Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 50(1); 2016 > Article
-
Original Article
Evaluation of the VE1 Antibody in Thyroid Cytology Using Ex Vivo Papillary Thyroid Carcinoma Specimens - Yon Hee Kim, Hyunee Yim, Yong-Hee Lee, Jae Ho Han, Kyi Beom Lee, Jeonghun Lee1, Euy Young Soh1, Seon-Yong Jeong2, Jang-Hee Kim
-
Journal of Pathology and Translational Medicine 2016;50(1):58-66.
DOI: https://doi.org/10.4132/jptm.2015.10.10
Published online: December 14, 2015
Department of Pathology, Ajou University School of Medicine, Suwon, Korea
1Department of Surgery, Ajou University School of Medicine, Suwon, Korea
2Department of Medical Genetics, Ajou University School of Medicine, Suwon, Korea
- Corresponding Author: Jang-Hee Kim, MD, PhD Department of Pathology, Ajou University School of Medicine, 206 World cup-ro, Yeongtong-gu, Suwon 16499, Korea Tel: +82-31-219-5925 Fax: +82-31-219-5934 E-mail: drjhk@ajou.ac.kr
© 2016 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background:
- Recently, VE1, a monoclonal antibody against the BRAFV600E mutant protein, has been investigated in terms of its detection of the BRAFV600E mutation. Although VE1 immunostaining and molecular methods used to assess papillary thyroid carcinoma in surgical specimens are in good agreement, evaluation of VE1 in thyroid cytology samples is rarely performed, and its diagnostic value in cytology has not been well established. In present study, we explored VE1 immunoexpression in cytology samples from ex vivo papillary thyroid carcinoma specimens in order to minimize limitations of low cellularity and sampling/targeting errors originated from thyroid fineneedle aspiration and compared our results with those obtained using the corresponding papillary thyroid carcinoma tissues.
-
Methods:
- The VE1 antibody was evaluated in 21 cases of thyroid cytology obtained directly from ex vivo thyroid specimens. VE1 immunostaining was performed using liquid-based cytology, and the results were compared with those obtained using the corresponding tissues.
-
Results:
- Of 21 cases, 19 classic papillary thyroid carcinomas had BRAFV600E mutations, whereas two follicular variants expressed wild-type BRAF. VE1 immunoexpression varied according to specimen type. In detection of the BRAFV600E mutation, VE1 immunostaining of the surgical specimen exhibited 100% sensitivity and 100% specificity, whereas VE1 immunostaining of the cytology specimen exhibited only 94.7% sensitivity and 0% specificity.
-
Conclusions:
- Our data suggest that VE1 immunostaining of a cytology specimen is less specific than that of a surgical specimen for detection of the BRAFV600E mutation, and that VE1 immunostaining of a cytology specimen should be further evaluated and optimized for clinical use.
- This study was approved by the Ajou University Hospital Institutional Review Board (AJIRB-BMR-OBS-13-342). Cytology samples were obtained from fresh ex vivo PTC tissues immediately following surgical resection in cases that provided informed consent. After gross examination of fresh PTC specimens, cytology samples were obtained by scraping representative cancerous areas. Smear slides were prepared and stained with hematoxylin and eosin to explore the adequacy of liquid-based cytology (LBC). In later evaluations, LBC slides were prepared using the BD SurePath method employing CytoRich Red (TriPath Inc., Burlington, NC, USA). PTC tissues were fixed in 4% buffered formalin and, after embedding in paraffin, processed for histology and ancillary tests.
- Immunohistochemistry and immunocytochemistry
- Formalin-fixed, paraffin-embedded tissue blocks that included the cytology-sampled lesion were sectioned at a 4-μm slice thickness and deparaffinized for immunohistochemistry (IHC). VE1 immunostaining was performed using the aid of a Benchmark XT automated IHC platform (Ventana Medical Systems, Tucson, AZ, USA), as described previously [16]. Briefly, after cell conditioning (conditioner 1) for 64 minutes and inhibition of the preprimary peroxidase, slides were incubated with the VE1 antibody (1:50, Spring Bioscience, Pleasant, CA, USA) at 37°C for 32 minutes. Primary antibodies were detected using an OptiView DAB IHC Detection kit (Ventana Medical Systems) following incubation with hematoxylin and a bluing reagent (4 minutes each). For immunocytochemistry (ICC), unstained LBC slides were fixed in 95% ethyl alcohol for a minimum of 30 minutes. The ICC protocol was identical to that of IHC, except that the cells were not conditioned.
- Two pathologists (J.-H.K. and Y.H.K), blinded to the molecular findings, assessed all IHC and ICC data independently; any difference in the interpretation was resolved by consensus. The extent of VE1 staining was graded from 0 to 3: 0, negative; 1, VE1 staining in <30% of cells; 2, VE1 staining in 30%–80% of cells; and 3, VE1 staining in >80% of cells. In terms of cytoplasmic staining of follicular cells, intensity was also graded from 0 to 3: 0, negative; 1, weak; 2, moderate; and 3, strong. In defining BRAFV600E mutation, VE1 immunostaining was considered positive if the intensity of cytoplasmic staining was grade 2 or 3, regardless of the overall extent of staining [13,16].
- In cases of discrepancy between immunostaining and molecular results, we repeated immunostaining with a different method, the Ultravision LP Detection System (Thermo Fisher Scientific, Fremont, CA, USA), and re-evaluated the results.
