49(6); 2015 > Article
-
Original Article
Therapeutic Effects of Umbilical Cord Blood Derived Mesenchymal Stem Cell-Conditioned Medium on Pulmonary Arterial Hypertension in Rats - Jae Chul Lee1,2,3, Choong Ik Cha3, Dong-Sik Kim,2, Soo Young Choe,1
-
Journal of Pathology and Translational Medicine 2015;49(6):472-480.
DOI: https://doi.org/10.4132/jptm.2015.09.11
Published online: October 16, 2015
1Department of Biology, School of Life Sciences, Chungbuk National University, Cheongju, Korea
2Department of Surgery, Brain Korea 21 PLUS Project for Medical Sciences and HBP Surgery and Liver Transplantation, Korea University College of Medicine, Seoul, Korea
3Department of Anatomy, Seoul National University College of Medicine, Seoul, Korea
-
Corresponding Author: Soo Young Choe, PhD Department of Biology, School of Life Sciences, Chungbuk National University, 1 Chungdae-ro, Seowon-gu, Cheongju 28644, Korea Tel: +82-43-261-2297 Fax: +82-43-275-2291 E-mail:leejc@chungbuk.ac.kr
Dong-Sik Kim, MD, PhD Department of Surgery, Brain Korea 21 PLUS Project for Medical Sciences and HBP Surgery and Liver Transplantation, Korea University College of Medicine, 145 Anam-ro, Seongbuk-gu, Seoul 02841, Korea Tel: +82-2-2286-1431 Fax: +82-2-2286-1428 E-mail:beas100@korea.ac.kr
