Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 50(1); 2016 > Article
-
Review
Overview of IgG4-Related Tubulointerstitial Nephritis and Its Mimickers - Hyeon Joo Jeong, Su-Jin Shin, Beom Jin Lim
-
Journal of Pathology and Translational Medicine 2016;50(1):26-36.
DOI: https://doi.org/10.4132/jptm.2015.11.09
Published online: December 14, 2015
Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
- Corresponding Author Hyeon Joo Jeong, MD Department of Pathology, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea Tel: +82-2-2228-1766 Fax: +82-2-362-0860 E-mail: jeong10@yuhs.ac
© 2016 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Abstract
- TUBULOINTERSTITIAL NEPHRITIS IN IMMUNOGLOBULIN G4-RELATED DISEASE
- TUBULOINTERSTITIAL NEPHRITIS IN LUPUS NEPHRITIS
- TUBULOINTERSTITIAL NEPHRITIS IN SJÖGREN SYNDROME
- TUBULOINTERSTITIAL NEPHRITIS IN ANTI-NEUTROPHIL CYTOPLASMIC ANTIBODY-ASSOCIATED VASCULITIS
- TUBULOINTERSTITIAL NEPHRITIS IN OTHER CONDITIONS SIMULATING IMMUNOGLOBULIN G4-TUBULOINTERSTITIAL NEPHRITIS
- CLINICAL AND HISTOLOGICAL OVERLAP BETWEEN IMMUNOGLOBULIN G4-TUBULOINTERSTITIAL NEPHRITIS AND TUBULOINTERSTITIAL NEPHRITIS OF AUTOIMMUNE DISEASES
- GLOMERULONEPHRITIS ASSOCIATED WITH IMMUNOGLOBULIN G4-TUBULOINTERSTITIAL NEPHRITIS
- NOTES
- REFERENCES
Abstract
- Tubulointerstitial nephritis (TIN) is the most common form of renal involvement in IgG4-related disease. It is characterized by a dominant infiltrate of IgG4-positive plasma cells in the interstitium and storiform fibrosis. Demonstration of IgG4-positive plasma cells is essential for diagnosis, but the number of IgG4-positive cells and the ratio of IgG4-positive/IgG-positive plasma cells may vary from case to case and depending on the methods of tissue sampling even in the same case. IgG4-positive plasma cells can be seen in TIN associated with systemic lupus erythematosus, Sjögren syndrome, or anti-neutrophil cytoplasmic antibody–associated vasculitis, which further add diagnostic confusion and difficulties. To have a more clear view of IgG4-TIN and to delineate differential points from other TIN with IgG4-positive plasma cell infiltrates, clinical and histological features of IgG4-TIN and its mimickers were reviewed. In the rear part, cases suggesting overlap of IgG4-TIN and its mimickers and glomerulonephritis associated with IgG4-TIN were briefly described.
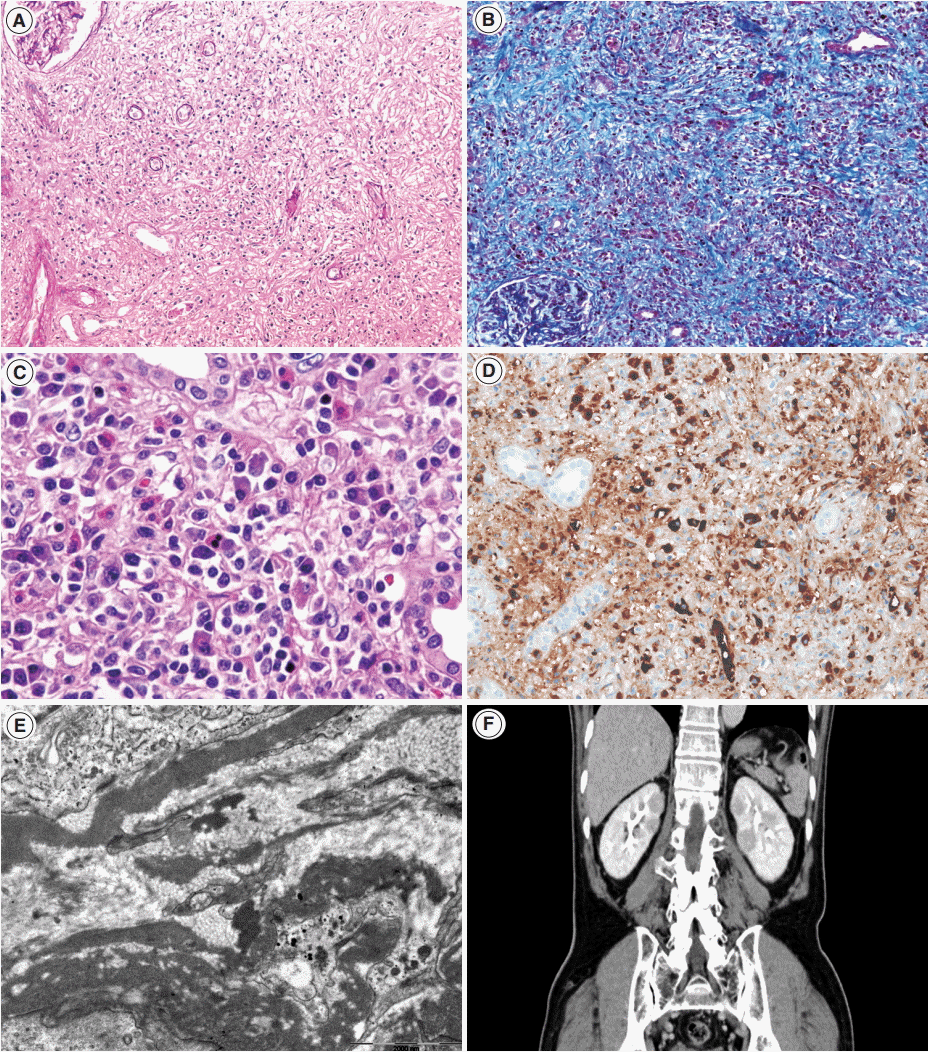
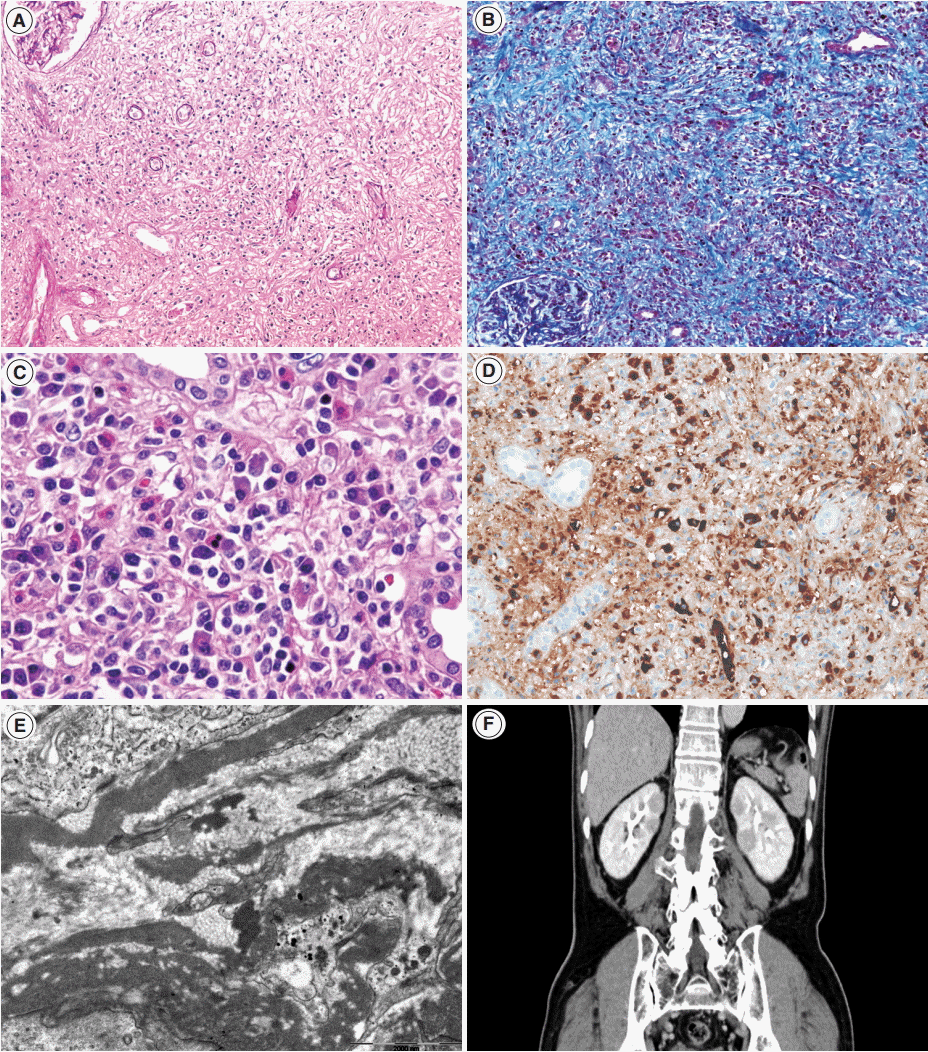
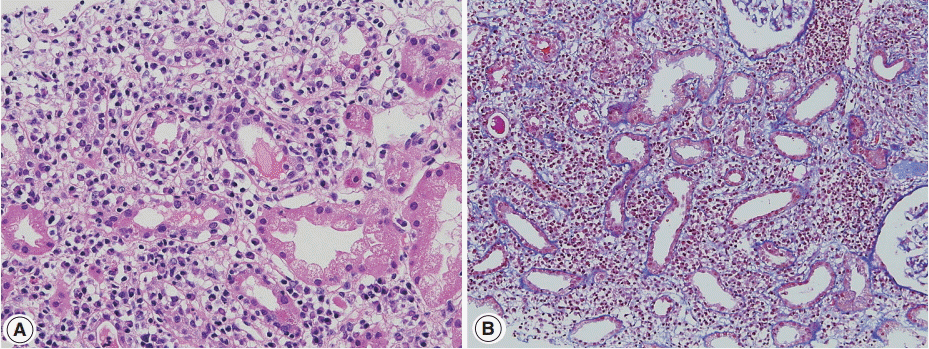
- Renal histology is fundamental in the diagnosis of TIN in IgG4-RD. Three features are characteristic: (1) interstitial lymphoplasmacytic infiltrates with dominant IgG4-positive plasma cells; (2) the ratio of IgG4-positive/IgG-positive plasma cells over 40%; and (3) obliterative phlebitis. A cut-off value of >10 IgG4-positive plasma cells/high-power field (HPF) and/or ratio of IgG4-positive/IgG-positive plasma cells >40% was used in the previous Japanese study [10]. Soon after, in the consensus guideline on IgG4-RD in 2012 [11], different cut-off values were applied in the number of IgG4-positive plasma cells according to the type of specimen received. In renal biopsy samples, >10 IgG4-positive plasma cells/HPF are enough, but >30 IgG4-positive plasma cells/HPF are required in nephrectomy specimens. The infiltrate may be patchy in distribution; therefore, the possibility of IgG4-RD should not be excluded based on negative biopsy results, especially in the presence of other supportive clinical and imaging features of IgG4-RD. The IgG4/IgG ratio of plasmacytic infiltration over 40% was maintained in the consensus guideline, which is a reasonable value as it demonstrated a sensitivity of 58.8% and a specificity of 90.2% in a meta-analysis [12]. In the lymphocytic infiltrates, T lymphocytes predominate over B cells. Eosinophils are common and may be numerous in some cases. Inflammatory infiltrates may extend into the renal capsule, which has not been known in TIN of other non-infectious causes [13,14] (Fig. 1A–D). Glomeruli are usually spared, but when glomerulonephritis is associated, membranous nephropathy is the most common [15]. Vascular changes are not common, but renal arteritis was reported in one case associated with TIN [16].