- Detection of the BRAFV600E mutation
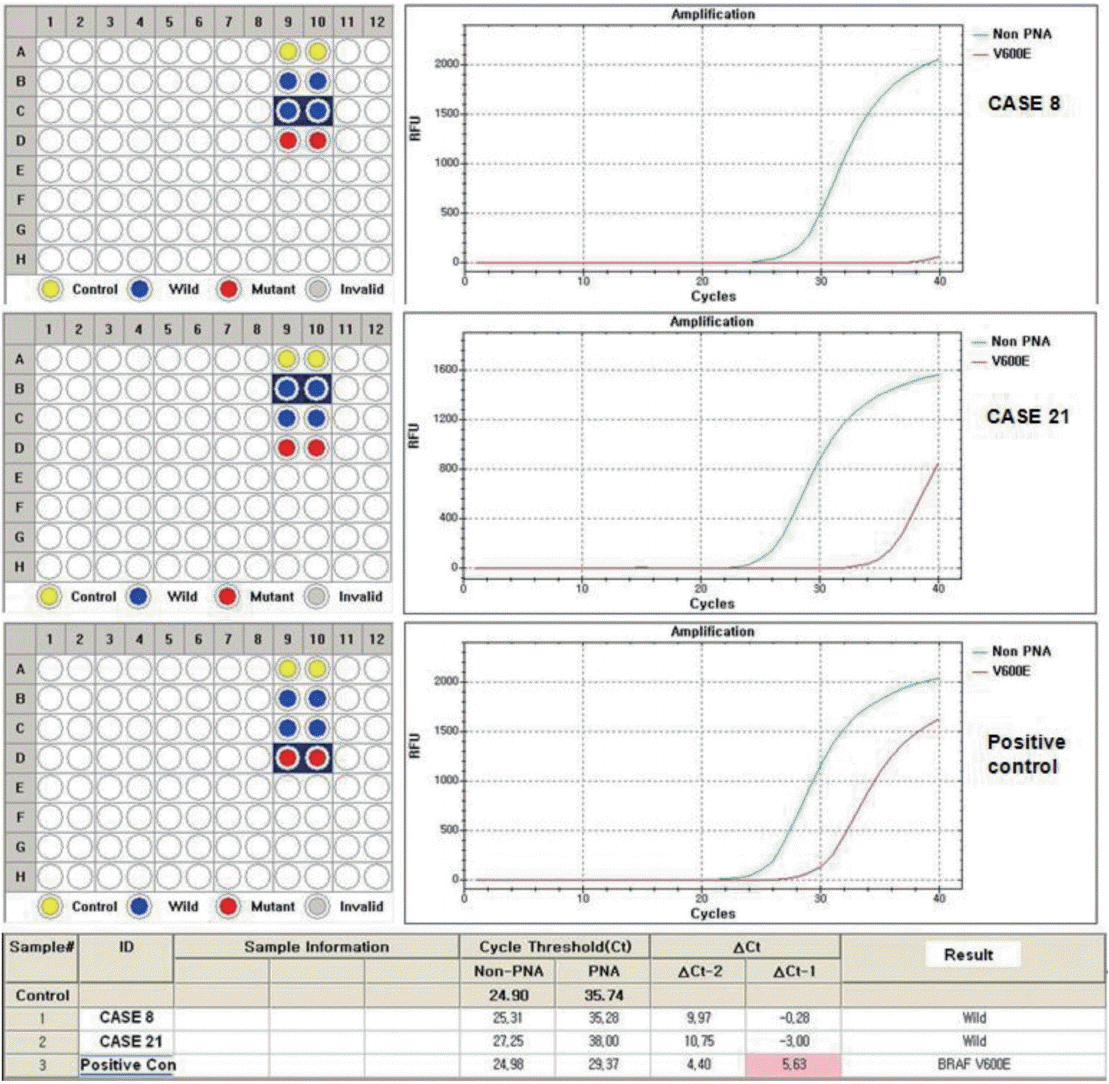
- For genomic DNA isolation, formalin-fixed, paraffin-embedded tissue blocks were sectioned at 10-μm thickness. Genomic DNA was extracted from manually microdissected tumor areas from each tissue section using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. To detect the BRAFV600E mutation, mutant enrichments with 3’-modified oligonucleotide sequencing were performed to confirm the presence or absence of the BRAFV600E mutation, employing primers and PCR conditions as described previously [7]. Results were analyzed using Sequencher 4.10 software (Gene Codes, Ann Arbor, MI, USA).
- In cases of discrepancy between immunostaining and molecular results, we repeated molecular testing with a different method, the PNAClamp Technology (Panagene, Daejeon, Korea), and re-evaluated the results.