© 2015 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Comparative analysis on the anti-inflammatory/immune effect of mesenchymal stem cell therapy for the treatment of pulmonary arterial hypertension
Seyeon Oh, Albert Y. Jang, Sehyun Chae, Seungbum Choi, Jeongsik Moon, Minsu Kim, Edda Spiekerkoetter, Roham T. Zamanian, Phillip C. Yang, Daehee Hwang, Kyunghee Byun, Wook-Jin Chung
Scientific Reports.2021;[Epub] CrossRef - MSCs Therapy Reverse the Gut Microbiota in Hypoxia-Induced Pulmonary Hypertension Mice
Lingjie Luo, Qinhua Chen, Lei Yang, Zhenxia Zhang, Jihong Xu, Deming Gou
Frontiers in Physiology.2021;[Epub] CrossRef - The promise of mesenchymal stem cell therapy for acute respiratory distress syndrome
Ben Antebi, Arezoo Mohammadipoor, Andriy I. Batchinsky, Leopoldo C. Cancio
Journal of Trauma and Acute Care Surgery.2018; 84(1): 183. CrossRef - Let-7a-transfected mesenchymal stem cells ameliorate monocrotaline-induced pulmonary hypertension by suppressing pulmonary artery smooth muscle cell growth through STAT3-BMPR2 signaling