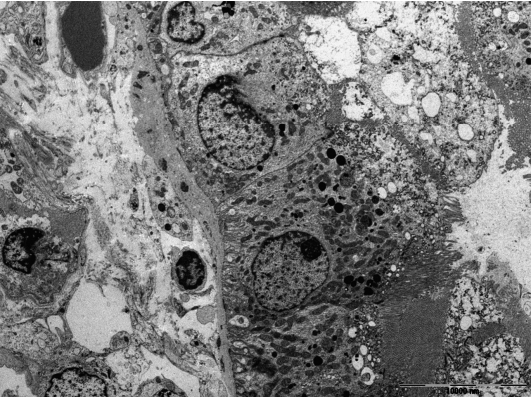
- Tubulointerstitial immune deposits may be seen in some cases [17]. IgG and C3 are deposited most commonly along the tubular basement membrane [18]. Interstitial immune deposits tend to be restricted in inflamed areas and they are regarded as a late change [18]. By electron microscopy, electron-dense deposits are frequently found in the tubular basement membrane and interstitium [13] (Fig. 1E). In an earlier report of idiopathic hypocomplementemic TIN, which is now regarded as a form of IgG4-TIN, fingerprint organoid deposits were present in the interstitium in two out of nine cases [4]. Glomerular mesangial and Bowman’s capsular deposits were also frequently observed under electron microscopy, even though glomeruli showed no significant changes by light and immunofluorescent microscopy [19].
- Most IgG4-TIN patients are males in mid-sixties. Renal involvement was reported in six of 132 (4.5%) [20], 10 of 114 (8.8%),21 54 of 235 (23.0%) [22], and 20 of 57 (35.1%) patients [23] with IgG4-RD. Patients present with acute renal failure, urinary abnormalities, or mass formation with urinary obstruction [7,19]. Urinalysis showed mild proteinuria in 82.6% and hematuria in 34.8% in one collective study [7]. If membranous nephropathy is associated, nephrotic range proteinuria may be present [15]. In the presence of renal failure, serum creatinine levels may be elevated. Serology shows polyclonal hypergammaglobulinemia and elevated IgG4 levels. Serum IgG4 levels (>135 mg/dL) may be elevated in up to 93% of IgG4-TIN [7,8,10], which are higher than the prevalence of about 70% in total IgG4-RD. This high rate may be related to the organ specificity, but it may reflect the increased number of involved organs because other organs are frequently involved at the time of diagnosis [22]. Serum IgG4 levels have been reported to decrease with steroid treatment, and increase with relapse. However, it is neither essential nor specific for the diagnosis. Elevated IgG4 serum levels were reported in 10.8% of SLE and 12.9% of rheumatoid arthritis patients [24]. IgG4 levels may even show a paradoxical response, showing an increase despite effective treatment [25]. Other serologic markers associated with autoimmune diseases or allergies have been frequently reported. Hypocomplementemia, elevated IgE levels, and eosinophilia have been reported in 56.0%–69.6%, 71.4%, and 33.0%–47.8%, respectively [7,8]. Antinuclear antibodies (ANA) and rheumatoid factors, usually in low titers, were reported in 31.0%–69.6% and 38.9%, respectively. Anti-DNA antibody was positive in a few cases.
- Although histology may be highly suggestive of IgG4-TIN, confirmatory diagnosis relies on both histological and clinical features. In addition to IgG4-positive plasma cells >10/HPF, the presence of at least one other feature from the imaging studies, serology, or other organ involvement categories was suggested for the diagnosis [8]. Cases showing clinical and laboratory features suggestive of SLE, Sjögren syndrome, or ANCA-associated vasculitis should be excluded [1]. Other organ involvement is frequent at the time of diagnosis of TIN (83.0%–95.7%). Among them, the salivary glands (82.6%), lymph nodes (43.5%), pancreas (39.1%), and lacrimal glands (30.4%) are most frequently involved either synchronously or metachronously (Table 1) [7,8,13,26-28]. In cases of renal involvement without extrarenal manifestation [7], imaging studies may be helpful (Fig. 1F). Four patterns of round or wedge-shaped renal cortical nodules, peripheral cortical lesions, mass-like lesions, and renal pelvic involvement, were reported [23].
- IgG4-TIN responds well to steroid therapy with decrease of serum creatinine levels. A recent report of repeated biopsy after steroid treatment showed advanced fibrosis but decreased inflammatory activity with fewer IgG4-positive plasma cells and reduced expression of connective tissue growth factor mRNA [29]. Regarding disease activity, elevated serum IgG4 levels and IgG4-positive plasmablast levels were suggested in one study [30].