MATERIALS AND METHODS
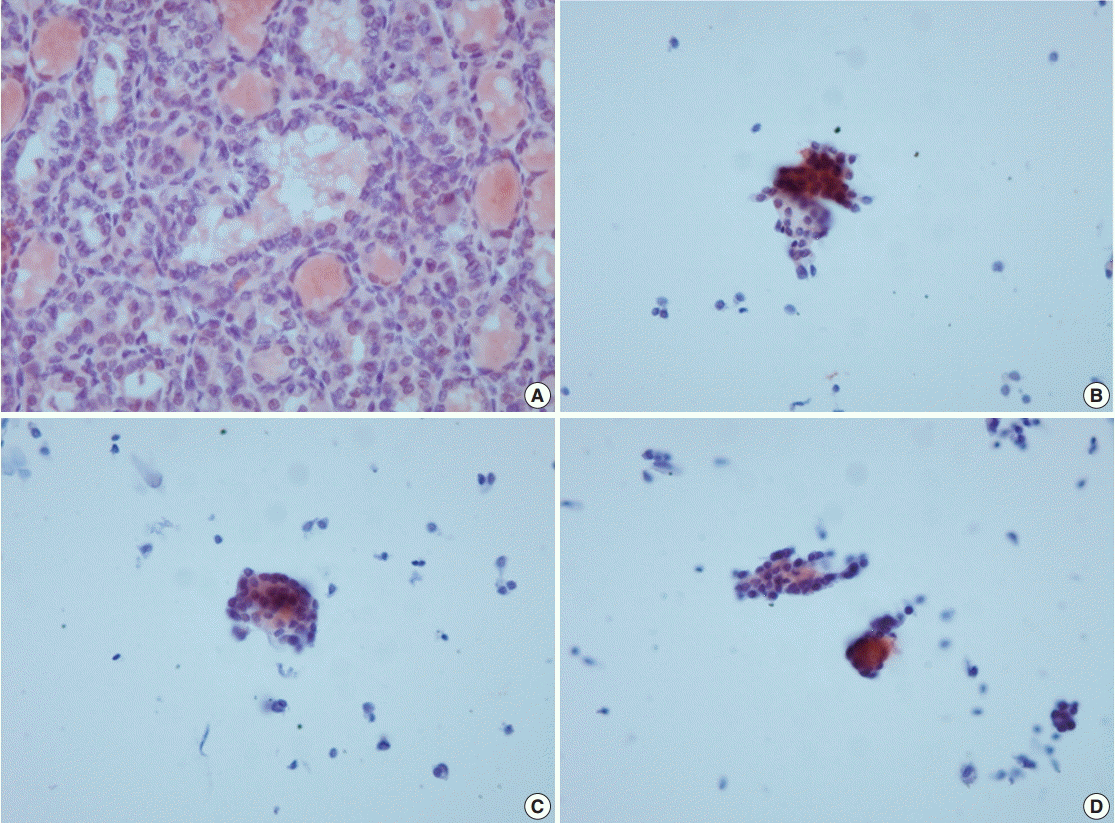
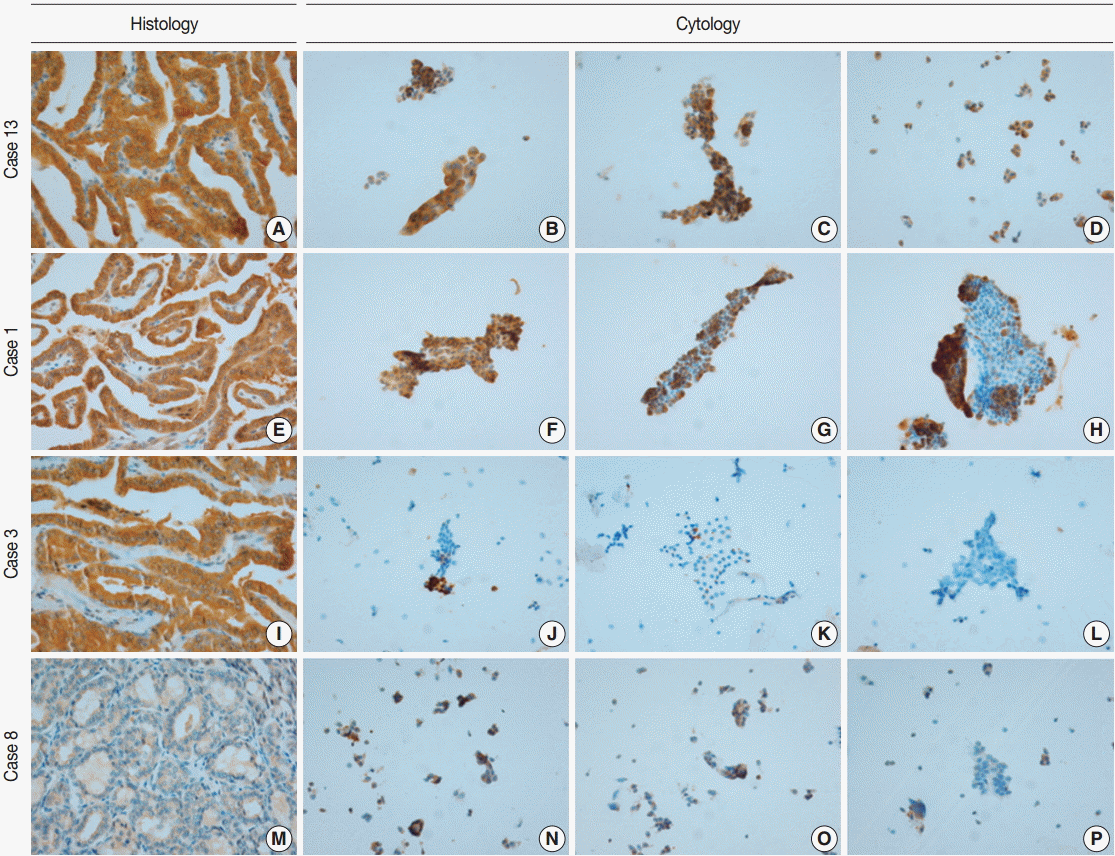
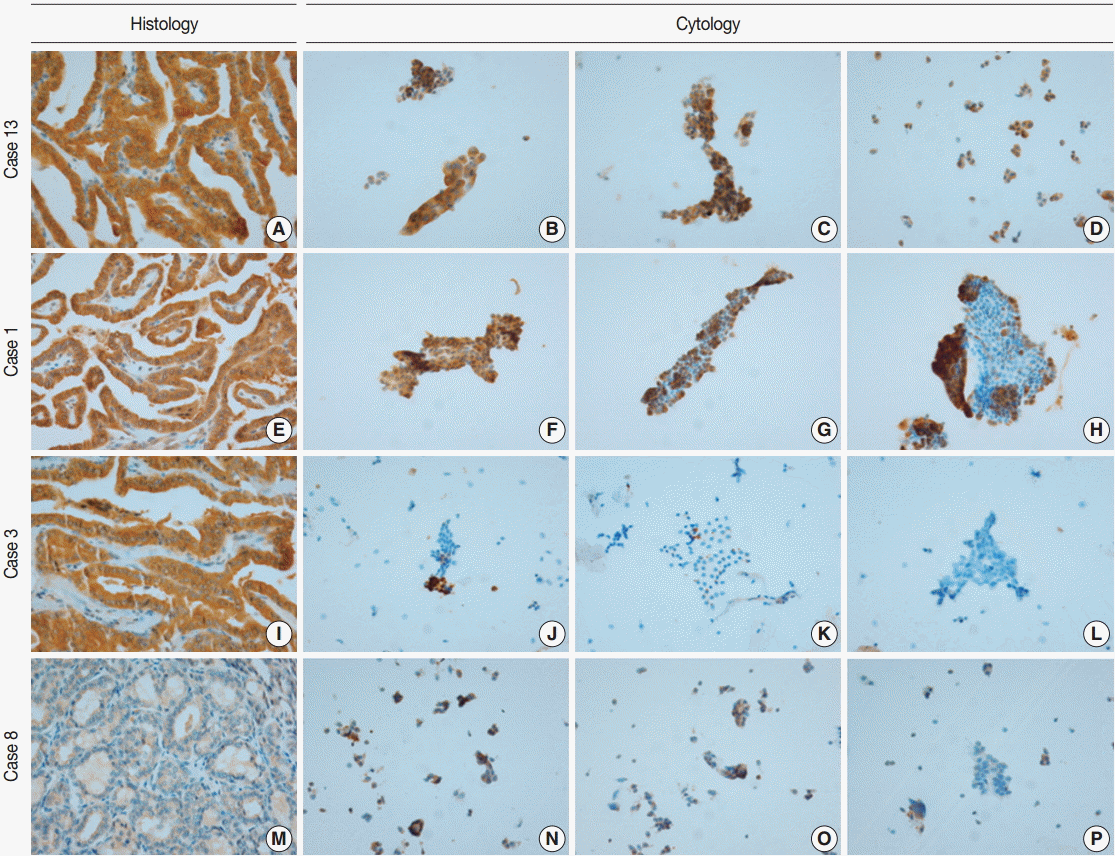
- VE1 immunostaining of LBC material and formalin-fixed, paraffin-embedded tissue sections of the corresponding areas was performed in 21 ex vivo PTC specimens. Clinicopathological characteristics of the 21 cases are summarized in Table 1. Of these, 19 were classic PTC cases, and two were follicular variants of PTC. The results of VE1 immunostaining according to BRAFV600E mutation status are shown in Table 2. Of the 21 cases, VE1 IHC of the 19 classic PTC cases exhibited diffuse immunoexpression with moderate or strong intensity, whereas staining in the two follicular variants was weak. Upon VE1 ICC, however, only 11 PTC cases (52.4%) exhibited diffuse immunoexpression (Table 3). The remaining cases yielded focal (2 cases, 9.5%) or multifocal (8 cases, 38.1%) immunostaining patterns (Fig. 1). Of the 21 cases with VE1 ICC, only 11 (52.4%) exhibited immunostaining intensity as strong as that of the corresponding VE1 IHC staining. In six cases (28.6%), immunostaining intensity was weaker than VE1 IHC staining, and in four cases (19.4%), VE1 immunostaining intensity was stronger than VE1 IHC staining (Table 4). VE1 immunostaining was interpreted as positive in 19 IHC and 20 ICC specimens (Table 5). We varied the molecular and immunohistochemical methods in cases of discrepancy between VE1 immunostaining and molecular results, but the results were similar (Appendices 1–3). In terms of the BRAFV600E mutation, VE1 immunostaining exhibited 100% sensitivity and 100% specificity with IHC but 94.7% sensitivity and 0% specificity with ICC.
RESULTS
- Clinical applications of VE1 immunostaining in terms of thyroid cytology evaluation are of great interest because PTC diagnosis in daily clinical practice is generally based on thyroid FNA cytology; immunostaining is simple, inexpensive, and routinely performed in most pathology laboratories. Moreover, VE1 immunostaining is not dependent on DNA quality or the proportion of tumor cells in a FNA sample and allows for in situ assessment of tumor cells expressing the BRAFV600E mutant protein at a single-cell level [13,16].