Gesheng Cheng, Xingye Wang, Yongxin Li, Lu He
Stem Cell Research & Therapy.2017;[Epub] CrossRef - Reduced immunoreactivities of B-type natriuretic peptide in pulmonary arterial hypertension rats after ranolazine treatment
Jae Chul Lee, Kwan Chang Kim, Soo Young Choe, Young Mi Hong
Anatomy & Cell Biology.2016; 49(1): 7. CrossRef - Right sided heart failure and pulmonary hypertension: New insights into disease mechanisms and treatment modalities
Diana Drogalis-Kim, John Jefferies, Ivan Wilmot, Juan Alejos
Progress in Pediatric Cardiology.2016; 43: 71. CrossRef - Notice of Retraction: Therapeutic Effects of Umbilical Cord Blood Derived Mesenchymal Stem Cell-Conditioned Medium on Pulmonary Arterial Hypertension in Rats
Jae Chul Lee, Choong Ik Cha, Dong-Sik Kim, Soo Young Choe
Journal of Pathology and Translational Medicine.2016; 50(4): 325. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure





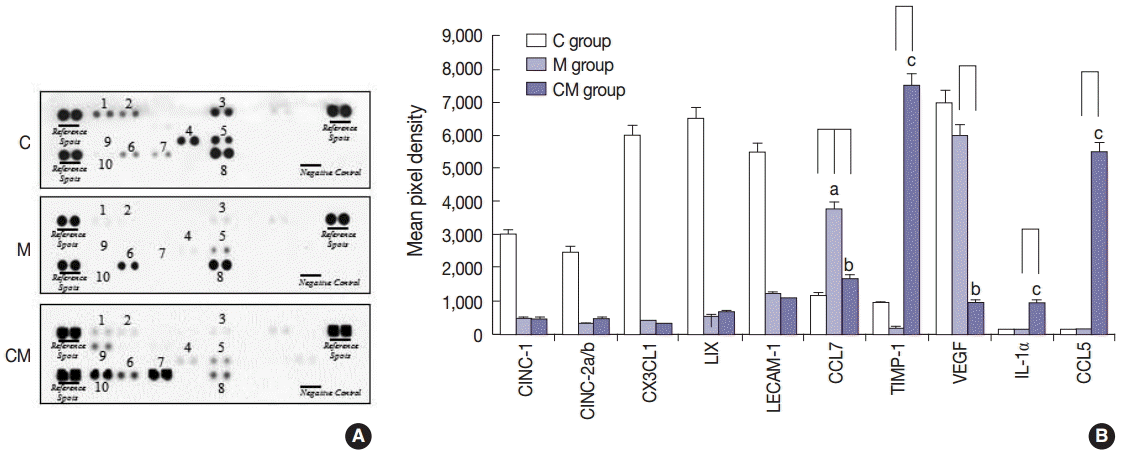
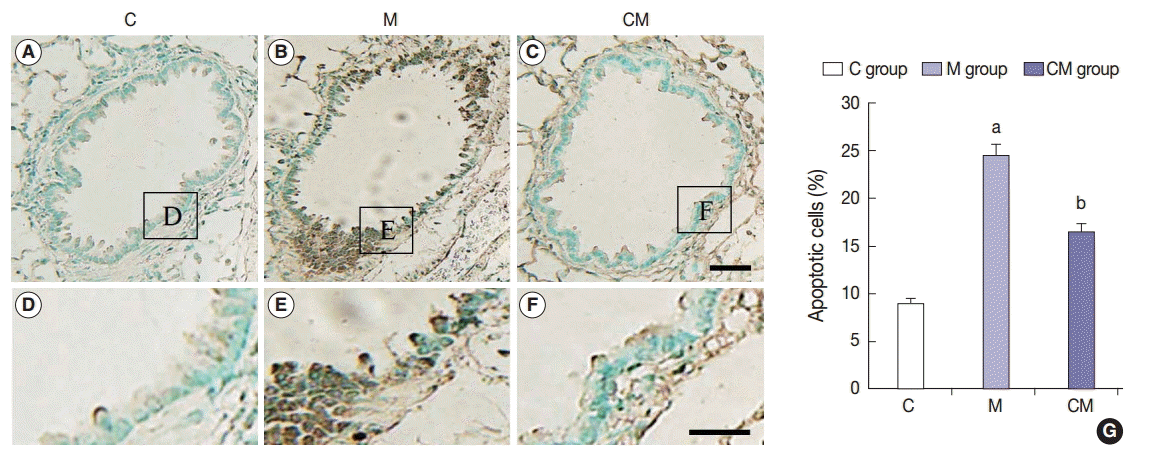
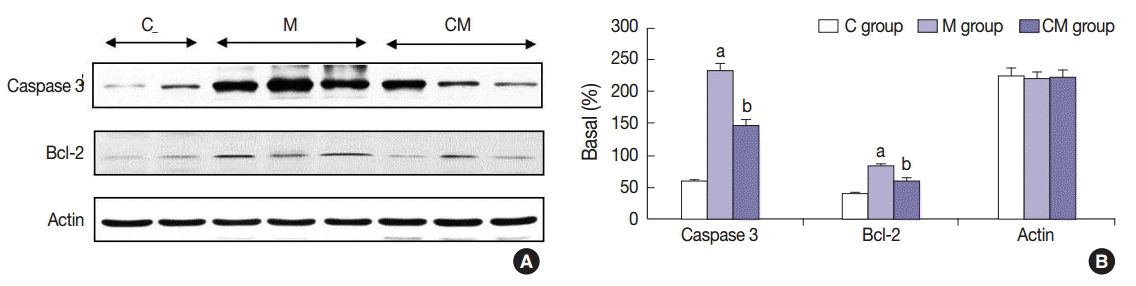
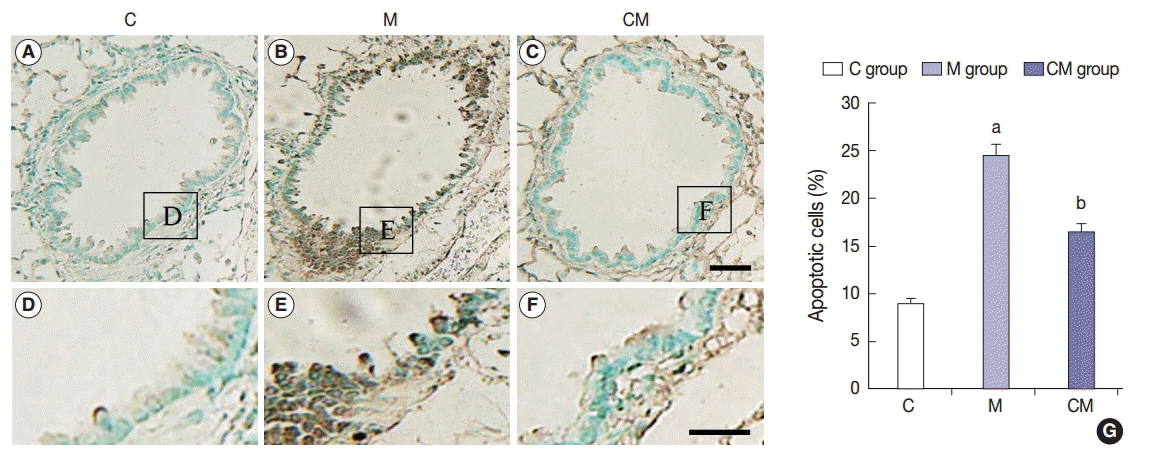
Fig. 1.
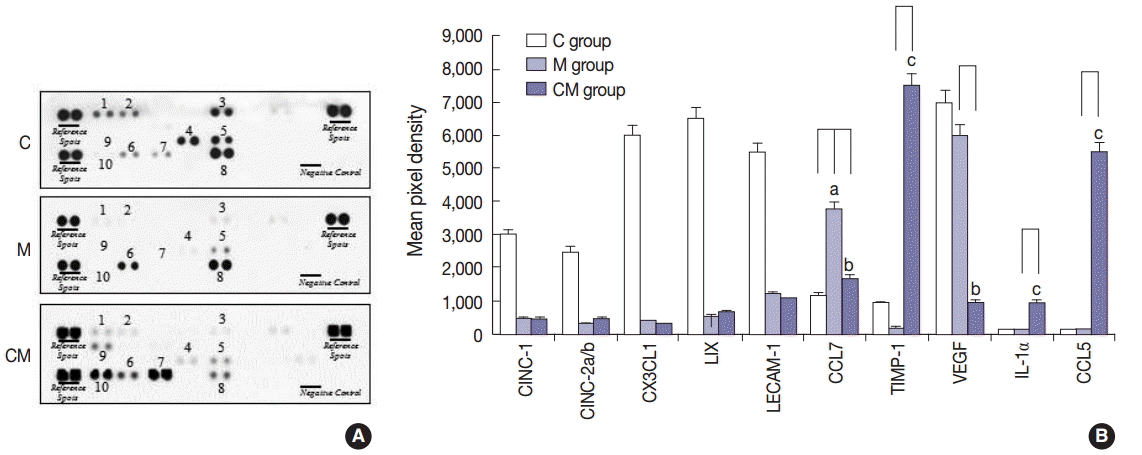
Fig. 2.