TUBULOINTERSTITIAL NEPHRITIS IN IMMUNOGLOBULIN G4-RELATED DISEASE
- Lupus nephritis is usually characterized by proliferative glomerulonephritis with massive immune deposits and accompanying mild to moderate interstitial inflammation. Rarely, it may present with predominant TIN without significant glomerular changes [31-37]. Up to now, about 20 cases of predominant lupus TIN have been reported. The patients presented with acute renal failure or renal insufficiency. Interstitial inflammatory infiltrate was composed of mixture of CD4+ and CD8+ T cells, B cells, macrophages, and plasma cells. IgG, C3, and C1q deposits were present in the tubular basement membranes, whereas glomerular deposits were negative or minimal. Electron-dense deposits could be seen in tubular basement membranes and interstitium (Fig. 2).
- IgG4-positive plasma cells may infiltrate in the interstitium and IgG4 deposits may be present in the peritubular interstitium and along the tubular basement membrane in lupus TIN [36], similar to IgG4-TIN. In contrast to IgG4-TIN, immune deposits in lupus TIN are rather diffuse. C1q deposits, if prominent, favors lupus TIN. However, if mass-effects on an imaging study or patchy inflammatory infiltrate extending to renal capsule and beyond are present, it is unlikely to be lupus TIN. Massive eosinophil infiltration is also exceptional for lupus.
- Distinction between IgG4-RD and SLE depends on the clinical diagnostic criteria, despite some differential histologic features. The American College of Rheumatology (ACR) and Systemic Lupus International Collaborating Clinics (SLICC) criteria may be applied to the diagnosis of SLE. Depending on the criteria applied, a variable proportion of TIN cases with IgG4-positive plasma cells may be categorized into SLE or other autoimmune diseases [10]. However, clinical distinction between IgG4-TIN and lupus TIN is not always simple. Signs of SLE may appear late and may not fulfill the diagnostic criteria at the time of biopsy [38]. Clinical and laboratory features which frequently present in IgG4-RD may also be present in SLE patients [36]. Serum gammaglobulin [35] may be elevated. Serum IgG4 levels were elevated in 10.8% of SLE patients [24]. Retroperitoneal fibrosis may be present [39]. A response to steroid treatment is also good in predominant lupus TIN [35]. Kiyama et al. [40] reported ANA subclasses in SLE and IgG4-RD, demonstrating IgG1, 2, or 3 subclasses in SLE and predominantly IgG2 in IgG4-RD, but very rare or no IgG4 in both conditions.
TUBULOINTERSTITIAL NEPHRITIS IN LUPUS NEPHRITIS
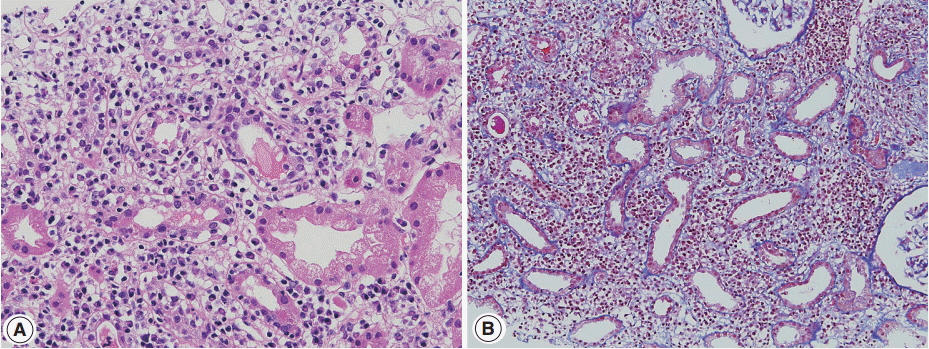
- Sjögren syndrome is characterized by keratoconjunctivitis sicca and xerostomia due to immunologic destruction of lacrimal and salivary glands. Renal involvement is infrequent. Distal renal tubular acidosis and acute kidney injury are the main clinical manifestations. Chronic TIN is the most commonly observed form on renal biopsy, but glomerulonephritis may also be observed [41,42]. In TIN of Sjögren syndrome, lymphocytes with a T-cell dominance and macrophages infiltrate in the interstitium along with mild tubulitis and tubular atrophy (Fig. 3A, B). Plasma cells may be numerous but storiform fibrosis is not a feature [43]. Immune deposits may be present in the tubular basement membrane.
- Clinically, symptoms of dry eye and mouth or arthralgia are more frequent in Sjögren syndrome than in IgG4-RD. The presence of anti–SS-A and anti–SS-B antibodies is characteristic and was present in 71% and 54%, respectively, in one large series [41]. Steroid therapy in Sjögren syndrome has limited effects compared with that in IgG4-RD [44]. However, clinical and laboratory features may infrequently overlap with IgG4-TIN. Serum IgG4 levels were reported to be elevated in 7.5% of the patients with primary Sjögren syndrome [24]. One TIN patient with Sjögren syndrome showed elevated serum IgG4 levels and renal interstitial IgG4-positive plasma cell infiltrate [45]. Marked hypocomplementemia was reported in a TIN case of Sjögren syndrome [46]. Some patients had ‘‘pseudolymphoma’’ lesions or autoimmune pancreatitis and sclerosing cholangitis [24]. By contrast, seven of 23 IgG4-TIN cases fulfilled the criteria of Sjögren syndrome [7], and anti–SS-A antibody was present in 4.4% of Mikulicz’s disease [47].
TUBULOINTERSTITIAL NEPHRITIS IN SJÖGREN SYNDROME
- Tubulointerstitial inflammation is frequently associated with ANCA-associated vasculitis. Interstitial inflammatory infiltrate is composed of lymphocytes, plasma cells, and some neutrophils. In typical cases, glomerular crescents or necrotizing lesions are commonly found with or without vasculitis and the distinction from IgG4-RD is not difficult. IgG4-positive plasma cells may be present, but the number of IgG4-positive plasma cells and/or IgG4-positive/IgG-positive plasma cell ratio is usually not high [9]. In addition, elevated C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR) is in favor of ANCA-associated vasculitis.
- Rarely, IgG4-dominant TIN may present with concomitant cytoplasmic ANCA and antibody to proteinase 3 [48,49]. Among the three types of ANCA-associated vasculitis, eosinophilic granulomatosis with polyangiitis (EGPA; Churg-Strauss syndrome) shows a close similarity with IgG4-RD, in terms of upper airway involvement and eosinophilia. It may be related to up-regulation of Th2 cytokines associated with increased IgG4 response [2]. Yamamoto et al. [50] reported elevated serum IgG4 levels and increased IgG4-positive/IgG-positive plasma cell ratio and also IgG4 renal infiltrate in EGPA patients. Vaglio et al. [51] showed that IgG4 levels correlated with the disease activity in EGPA patients. Chang et al. [52] showed increased IgG4-positive cells in 18.6% of 43 cases of granulomatosis with polyangiitis (Wegener’s granulomatosis) including four kidney samples, but the cases were limited to sinonasal or orbital/periorbital biopsies. A case of ANCA-negative EGPA showed salivary gland swelling, high serum IgG4 levels, membranous nephropathy with eosinophil-rich TIN, and leukocytoclastic vasculitis [53].
TUBULOINTERSTITIAL NEPHRITIS IN ANTI-NEUTROPHIL CYTOPLASMIC ANTIBODY-ASSOCIATED VASCULITIS
- Tubulointerstitial nephritis and uveitis with dominant IgG4-positive plasma cells was suggested as a form of IgG4-TIN [55], but it was not supported by others [56]. Sakairi et al. [57] reported a case of ANCA-negative renal small-vessel vasculitis with IgG4-TIN. Recently, a case of multiple organ involvement remarkably similar to that of IgG4-RD was reported. The patient showed multiple hypodense renal lesions in radiographic examination and lymphoplasmacytic infiltrates with storiform fibrosis, but did not have accompanying elevated serum IgG4 and IgG4-positive plasma cell infiltration [58]. IgG4-TIN was reported in a patient with chronic lymphocytic leukemia [59], and in a renal allograft patient [60].