- In the present study, we evaluated the VE1 antibody in thyroid cytology using LBC specimens obtained directly from surgically resected ex vivo PTC specimens, because previous studies have suggested that the lower sensitivity and specificity of VE1 ICC compared to those of VE1 IHC might be related to the limitations of thyroid FNA cytology such as the extent of cellularity and the representative nature of the obtained thyroid tissue [13,15,16]. In the present study, all 21 LBC samples contained predominantly tumor cells, representing the cancerous area of each PTC specimen, and had an optimal cellularity for evaluation with VE1 ICC. Our data showed that the VE1 antibody had a higher sensitivity (94.7%) than that afforded by FNA cytology. Rossi et al. [15] and Lee et al. [13] reported sensitivities of 82.0% and 88.8%, respectively, when the VE1 antibody was evaluated in LBC samples. Wobker et al. [14] evaluated the VE1 antibody using smears of thyroid FNA material, but the detection sensitivity (63.6%) was less than that afforded by LBC samples.
- Although the cytology specimen in the present study was more representative of the corresponding histology than the cytology of FNA samples, the VE1 immunostaining patterns in ICC differed from those in IHC. All PTCs with BRAFV600E mutation showed diffuse positivity in VE1 IHC, as in previous studies [10,12,17], suggesting that the BRAFV600E mutation represents a clonal event during PTC development [17]. Using ICC, however, only 11 PTCs (57.9%) with the BRAFV600E mutation revealed diffuse positivity; other cases exhibited focal or multifocal positivity. Variations in the intensities and proportions of VE1-positive tumor cells in the same samples were also noted in earlier studies using FNA material [14-16]. Staining variability can be influenced by storage duration, technical problems, or fixation type [14,15,18]. In the present study, ICC on LBC was performed within 48 hours after sampling. In an attempt to eliminate technical problems, VE1 ICC was performed using different methods, but the results were similar (Appendix 1). It has been suggested that ethanol-based fixation destabilizes proteins not only in histology [19], but also in cytology [18]. We used a methanol- and isopropanol-based preservative (CytoRich Red) containing 1% formalin as a fixative, which is known to be more compatible with ICC than an ethanol-based fixative [18]. Nonetheless, we found that VE1 ICC was less sensitive than IHC in detecting BRAFV600E mutation. Previous studies found that the extent of disagreement between ICC and IHC was 7.2%–34.7% and suggested that differences in fixation methods might explain the observed discrepancies [14-16]. Our results also indicate that differences in fixation between ICC and IHC are a major contributing factor resulting in different VE1 immunoexpression in the same tissue samples.
- Upon IHC, VE1 was detected with high specificity, but the ICC specificity was 0% because one PTC harboring the BRAFV600E mutation was negative for VE1, while two follicular variant PTCs lacking the BRAFV600E mutation were positive for VE1 immunostaining. We varied the molecular and immunohistochemical methods used, but the results were similar (Appendices 2, 3). To evaluate the specificity, the number of wild-type PTC samples in the present study was too small. Nonetheless, false positivity of VE1 in thyroid cytology should not be underestimated. Nonspecific staining of colloids, macrophages, and follicles containing colloids or stroma has been suggested to hamper the interpretation of VE1 ICC [13-16]. One recent study showed that the VE1 antibody cross-reacted with certain ciliary structural proteins, inducing VE1 false positivity [20]. Some proteins expressed in endocrine organs, including α-ketoglutarate-dependent dioxygenase alk-B homolog 7, eukaryotic translation initiation factor 2-α kinase 4, polo-like kinase-1δ, potassium channel tetramerization domain-containing 4, and solute carrier family 4 (anion exchanger) member 3, also share sequence similarities with the peptide immunogen used to generate the VE1 antibody [20]. Such cross-reactivities might possibly explain the nonspecific staining of, or false-positivity for, VE1 in thyroid PTC samples.
- This study has several limitations, mostly stemming from its small number of cases. Nonetheless, the results from the present study suggest that VE1 ICC is less specific than VE1 IHC in detecting the BRAFV600E mutation. For clinical application of the VE1 antibody in thyroid cytology, further evaluation and optimization of VE1 immunostaining in cytology specimens are essential.