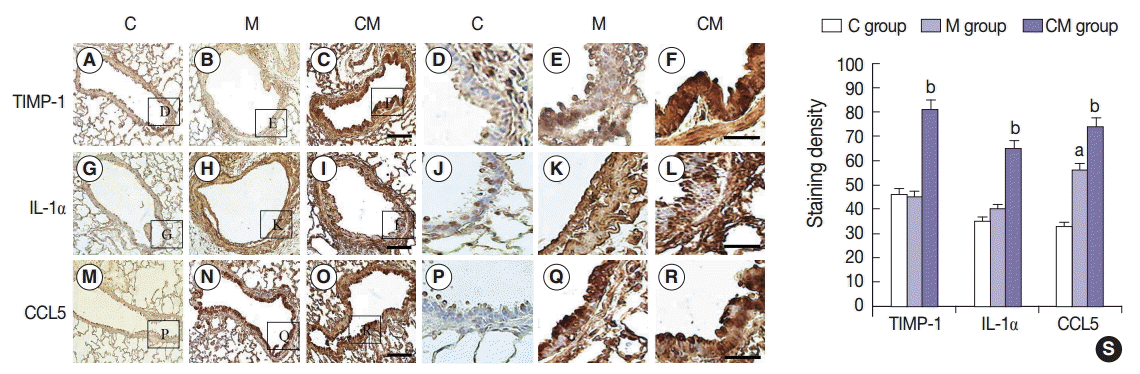
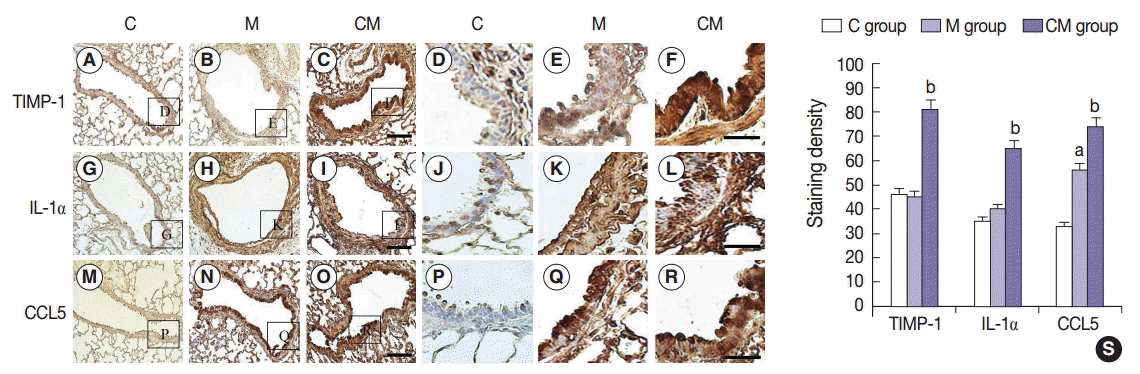
Fig. 3.
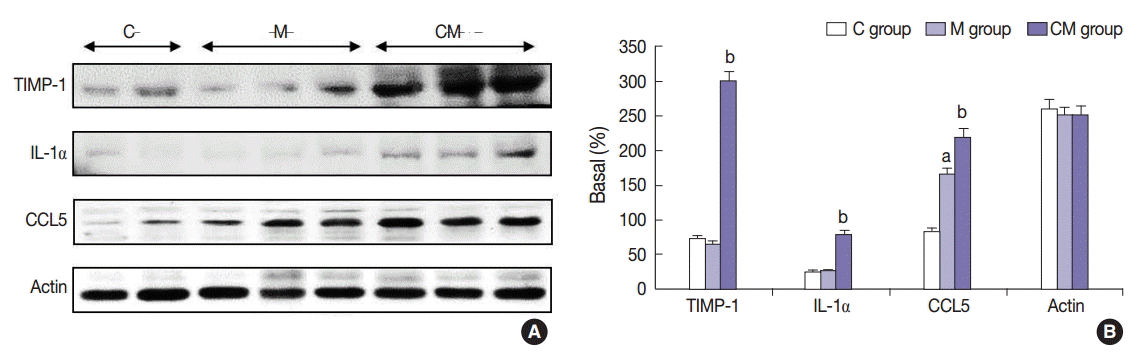
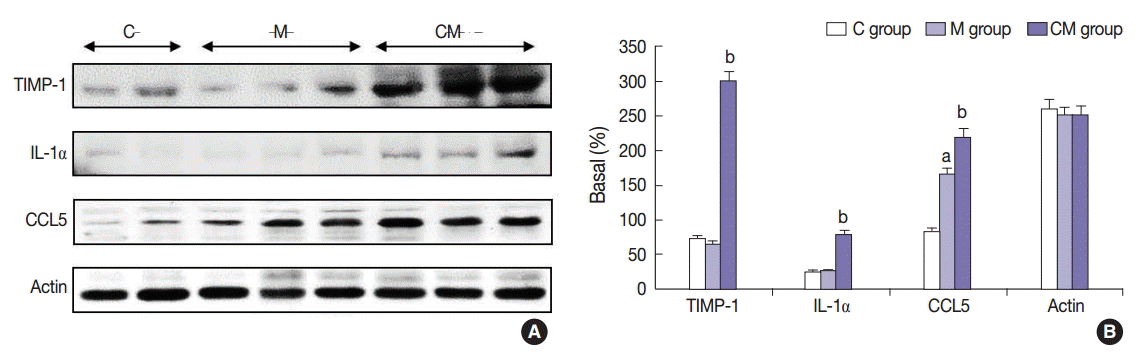
Fig. 4.