TUBULOINTERSTITIAL NEPHRITIS IN OTHER CONDITIONS SIMULATING IMMUNOGLOBULIN G4-TUBULOINTERSTITIAL NEPHRITIS
- Cases showing clinical, laboratory, and histological overlap of IgG4-TIN and TIN of autoimmune diseases have been introduced in previous sections [24,45,47,53], and summarized in Table 2. These overlapping features may partly have roots in autoimmune mechanisms. IgG4-RD demonstrates immunologic derangement in cytokine profiles and activation of regulatory T cells [2]. Practically, the overlap causes dilemma and difficulties in diagnosis. Even though each disease accompanies unique histological features, confirmatory diagnosis is made by the diagnostic criteria of each disease. The ACR and SLICC criteria for SLE have been well established and used, but they have been modified by consensus among the experts, statistical results, and pathogenetic mechanisms [61]. Sjögren syndrome also has complex diagnostic criteria incorporating clinical features and histological findings. As mentioned previously, the diagnostic criteria of IgG4-RD are also composed of a combination of histological, clinical, imaging, and laboratory features. Furthermore, these complex diagnostic criteria may evolve as our understanding of the pathogenetic mechanisms and clinical course of the disease extends, as in SLE. In fact, some cases that had originally been diagnosed as rheumatoid arthritis, Sjögren syndrome or antiphospholipid syndrome were re-categorized into IgG4-RD [62]. Even if we apply the above diagnostic criteria, symptoms are protean and laboratory abnormalities and organ involvement may vary in each individual patient. Clinical and laboratory findings supportive of autoimmune diseases or IgG4-RD may not be apparent at the time of biopsy [45]. Furthermore, in some cases it is not possible to distinguish IgG4-TIN from TIN of autoimmune diseases due to overlapping clinical and laboratory features. Cases in this gray zone should be collected and reserved in a separate category until we have more clear understanding on the pathogenetic mechanisms of IgG4-RD and so we can unveil the possible link between them.
CLINICAL AND HISTOLOGICAL OVERLAP BETWEEN IMMUNOGLOBULIN G4-TUBULOINTERSTITIAL NEPHRITIS AND TUBULOINTERSTITIAL NEPHRITIS OF AUTOIMMUNE DISEASES
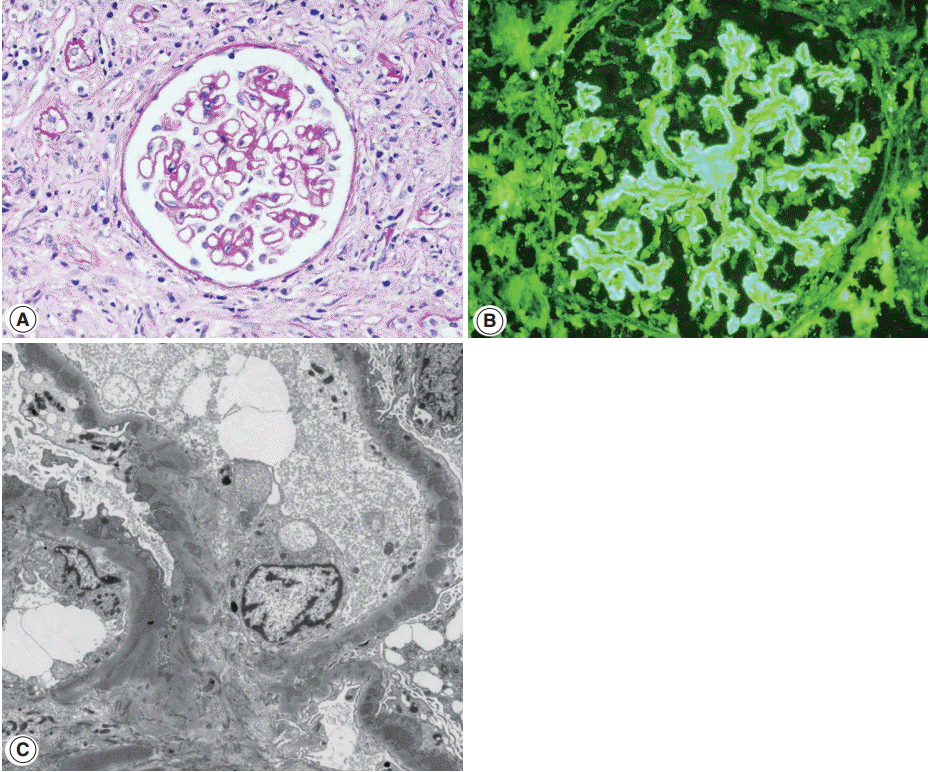
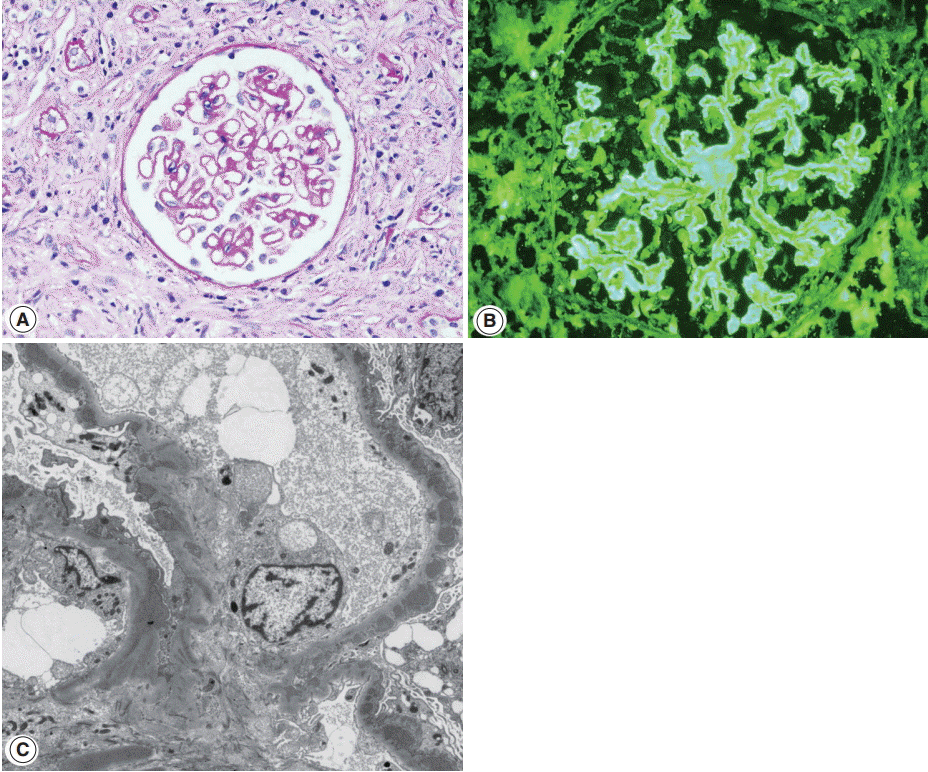
- Glomerulonephritis may develop in the setting of IgG4-RD. Membranous nephropathy is the most common, and about 30 cases have been reported in the English literature so far [26,63-69]. Membranous nephropathy presented concurrently with TIN in most case reports, but in one collective series, four of nine cases presented with only glomerulonephritis [15]. Nephrotic syndrome is a frequent manifestation at the time of biopsy [15]. Glomerular histology is similar to that of idiopathic membranous nephropathy, except for a negative staining against phospholipase A2 receptor (PLA2R) antibodies. By light microscopy, glomerular cellularity is normal and the basement membrane is mildly thickened with occasional spikes (Fig. 4A). Immunofluorescence shows granular staining of IgG along the peripheral capillary walls (Fig. 4B). IgG4 is usually dominant among the IgG subclasses and C3 deposits are frequent. On electron microscopy, electron dense-deposits are present mainly in the subepithelial and occasionally in the intramembranous areas, ranging from stage I to III.