DISCUSSION
Acknowledgments
| Distribution of VE1 |
ICC |
||
|---|---|---|---|
| < 30% | 30-80% | > 80% | |
| IHC | |||
| < 30% | - | - | - |
| 30%-80% | - | - | - |
| > 80% | 2 | 8 | 11 |
| Intensity of VE1 |
ICC |
||
|---|---|---|---|
| Weak | Moderate | Strong | |
| IHC | |||
| Weak | - | 2 | - |
| Moderate | - | 1 | 2 |
| Strong | 1 | 5 | 10 |
| BRAFV600E mutation |
VE1 ICC |
VE1 IHC |
||
|---|---|---|---|---|
| Negative | Positive | Negative | Positive | |
| Absence | 0 | 2 | 2 | 0 |
| Presence | 1 | 18 | 0 | 19 |
- 1. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417: 949-54. PubMed
- 2. Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature 2002; 418: 934.ArticlePubMedPDF
- 3. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer 2005; 12: 245-62. ArticlePubMed
- 4. Kim JH, Paulus W, Heim S. BRAF V600E mutation is a useful marker for differentiating Rathke’s cleft cyst with squamous metaplasia from papillary craniopharyngioma. J Neurooncol 2015; 123: 189-91. ArticlePubMedPDF
- 5. Ascierto PA, Minor D, Ribas A, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 2013; 31: 3205-11. ArticlePubMed
- 6. Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairycell leukemia. N Engl J Med 2011; 364: 2305-15. PubMedPMC
- 7. Lee ST, Kim SW, Ki CS, et al. Clinical implication of highly sensitive detection of the BRAF V600E mutation in fine-needle aspirations of thyroid nodules: a comparative analysis of three molecular assays in 4585 consecutive cases in a BRAF V600E mutation-prevalent area. J Clin Endocrinol Metab 2012; 97: 2299-306. ArticlePubMedPDF
- 8. Kim YH, Choi SE, Yoon SO, Hong SW. A testing algorithm for detection of the B-type Raf kinase V600E mutation in papillary thyroid carcinoma. Hum Pathol 2014; 45: 1483-8. ArticlePubMed
- 9. Ritterhouse LL, Barletta JA. BRAF V600E mutation-specific antibody: a review. Semin Diagn Pathol 2015; 32: 400-8. ArticlePubMed
- 10. Zagzag J, Pollack A, Dultz L, et al. Clinical utility of immunohistochemistry for the detection of the BRAF V600E mutation in papillary thyroid carcinoma. Surgery 2013; 154: 1199-204. ArticlePubMedPMC
- 11. Routhier CA, Mochel MC, Lynch K, Dias-Santagata D, Louis DN, Hoang MP. Comparison of 2 monoclonal antibodies for immunohistochemical detection of BRAF V600E mutation in malignant melanoma, pulmonary carcinoma, gastrointestinal carcinoma, thyroid carcinoma, and gliomas. Hum Pathol 2013; 44: 2563-70. ArticlePubMed
- 12. Koperek O, Kornauth C, Capper D, et al. Immunohistochemical detection of the BRAF V600E-mutated protein in papillary thyroid carcinoma. Am J Surg Pathol 2012; 36: 844-50. ArticlePubMed
- 13. Lee SR, Yim H, Han JH, et al. VE1 antibody is not highly specific for the BRAF V600E mutation in thyroid cytology categories with the exception of malignant cases. Am J Clin Pathol 2015; 143: 437-44. ArticlePubMedPDF
- 14. Wobker SE, Kim LT, Hackman TG, Dodd LG. Use of BRAF V600E immunocytochemistry on FNA direct smears of papillary thyroid carcinoma. Cancer Cytopathol 2015; 123: 531-9. ArticlePubMed
- 15. Rossi ED, Martini M, Capodimonti S, et al. Analysis of immunocytochemical and molecular BRAF expression in thyroid carcinomas: a cytohistologic institutional experience. Cancer Cytopathol 2014; 122: 527-35. ArticlePubMed
- 16. Zimmermann AK, Camenisch U, Rechsteiner MP, Bode-Lesniewska B, Rössle M. Value of immunohistochemistry in the detection of BRAF(V600E) mutations in fine-needle aspiration biopsies of papillary thyroid carcinoma. Cancer Cytopathol 2014; 122: 48-58. ArticlePubMed
- 17. Ghossein RA, Katabi N, Fagin JA. Immunohistochemical detection of mutated BRAF V600E supports the clonal origin of BRAF-induced thyroid cancers along the spectrum of disease progression. J Clin Endocrinol Metab 2013; 98: E1414-21. Article