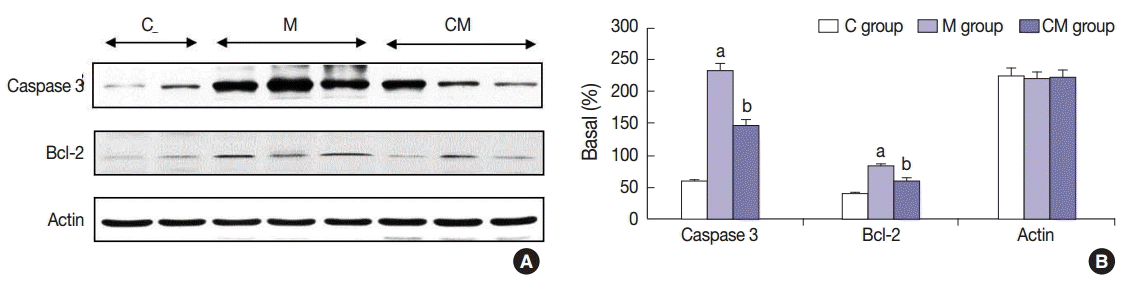
Fig. 5.
| Day | Group | Body weight (g) | RV (g) | LV + IVS (g) | RV / (LV + IVS) (%) |
|---|---|---|---|---|---|
| 7 | Control | 318.63 ± 14.78 | 0.132 ± 0.02 | 0.611 ± 0.02 | 0.21 ± 0.01 |
| M | 278.50 ± 32.71 | 0.155 ± 0.03 | 0.543 ± 0.03 | 0.28 ± 0.02 | |
| CM | 280.46 ± 29.82 | 0.164 ± 0.02 | 0.561 ± 0.03 | 0.29 ± 0.02 | |
| 14 | Control | 343.65 ± 24.52 | 0.156 ± 0.02 | 0.731 ± 0.03 | 0.21 ± 0.02 |
| M | 256.71 ± 45.57 |
0.234 ± 0.03 | 0.671 ± 0.02 | 0.34 ± 0.03 |
|
| CM | 271.21 ± 38.82 | 0.224 ± 0.04 | 0.699 ± 0.03 | 0.32 ± 0.02 | |
| 21 | Control | 393.81 ± 24.62 | 0.166 ± 0.03 | 0.782 ± 0.03 | 0.21 ± 0.02 |
| M | 249.67 ± 47.29 |
0.314 ± 0.06 |
0.677 ± 0.05 | 0.46 ± 0.05 |
|
| CM | 271.00 ± 51.55 |
0.284 ± 0.05 | 0.631 ± 0.03 | 0.45 ± 0.03 | |
| 28 | Control | 394.00 ± 41.61 | 0.171 ± 0.02 | 0.801 ± 0.03 | 0.21 ± 0.02 |
| M | 229.71 ± 44.82 |
0.394 ± 0.08 |
0.751 ± 0.06 | 0.52 ± 0.07 |
|
| CM | 319.29 ± 36.62 |
0.261 ± 0.06 |
0.732 ± 0.04 | 0.35 ± 0.04 |
| Day | C group | M group | CM group |
|---|---|---|---|
| 7 | 22.7 ± 0.6 | 24.5 ± 2.1 | 23.2 ± 3.4 |
| 14 | 22.0 ± 1.1 | 37.8 ± 3.2 |
30.9 ± 4.6 |
| 21 | 23.3 ± 0.9 | 50.2 ± 4.7 |
39.2 ± 5.2 |
| 28 | 22.9 ± 2.1 | 58.0 ± 6.4 |
37.8 ± 4.1 |
Values are presented as mean ± standard deviation. hUCB-MSCs-CM, conditioned medium from human umbilical-cord blood derived mesenchymal cells; PAH, pulmonary artery hypertension; RV, right ventricle; LV, left ventricle; IVS, interventricular septum; M, monocrotaline; CM, hUCB-MSCs-CM. p < .05 compared with the C group; p < .05 compared with the M group.
Values are presented as mean ± standard deviation. hUCB-MSCs-CM, conditioned medium from human umbilical-cord blood derived mesenchymal cells; PAH, pulmonary artery hypertension; C, control; M, monocrotaline; CM, hUCB-MSCs-CM. p < .05 compared with the C group; p < .05 compared with the M group.

 E-submission
E-submission