- Membranous nephropathy of IgG4-RD is sometimes difficult to differentiate from lupus membranous nephritis. Although glomerular IgA, IgM, or C1q deposits are rare in IgG4-RD, strong C1q deposits have been reported in a few cases of IgG4-RD. Mesangial or subendothelial deposits were present in three and four of nine cases of membranous nephropathy associated with IgG4-RD, respectively [15]. Tubuloreticular inclusions, which are regarded as a characteristic feature of lupus nephritis [70], were observed in one case of IgG4-RD [15]. Some authors proposed differential IgG subclass staining, which demonstrated strong IgG2 staining in lupus but not in IgG4-RD. However, it has not been verified in other studies [15]. IgG3 staining intensity similar to or exceeding that of IgG4 [71] or IgG2 [72] was reported in membranous lupus nephritis, but its comparison with that in IgG4-RD has not been done. Similar to TIN, distinction between membranous nephropathy associated with IgG4-RD and lupus membranous nephritis could not be made with certainty in a few cases. In a recent report of a case of membranous nephropathy, clinical features of both SLE and IgG4-RD were present [69]. Features suggestive of SLE were vitiligo, elevated ESR and CRP, hypocomplementemia, positive ANA and weakly positive anti-dsDNA antibody and atypical pANCA, while increased serum IgG4 levels, sialadenitis, lymphadenopathy, large kidneys, and marked hepatomegaly favored IgG4-RD. We experienced a case of membranous nephropathy in a patient satisfying ARA and SLICC criteria for SLE, but the patient also had retroperitoneal fibrosis.
- Except for membranous nephropathy, Henoch-Schönlein purpura nephritis [73-75], membranoproliferative [76], and endocapillary proliferative glomerulonephritis [77] have been reported anecdotally. Cases with Henoch-Schönlein purpura nephritis had mesangial IgA deposition in addition to TIN. It is unclear whether these glomerulonephritides have a direct relation with IgG4-TIN or develop co-incidentally. In a case of endocapillary proliferative glomerulonephritis with crescent formation, the patient had hydroureteronephrosis due to retroperitoneal fibrosis and elevated circulating immune complexes [77], which might raise a suspicion of urinary tract infection causing glomerulonephritis.
GLOMERULONEPHRITIS ASSOCIATED WITH IMMUNOGLOBULIN G4-TUBULOINTERSTITIAL NEPHRITIS
| References | No. of cases | Age (median, yr) | Male:Femlae | Extrarenal lesion | Serum IgG (median, mg/dL) | Serum lgG4 (median, mg/dL) | SCr at biopsy (mean) | Elevated Cr (>1.2 mg/dL) | ANA (+) | RF (+) | Proteinuria (> 1 g/day) | Hematuria | Renal biopsy finding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saeki et al. [7] | 23 | 40-83 (64) | 20:3 | Sa (19), LN (10), Pa (9), La (7), Lu (6), Li (1), Pr (1) | 2,721-8,841 (4,387) | 305-4,630 (1,330) | 0.67-6.87 (1.98) | 13/23 | 16/23 | 7/18 | 2/23 | 8/23 | TIN (23/23), MGN (1/23), mild MPGN (3/23), focal segmental EC (1/23) |
| Raissian et al. [8] | 35 | 20-84 (67) | 30:5 | Sa (6), LN (8), Pa (15), La (1), Lu (8), Li (7), RP(3) | - | - | 0.9-9.0 (3.57) | 27/35 | 10/32 | - | 8/27 | 6/27 | TIN (35/35), MGN (2/35), many eosinophils (4/35) |
| Kawano et al. [28] | 20 | 55-83 (70) | 18:2 | Jo (1), La (2), Li (1), LN (5), Lu (6), Ne (1), Pa (7), Pr (2), RP (1), Sa (12) | 1,679-5,380 (3,596) | 408-1,860 (828) | 0.59-7.26 (1.36) | 12/20 | - | - | 2/15 | - | TIN (20/20), MPGN (1), IgAN (1), EC (2), HSPN (2), MGN (3) |
| Yamaguchi et al. [13] | 16 | 45-78 (62) | 12:4 | Pa (8), Sa (7), RP (1), Lu (1), Li (1) | 1,569-6,328 (3,604) | 142-2,120 (958) | 0.84-6.17 (1.6) | 12/16 | - | - | 3/11 | - | TIN (16/16), MGN (2) |
SCr, serum creatinine; Cr, creatinine; ANA, antinuclear antibodies; RF, rheumatoid factor; Sa, salivary gland; LN, lymph node; Pa, pancreas; La, lacrimal gland; Lu, lung; Li, liver; Pr, prostate; TIN, tubulointerstitial nephritis; MGN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis; EC, endocapillary hypercellularity; RP, retroperitoneum; Jo, joint; Ne, nerve; IgAN, IgA nephropathy; HSPN, Henoch-Schönlein purpura nephritis.
| Age | Sex | Serum IgG (mg/dL) | Serum IgG4 (mg/dL) | Antibody | SCr (mg/dL) | Proteinuria | Systemic complications | IgG4/HPF | IgG4/IgG (%) | IF | EM | Diagnosis of kidney biopsy | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| lgG4-RD and Sjogren's syndrome | 49 | F | 6,000 | 2,790 | Anti-SS-A (1:16) | - | - | Chronic hepatitis (3), portal hypertension (2), retroperitoneal ficrosis (1), renal involve (1) | 21.7 | 42.1 | - | - | - | Yamamoto et al. [47] |
| 43 | F | 1,898 | 188 | Anti-SS-A (1:16) | - | - | - | - | - | - | - | |||
| 48 | F | 3,009 | 768 | Anti-SS-A (1:4) | . | . | 11.3 | 23.5 | . | . | . | |||
| 56 | F | 1,890 | 694 | Anti-SS-A (1:16) | - | - | 9.7 | 19.4 | - | - | - | |||
| 59 | M | 1,880 | 339 | Anti-SS-A (1:64) | - | - | - | - | - | - | - | |||
| 73 | M | 1,912 | 374 | Anti-SS-A (1:16) | - | - | 6.7 | 21.3 | - | - | - | |||
| 61 | M | 2,558 | 774 | Anti-SS-A (1:16) | - | - | 14.7 | 33.4 | - | - | - | |||
| lgG4-RD and Sjogren's syndrome | 62 | F | 8,478 | 647 | ANA (1:10,240, homogeneous), anti-SS-A (+), anti-SS-B (+) | 0.92 | 0.82 g/gCr | General malaise, dry mouth, Raynaud’s phenomenon, anemia, lower extremity weakness, hypergammaglobulinemia | 15 | - | No immunoglobulin or complement deposition | - | Chronic plasma cell-rich TIN | Kawano et al. [45] |
| lgG4-RD and Churg-Strauss Syndrome | 68 | F | 1,997 | 275 | ANCA (–), anti–SS-A (–), RF (+) | 0.9 | 1.2 g/day | Asthma, multifocal pulmonary infiltrates, marked eosinophilia, a rash on feet, right median nerve paralysis, salivary gland swelling | - | 10 | IgG, C3 (granular, capillary), lgG1, lgG4 (+) | Electron-dense to electron-lucent subepithelial deposits in glomerular capillary walls | MGN (stage lll-IV) with eosinophil-rich TIN | Ayuzawa et al. [53] |
| IgG4-RD and Lupus nephritis | 71 | F | lgG1:1,230, lgG2:735, lgG3:418 | 37.1 | ANA (1.320 homogeneous) | 9.65 | 2.6 g/day | Abdominal pain, vomiting, diarrhea, epigastric tenderness, bilateral lower extremity pitting edema, marked leukocytosis, hypoalbuminemia, no skin changes | 13 | - | IgG, K, L (2+, granular, mesangial), IgM, IgA, C3 (1+, granular, mesangial) | Small paramesangial and scattered small electron dense to electron lucent subepithelial and intramembranous deposits | IgG4-related TIN with MGN, and/or lupus membranous nephritis with TIN | Zaarour et al. [54] |
| ANCA (–), anti–SS-A (–), anti–SS-B (–), anti-dsDNS (–), anti-Sm (–), anti-GBM (–) |
IgG4-TIN, tubulointerstitial nephritis with dominant IgG4-positive cell infiltrate; SCr, serum creatinine; HPF, high-power field; IF, immunofluorescence; EM, electron microscopy; IgG4-RD, IgG4-related disease; ANA, antinuclear antibodies; TIN, tubulointerstitial nephritis; ANCA, anti-neutrophil cytoplasmic antibody; RF, rheumatoid factor; MGN, membranous nephropathy; GBM, glomerular basement membrane; K. kappa light chain; L, lambda light chain.