- 18. Kawahara A, Taira T, Abe H, et al. Fixation effect of SurePath preservative fluids using epidermal growth factor receptor mutation-specific antibodies for immunocytochemistry. Cancer Cytopathol 2014; 122: 145-52. ArticlePubMed
- 19. Dvorak K, Aggeler B, Palting J, McKelvie P, Ruszkiewicz A, Waring P. Immunohistochemistry with the anti-BRAF V600E (VE1) antibody: impact of pre-analytical conditions and concordance with DNA sequencing in colorectal and papillary thyroid carcinoma. Pathology 2014; 46: 509-17. ArticlePubMedPMC
- 20. Jones RT, Abedalthagafi MS, Brahmandam M, et al. Cross-reactivity of the BRAF VE1 antibody with epitopes in axonemal dyneins leads to staining of cilia. Mod Pathol 2015; 28: 596-606. ArticlePubMedPDF
REFERENCES
Appendix
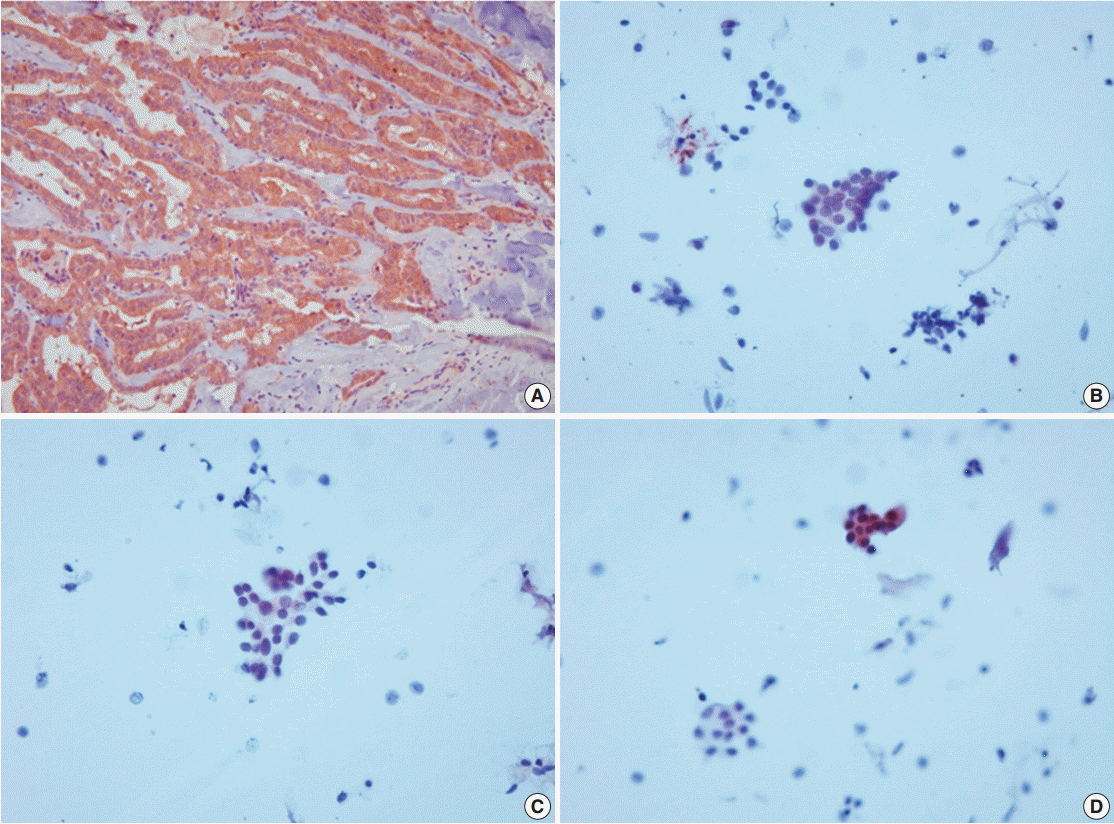
Figure & Data
References
Citations

- Principles of Analytic Validation of Immunohistochemical Assays: Guideline Update
Jeffrey D. Goldsmith, Megan L. Troxell, Sinchita Roy-Chowdhuri, Carol F. Colasacco, Mary Elizabeth Edgerton, Patrick L. Fitzgibbons, Regan Fulton, Thomas Haas, Patricia L. Kandalaft, Tanja Kalicanin, Christina Lacchetti, Patti Loykasek, Nicole E. Thomas,
Archives of Pathology & Laboratory Medicine.2024; 148(6): e111. CrossRef - Inter- and Intra-observer Reproducibility of Thyroid Fine Needle Aspiration Cytology: An investigation of Bethesda 2023 Using Immunohistochemical BRAFV600E Antibody
Erdogan Bahattin, Dündar Emine, Çetin Kısmet Çivi, Yılmaz Fatih
Journal of Cytology.2024; 41(4): 221. CrossRef - VE1 immunohistochemistry is an adjunct tool for detection of BRAFV600E mutation: Validation in thyroid cancer patients
Faiza A. Rashid, Sobia Tabassum, Mosin S. Khan, Hifzur R. Ansari, Muhammad Asif, Ahmareen K. Sheikh, Syed Sameer Aga
Journal of Clinical Laboratory Analysis.2021;[Epub] CrossRef - Effective utilization of liquid-based cytology for thyroid lesions
Yukie YAMAYA
The Journal of the Japanese Society of Clinical Cytology.2021; 60(3): 164. CrossRef - Diagnostic Efficacy of BRAFV600E Immunocytochemistry in Thyroid Aspirates in Bethesda Category IV and Papillary Thyroid Carcinoma
Nidhi Anand, Tushar Agrawal, Anurag Gupta, Saumya Shukla, Roma Pradhan, Nuzhat Husain
Journal of Cytology.2021; 38(3): 113. CrossRef - The immunocytochemical expression of VE‐1 (BRAF V600E‐related) antibody identifies the aggressive variants of papillary thyroid carcinoma on liquid‐based cytology
Patrizia Straccia, Chiara Brunelli, Esther D. Rossi, Paola Lanza, Maurizio Martini, Teresa Musarra, Celestino Pio Lombardi, Alfredo Pontecorvi, Guido Fadda
Cytopathology.2019; 30(5): 460. CrossRef - Utility of the BRAF p.V600E immunoperoxidase stain in FNA direct smears and cell block preparations from patients with thyroid carcinoma
Amber L. Smith, Michelle D. Williams, John Stewart, Wei‐Lien Wang, Savitri Krishnamurthy, Maria E. Cabanillas, Sinchita Roy‐Chowdhuri
Cancer Cytopathology.2018; 126(6): 406. CrossRef - Refinement of the criteria for ultrastructural peritubular capillary basement membrane multilayering in the diagnosis of chronic active/acute antibody-mediated rejection
Heounjeong Go, Sung Shin, Young Hoon Kim, Duck Jong Han, Yong Mee Cho
Transplant International.2017; 30(4): 398. CrossRef - Thyroid Fine-Needle Aspiration Cytology Practice in Korea
Yoon Jin Cha, Ju Yeon Pyo, SoonWon Hong, Jae Yeon Seok, Kyung-Ju Kim, Jee-Young Han, Jeong Mo Bae, Hyeong Ju Kwon, Yeejeong Kim, Kyueng-Whan Min, Soonae Oak, Sunhee Chang
Journal of Pathology and Translational Medicine.2017; 51(6): 521. CrossRef - Use of monoclonal antibodies to detect specific mutations in formalin-fixed, paraffin-embedded tissue sections
Zhenying Guo, Ricardo V. Lloyd
Human Pathology.2016; 53: 168. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

Fig. 1.