- 1. Saeki T, Kawano M. IgG4-related kidney disease. Kidney Int 2014; 85: 251-7. ArticlePubMed
- 2. Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 2012; 366: 539-51. ArticlePubMed
- 3. Divatia M, Kim SA, Ro JY. IgG4-related sclerosing disease, an emerging entity: a review of a multi-system disease. Yonsei Med J 2012; 53: 15-34. ArticlePubMedPMC
- 4. Kambham N, Markowitz GS, Tanji N, Mansukhani MM, Orazi A, D’Agati VD. Idiopathic hypocomplementemic interstitial nephritis with extensive tubulointerstitial deposits. Am J Kidney Dis 2001; 37: 388-99. ArticlePubMed
- 5. Uchiyama-Tanaka Y, Mori Y, Kimura T, et al. Acute tubulointerstitial nephritis associated with autoimmune-related pancreatitis. Am J Kidney Dis 2004; 43: e18-25. ArticlePubMed
- 6. Takeda S, Haratake J, Kasai T, Takaeda C, Takazakura E. IgG4-associated idiopathic tubulointerstitial nephritis complicating autoimmune pancreatitis. Nephrol Dial Transplant 2004; 19: 474-6. ArticlePubMed
- 7. Saeki T, Nishi S, Imai N, et al. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int 2010; 78: 1016-23. ArticlePubMed
- 8. Raissian Y, Nasr SH, Larsen CP, et al. Diagnosis of IgG4-related tubulointerstitial nephritis. J Am Soc Nephrol 2011; 22: 1343-52. ArticlePubMedPMC
- 9. Houghton DC, Troxell ML. An abundance of IgG4+ plasma cells is not specific for IgG4-related tubulointerstitial nephritis. Mod Pathol 2011; 24: 1480-7. ArticlePubMedPDF
- 10. Kawano M, Saeki T, Nakashima H, et al. Proposal for diagnostic criteria for IgG4-related kidney disease. Clin Exp Nephrol 2011; 15: 615-26. ArticlePubMedPDF
- 11. Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 2012; 25: 1181-92. PubMed
- 12. Deng C, Li W, Chen S, et al. Histopathological diagnostic value of the IgG4+/IgG+ ratio of plasmacytic infiltration for IgG4-related diseases: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2015; 94: e579.PubMedPMC
- 13. Yamaguchi Y, Kanetsuna Y, Honda K, et al. Characteristic tubulointerstitial nephritis in IgG4-related disease. Hum Pathol 2012; 43: 536-49. ArticlePubMed
- 14. Yoshita K, Kawano M, Mizushima I, et al. Light-microscopic characteristics of IgG4-related tubulointerstitial nephritis: distinction from non-IgG4-related tubulointerstitial nephritis. Nephrol Dial Transplant 2012; 27: 2755-61. ArticlePubMed
- 15. Alexander MP, Larsen CP, Gibson IW, et al. Membranous glomerulonephritis is a manifestation of IgG4-related disease. Kidney Int 2013; 83: 455-62. ArticlePubMed
- 16. Sharma SG, Vlase HL, D’Agati VD. IgG4-related tubulointerstitial nephritis with plasma cell-rich renal arteritis. Am J Kidney Dis 2013; 61: 638-43. ArticlePubMed
- 17. Nagamachi S, Ohsawa I, Sato N, et al. Immune complex-mediated complement activation in a patient with IgG4-related tubulointerstitial nephritis. Case Rep Nephrol Urol 2011; 1: 7-14. ArticlePubMedPMC
- 18. Cornell LD, Chicano SL, Deshpande V, et al. Pseudotumors due to IgG4 immune-complex tubulointerstitial nephritis associated with autoimmune pancreatocentric disease. Am J Surg Pathol 2007; 31: 1586-97. ArticlePubMed
- 19. Nishi S, Imai N, Yoshida K, Ito Y, Saeki T. Clinicopathological findings of immunoglobulin G4-related kidney disease. Clin Exp Nephrol 2011; 15: 810-9. ArticlePubMedPDF
- 20. Koizumi S, Kamisawa T, Kuruma S, et al. Organ correlation in IgG4-related diseases. J Korean Med Sci 2015; 30: 743-8. ArticlePubMedPMCPDF
- 21. Zen Y, Nakanuma Y. IgG4-related disease: a cross-sectional study of 114 cases. Am J Surg Pathol 2010; 34: 1812-9. PubMed
- 22. Inoue D, Yoshida K, Yoneda N, et al. IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore) 2015; 94: e680.PubMedPMC
- 23. Vlachou PA, Khalili K, Jang HJ, Fischer S, Hirschfield GM, Kim TK. IgG4-related sclerosing disease: autoimmune pancreatitis and extrapancreatic manifestations. Radiographics 2011; 31: 1379-402. ArticlePubMed
- 24. Mavragani CP, Fragoulis GE, Rontogianni D, Kanariou M, Moutsopoulos HM. Elevated IgG4 serum levels among primary Sjogren’s syndrome patients: do they unmask underlying IgG4-related disease? Arthritis Care Res (Hoboken) 2014; 66: 773-7. PubMed
- 25. Goh TL, Cicovic A, Sapsford T, Semple D. A case of immunoglobulin G4 (IgG4) tubulointerstitial nephritis with delayed elevation of serum IgG4 levels. Intern Med J 2015; 45: 788-90. PubMed
- 26. Watson SJ, Jenkins DA, Bellamy CO. Nephropathy in IgG4-related systemic disease. Am J Surg Pathol 2006; 30: 1472-7. ArticlePubMed
- 27. Yoneda K, Murata K, Katayama K, et al. Tubulointerstitial nephritis associated with IgG4-related autoimmune disease. Am J Kidney Dis 2007; 50: 455-62. ArticlePubMed
- 28. Kawano M, Mizushima I, Yamaguchi Y, et al. Immunohistochemical characteristics of IgG4-related tubulointerstitial nephritis: detailed analysis of 20 Japanese cases. Int J Rheumatol 2012; 2012: 609795.ArticlePubMedPMCPDF
- 29. Arai H, Hayashi H, Takahashi K, et al. Tubulointerstitial fibrosis in patients with IgG4-related kidney disease: pathological findings on repeat renal biopsy. Rheumatol Int 2015; 35: 1093-101. ArticlePubMedPDF
- 30. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol 2015; 67: 2466-75. ArticlePubMedPMC
- 31. Cunningham E, Provost T, Brentjens J, Reichlin M, Venuto RC. Acute renal failure secondary to interstitial lupus nephritis. Arch Intern Med 1978; 138: 1560-1. ArticlePubMed
- 32. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercise. Case 53-1976. N Engl J Med 1976; 295: 1526-32. ArticlePubMed
- 33. Gur H, Kopolovic Y, Gross DJ. Chronic predominant interstitial nephritis in a patient with systemic lupus erythematosus: a follow up of three years and review of the literature. Ann Rheum Dis 1987; 46: 617-23. ArticlePubMedPMC
- 34. Tron F, Ganeval D, Droz D. Immunologically-mediated acute renal failure of nonglomerular origin in the course of systemic lupus erythematosus [SLE]: report of two cases. Am J Med 1979; 67: 529-32. ArticlePubMed
- 35. Mori Y, Kishimoto N, Yamahara H, et al. Predominant tubulointerstitial nephritis in a patient with systemic lupus nephritis. Clin Exp Nephrol 2005; 9: 79-84. ArticlePubMedPDF
- 36. Omokawa A, Wakui H, Okuyama S, et al. Predominant tubulointerstitial nephritis in a patient with systemic lupus erythematosus: phenotype of infiltrating cells. Clin Nephrol 2008; 69: 436-44. ArticlePubMed
- 37. Singh AK, Ucci A, Madias NE. Predominant tubulointerstitial lupus nephritis. Am J Kidney Dis 1996; 27: 273-8. ArticlePubMed
- 38. Alpers CE, Hopper J Jr, Bernstein MJ, Biava CG. Late development of systemic lupus erythematosus in patients with glomerular “fingerprint” deposits. Ann Intern Med 1984; 100: 66-8. ArticlePubMed
- 39. Okada H, Takahira S, Sugahara S, Nakamoto H, Suzuki H. Retroperitoneal fibrosis and systemic lupus erythematosus. Nephrol Dial Transplant 1999; 14: 1300-2. PubMed
- 40. Kiyama K, Yoshifuji H, Kandou T, et al. Screening for IgG4-type anti-nuclear antibodies in IgG4-related disease. BMC Musculoskelet Disord 2015; 16: 129.ArticlePubMedPMCPDF
- 41. Ramos-Casals M, Brito-Zerón P, Seror R, et al. Characterization of systemic disease in primary Sjogren’s syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology (Oxford) 2015; 54: 2230-8. PubMedPMC
- 42. Kidder D, Rutherford E, Kipgen D, Fleming S, Geddes C, Stewart GA. Kidney biopsy findings in primary Sjögren syndrome. Nephrol Dial Transplant 2015; 30: 1363-9. ArticlePubMed
- 43. Pijpe J, Vissink A, Van der Wal JE, Kallenberg CG. Interstitial nephritis with infiltration of IgG-kappa positive plasma cells in a patient with Sjögren’s syndrome. Rheumatology (Oxford) 2004; 43: 108-10. PubMed
- 44. Umehara H, Okazaki K, Masaki Y, et al. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol 2012; 22: 1-14. ArticlePubMedPMC
- 45. Kawano M, Suzuki Y, Yamada K, et al. Primary Sjögren’s syndrome with chronic tubulointerstitial nephritis and lymphadenopathy mimicking IgG4-related disease. Mod Rheumatol 2015; 25: 637-41. ArticlePubMed
- 46. Yukawa N, Tsuboi N, Yukawa S, et al. Marked hypocomplementemia and tubulointerstitial nephritis in a male patient with Sjögren’s syndrome. Mod Rheumatol 2004; 14: 164-8. ArticlePubMed
- 47. Yamamoto M, Takahashi H, Shinomura Y. Are Sjögren’s syndrome and IgG4-related disease able to coexist? Mod Rheumatol 2015; 25: 970-1. ArticlePubMed
- 48. Kronbichler A, Gut N, Zwerina J, Neuwirt H, Rudnicki M, Mayer G. Extending the spectrum of a chameleon: IgG4-related disease appearing as interstitial nephritis and mimicking anti-neutrophil cytoplasmic antibody-associated vasculitis. Rheumatology (Oxford) 2015; 54: 1936-8. PubMed
- 49. Perez Alamino R, Martínez C, Espinoza LR. IgG4-associated vasculitis. Curr Rheumatol Rep 2013; 15: 348.PubMed
- 50. Yamamoto M, Takahashi H, Suzuki C, et al. Analysis of serum IgG subclasses in Churg-Strauss syndrome: the meaning of elevated serum levels of IgG4. Intern Med 2010; 49: 1365-70. ArticlePubMed
- 51. Vaglio A, Strehl JD, Manger B, et al. IgG4 immune response in Churg-Strauss syndrome. Ann Rheum Dis 2012; 71: 390-3. ArticlePubMed
- 52. Chang SY, Keogh KA, Lewis JE, et al. IgG4-positive plasma cells in granulomatosis with polyangiitis (Wegener’s): a clinicopathologic and immunohistochemical study on 43 granulomatosis with polyangiitis and 20 control cases. Hum Pathol 2013; 44: 2432-7. ArticlePubMed
- 53. Ayuzawa N, Ubara Y, Keiichi S, et al. Churg-Strauss syndrome with a clinical condition similar to IgG4-related kidney disease: a case report. Intern Med 2012; 51: 1233-8. ArticlePubMed
- 54. Zaarour M, Weerasinghe C, Eter A, El-Sayegh S, El-Charabaty E. An overlapping case of lupus nephritis and IgG4-related kidney disease. J Clin Med Res 2015; 7: 575-81. ArticlePubMedPMC
- 55. Sugimoto T, Tanaka Y, Morita Y, Kume S, Uzu T, Kashiwagi A. Is tubulointerstitial nephritis and uveitis syndrome associated with IgG4-related systemic disease? Nephrology (Carlton) 2008; 13: 89.ArticlePubMed
- 56. Houghton D, Troxell M, Fox E, Rosenbaum J. TINU (tubulointerstitial nephritis and uveitis) syndrome is not usually associated with IgG4 sclerosing disease. Am J Kidney Dis 2012; 59: 583-4. Article
- 57. Sakairi T, Okabe S, Hiromura K, et al. A case of ANCA-negative renal small-vessel vasculitis with tubulointerstitial infiltration of IgG4-positive plasma cells. Mod Rheumatol 2014 May 20 [Epub]. http://dx.doi.org/10.3109/14397595.2014.915510. Article
- 58. Hara S, Kawano M, Mizushima I, et al. A condition closely mimicking IgG4-related disease despite the absence of serum IgG4 elevation and IgG4-positive plasma cell infiltration. Mod Rheumatol 2014 Jun 2 [Epub]. http://dx.doi.org/10.3109/14397595.2014.916836. Article
- 59. Malone AF, Sparks MA, Howell DN, Middleton JP, Smith SR, Lehrich RW. IgG4-related tubulointerstitial nephritis associated with chronic lymphocytic leukemia. J Nephrol 2013; 26: 1195-8. ArticlePubMed
- 60. Nishikawa K, Takeda A, Masui S, et al. A case of IgG4-positive plasma cell-rich tubulointerstitial nephritis in a kidney allograft mimicking IgG4-related kidney disease. Nephrology (Carlton) 2014; 19 Suppl 3: 52-6. PubMed
- 61. Rekvig OP, Van der Vlag J. The pathogenesis and diagnosis of systemic lupus erythematosus: still not resolved. Semin Immunopathol 2014; 36: 301-11. ArticlePubMedPDF
- 62. Soliotis F, Mavragani CP, Plastiras SC, Rontogianni D, Skopouli FN, Moutsopoulos HM. IgG4-related disease: a rheumatologist’s perspective. Clin Exp Rheumatol 2014; 32: 724-7. PubMed
- 63. Cravedi P, Abbate M, Gagliardini E, et al. Membranous nephropathy associated with IgG4-related disease. Am J Kidney Dis 2011; 58: 272-5. ArticlePubMed
- 64. Fervenza FC, Downer G, Beck LH Jr, Sethi S. IgG4-related tubulointerstitial nephritis with membranous nephropathy. Am J Kidney Dis 2011; 58: 320-4. ArticlePubMed
- 65. Jindal N, Yadav D, Passero C, et al. Membranous nephropathy: a rare renal manifestation of IgG4-related systemic disease. Clin Nephrol 2012; 77: 321-8. ArticlePubMed
- 66. Li XL, Yan TK, Li HF, et al. IgG4-related membranous nephropathy with high blood and low urine IgG4/IgG ratio: a case report and review of the literature. Clin Rheumatol 2014; 33: 145-8. ArticlePubMedPDF
- 67. Palmisano A, Corradi D, Carnevali ML, et al. Chronic periaortitis associated with membranous nephropathy: clues to common pathogenetic mechanisms. Clin Nephrol 2010; 74: 485-90. ArticlePubMed
- 68. Saeki T, Imai N, Ito T, Yamazaki H, Nishi S. Membranous nephropathy associated with IgG4-related systemic disease and without autoimmune pancreatitis. Clin Nephrol 2009; 71: 173-8. ArticlePubMed
- 69. Stylianou K, Maragkaki E, Tzanakakis M, Stratakis S, Gakiopoulou H, Daphnis E. Acute interstitial nephritis and membranous nephropathy in the context of IgG4-related disease. Case Rep Nephrol Dial 2015; 5: 44-8. ArticlePubMedPMCPDF
- 70. Jennette JC, Iskandar SS, Dalldorf FG. Pathologic differentiation between lupus and nonlupus membranous glomerulopathy. Kidney Int 1983; 24: 377-85. ArticlePubMed
- 71. Haas M. IgG subclass deposits in glomeruli of lupus and nonlupus membranous nephropathies. Am J Kidney Dis 1994; 23: 358-64. ArticlePubMed