| Case No. | Sex | Age (yr) | Histological type | Tumor diameter (cm) | T stage | N stage | BRAFV600E mutation |
|---|---|---|---|---|---|---|---|
| 1 | F | 49 | Classic | 1 | T3 | N1a | Present |
| 2 | F | 51 | Classic | 1.2 | T3 | N0 | Present |
| 3 | M | 43 | Classic | 0.7 | T3 | N1a | Present |
| 4 | F | 30 | Classic | 0.9 | T3 | N0 | Present |
| 5 | F | 35 | Classic | 1.2 | T3 | N1a | Present |
| 6 | M | 48 | Classic | 0.9 | T1a | N0 | Present |
| 7 | F | 30 | Classic | 1.2 | T3 | N1a | Present |
| 8 | M | 26 | FVPTC | 1.4 | T3 | N1a | Absent |
| 9 | F | 60 | Classic | 1.8 | T3 | N1a | Present |
| 10 | F | 56 | Classic | 1 | T3 | N1a | Present |
| 11 | F | 63 | Classic | 0.8 | T3 | N1b | Present |
| 12 | M | 69 | Classic | 1.3 | T3 | N1a | Present |
| 13 | F | 48 | Classic | 0.8 | T3 | N0 | Present |
| 14 | F | 59 | Classic | 1 | T3 | N0 | Present |
| 15 | F | 46 | Classic | 1.5 | T1b | N1a | Present |
| 16 | F | 62 | Classic | 1.2 | T1b | N0 | Present |
| 17 | F | 54 | Classic | 1 | T3 | N0 | Present |
| 18 | F | 63 | Classic | 3.3 | T3 | N1a | Present |
| 19 | F | 42 | Classic | 0.7 | T1a | N1a | Present |
| 20 | M | 69 | Classic | 0.8 | T1a | N0 | Present |
| 21 | F | 52 | FVPTC | 1.2 | T3 | N0 | Absent |
| Case No. | Liquid-based cytology |
Histology |
BRAFV600E mutation | ||
|---|---|---|---|---|---|
| Distribution | Intensity | Distribution | Intensity | ||
| 1 | 2+ | 3+ | 3+ | 3+ | Present |
| 2 | 2+ | 2+ | 3+ | 3+ | Present |
| 3 | 1+ | 1+ | 3+ | 3+ | Present |
| 4 | 2+ | 2+ | 3+ | 3+ | Present |
| 5 | 3+ | 3+ | 3+ | 3+ | Present |
| 6 | 1+ | 2+ | 3+ | 2+ | Present |
| 7 | 2+ | 3+ | 3+ | 3+ | Present |
| 8 | 2+ | 2+ | 3+ | 1+ | Absent |
| 9 | 2+ | 3+ | 3+ | 3+ | Present |
| 10 | 2+ | 2+ | 3+ | 3+ | Present |
| 11 | 3+ | 3+ | 3+ | 2+ | Present |
| 12 | 3+ | 3+ | 3+ | 2+ | Present |
| 13 | 3+ | 3+ | 3+ | 3+ | Present |
| 14 | 3+ | 3+ | 3+ | 3+ | Present |
| 15 | 3+ | 3+ | 3+ | 3+ | Present |
| 16 | 3+ | 3+ | 3+ | 3+ | Present |
| 17 | 3+ | 3+ | 3+ | 3+ | Present |
| 18 | 3+ | 3+ | 3+ | 3+ | Present |
| 19 | 3+ | 2+ | 3+ | 3+ | Present |
| 20 | 3+ | 2+ | 3+ | 3+ | Present |
| 21 | 2+ | 2+ | 3+ | 1+ | Absent |
| Distribution of VE1 | ICC |
||
|---|---|---|---|
| < 30% | 30-80% | > 80% | |
| IHC | |||
| < 30% | - | - | - |
| 30%-80% | - | - | - |
| > 80% | 2 | 8 | 11 |
| Intensity of VE1 | ICC |
||
|---|---|---|---|
| Weak | Moderate | Strong | |
| IHC | |||
| Weak | - | 2 | - |
| Moderate | - | 1 | 2 |
| Strong | 1 | 5 | 10 |
| BRAFV600E mutation | VE1 ICC |
VE1 IHC |
||
|---|---|---|---|---|
| Negative | Positive | Negative | Positive | |
| Absence | 0 | 2 | 2 | 0 |
| Presence | 1 | 18 | 0 | 19 |
FVPTC, follicular variant papillary thyroid carcinoma.
Distributions of VE1-positive cells: 0+, 0%; 1+, < 30%; 2+, 30%–80%; and 3+, > 80%. Intensities of VE1-positive cells: 0+, none; 1+, weak; 2+, moderate; and 3+, strong.
ICC, immunocytochemistry; IHC, immunohistochemistry.
ICC, immunocytochemistry; IHC, immunohistochemistry.
ICC, immunocytochemistry; IHC, immunohistochemistry.

 E-submission
E-submission