- 72. Omokawa A, Komatsuda A, Nara M, et al. Distribution of glomerular IgG subclass deposits in patients with membranous nephropathy and anti-U1 ribonucleoprotein antibody. Nephrol Dial Transplant 2012; 27: 1937-41. ArticlePubMed
- 73. Tamai R, Hasegawa Y, Hisano S, Miyake K, Nakashima H, Saito T. A case of IgG4-related tubulointerstitial nephritis concurrent with Henoch-Schonlein purpura nephritis. Allergy Asthma Clin Immunol 2011; 7: 5.PubMedPMC
- 74. Ito K, Yamada K, Mizushima I, et al. Henoch-Schönlein purpura nephritis in a patient with IgG4-related disease: a possible association. Clin Nephrol 2013; 79: 246-52. ArticlePubMed
- 75. Yang H, Choi SK, Kim B, et al. IgG4-related tubulointerstitial nephritis accompanied by Henoch-Schonlein purpura. Korean J Med 2014; 87: 96-100. Article
- 76. Morimoto J, Hasegawa Y, Fukushima H, et al. Membranoproliferative glomerulonephritis-like glomerular disease and concurrent tubulointerstitial nephritis complicating IgG4-related autoimmune pancreatitis. Intern Med 2009; 48: 157-62. ArticlePubMed
- 77. Katano K, Hayatsu Y, Matsuda T, et al. Endocapillary proliferative glomerulonephritis with crescent formation and concurrent tubulointerstitial nephritis complicating retroperitoneal fibrosis with a high serum level of IgG4. Clin Nephrol 2007; 68: 308-14. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Glycosylation in kidney diseases
Yingying Ling, Fei Cai, Tao Su, Yi Zhong, Ling Li, Bo Meng, Guisen Li, Meng Gong, Hao Yang, Xinfang Xie, Zhenyu Sun, Yang Zhao, Fang Liu, Yong Zhang
Precision Clinical Medicine.2025;[Epub] CrossRef - IgG4-related kidney disease: Clinicopathologic features, differential diagnosis, and mimics
Sarwat I. Gilani, Alessia Buglioni, Lynn D. Cornell
Seminars in Diagnostic Pathology.2024; 41(2): 88. CrossRef - Utilizing Immunoglobulin G4 Immunohistochemistry for Risk Stratification in Patients with Papillary Thyroid Carcinoma Associated with Hashimoto Thyroiditis
Faridul Haq, Gyeongsin Park, Sora Jeon, Mitsuyoshi Hirokawa, Chan Kwon Jung
Endocrinology and Metabolism.2024; 39(3): 468. CrossRef - IgG4-assoziierte Nierenerkrankungen
Christina Thompson, Frank O. Henes, Oliver M. Steinmetz, Simon Melderis
Die Nephrologie.2023; 18(4): 249. CrossRef - Concurrent anti-neutrophil cytoplasmic antibody-associated glomerulonephritis and IgG4-associated tubulointerstitial nephritis with C3 glomerulonephritis
Jianan Feng, Jinyu Yu, Xueyao Wang, Yue Wang, Yang Liu, Zhonggao Xu, Weixia Sun
Medicine.2020; 99(5): e18857. CrossRef - A case of eosinophilic granulomatosis with polyangiitis as a mimicker of IgG4-related disease
Ryuichiro Kanda, Satoshi Kubo, Kazuhisa Nakano, Akio Kawabe, Aya Nawata, Kentaro Hanami, Shingo Nakayamada, Yoshiya Tanaka
Modern Rheumatology Case Reports.2020; 4(2): 278. CrossRef - Renal tubular acidosis as the initial presentation of Sjögren’s syndrome
Karen Ho, Pouneh Dokouhaki, Mark McIsaac, Bhanu Prasad
BMJ Case Reports.2019; 12(8): e230402. CrossRef - Hypocomplementemic interstitial nephritis with long-term follow-up
Alyssa Penning, Claire Kassakian, Donald C Houghton, Nicole K Andeen
Journal of Clinical Nephrology.2019; 3(1): 042. CrossRef - Immunoglobulin G4-related kidney diseases: An updated review
Maurizio Salvadori, Aris Tsalouchos
World Journal of Nephrology.2018; 7(1): 29. CrossRef - Systemic lupus erythematosus in a patient with an organic lesion of the central nervous system: practicaldifferential diagnosis
E. V. Lebedeva, M. V. Novoseltsev, A. N. Lvov, I. V. Khamaganova
Klinicheskaya dermatologiya i venerologiya.2018; 17(6): 21. CrossRef - Concurrent IgG4-related tubulointerstitial nephritis and IgG4 myeloperoxidase-anti-neutrophil cytoplasmic antibody positive crescentic glomerulonephritis
Tao Su, Li Yang, Zhao Cui, Su-xia Wang, Ming-hui Zhao
Medicine.2017; 96(20): e6707. CrossRef - IgG4-Related Kidney Disease: Report of a Case Presenting as a Renal Mass
Daniele Bianchi, Luca Topazio, Gabriele Gaziev, Valerio Iacovelli, Pierluigi Bove, Alessandro Mauriello, Enrico Finazzi Agrò
Case Reports in Surgery.2017; 2017: 1. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure




Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
| References | No. of cases | Age (median, yr) | Male:Femlae | Extrarenal lesion | Serum IgG (median, mg/dL) | Serum lgG4 (median, mg/dL) | SCr at biopsy (mean) | Elevated Cr (>1.2 mg/dL) | ANA (+) | RF (+) | Proteinuria (> 1 g/day) | Hematuria | Renal biopsy finding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saeki et al. [7] | 23 | 40-83 (64) | 20:3 | Sa (19), LN (10), Pa (9), La (7), Lu (6), Li (1), Pr (1) | 2,721-8,841 (4,387) | 305-4,630 (1,330) | 0.67-6.87 (1.98) | 13/23 | 16/23 | 7/18 | 2/23 | 8/23 | TIN (23/23), MGN (1/23), mild MPGN (3/23), focal segmental EC (1/23) |
| Raissian et al. [8] | 35 | 20-84 (67) | 30:5 | Sa (6), LN (8), Pa (15), La (1), Lu (8), Li (7), RP(3) | - | - | 0.9-9.0 (3.57) | 27/35 | 10/32 | - | 8/27 | 6/27 | TIN (35/35), MGN (2/35), many eosinophils (4/35) |
| Kawano et al. [28] | 20 | 55-83 (70) | 18:2 | Jo (1), La (2), Li (1), LN (5), Lu (6), Ne (1), Pa (7), Pr (2), RP (1), Sa (12) | 1,679-5,380 (3,596) | 408-1,860 (828) | 0.59-7.26 (1.36) | 12/20 | - | - | 2/15 | - | TIN (20/20), MPGN (1), IgAN (1), EC (2), HSPN (2), MGN (3) |
| Yamaguchi et al. [13] | 16 | 45-78 (62) | 12:4 | Pa (8), Sa (7), RP (1), Lu (1), Li (1) | 1,569-6,328 (3,604) | 142-2,120 (958) | 0.84-6.17 (1.6) | 12/16 | - | - | 3/11 | - | TIN (16/16), MGN (2) |
| Age | Sex | Serum IgG (mg/dL) | Serum IgG4 (mg/dL) | Antibody | SCr (mg/dL) | Proteinuria | Systemic complications | IgG4/HPF | IgG4/IgG (%) | IF | EM | Diagnosis of kidney biopsy | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| lgG4-RD and Sjogren's syndrome | 49 | F | 6,000 | 2,790 | Anti-SS-A (1:16) | - | - | Chronic hepatitis (3), portal hypertension (2), retroperitoneal ficrosis (1), renal involve (1) | 21.7 | 42.1 | - | - | - | Yamamoto et al. [47] |
| 43 | F | 1,898 | 188 | Anti-SS-A (1:16) | - | - | - | - | - | - | - | |||
| 48 | F | 3,009 | 768 | Anti-SS-A (1:4) | . | . | 11.3 | 23.5 | . | . | . | |||
| 56 | F | 1,890 | 694 | Anti-SS-A (1:16) | - | - | 9.7 | 19.4 | - | - | - | |||
| 59 | M | 1,880 | 339 | Anti-SS-A (1:64) | - | - | - | - | - | - | - | |||
| 73 | M | 1,912 | 374 | Anti-SS-A (1:16) | - | - | 6.7 | 21.3 | - | - | - | |||
| 61 | M | 2,558 | 774 | Anti-SS-A (1:16) | - | - | 14.7 | 33.4 | - | - | - | |||
| lgG4-RD and Sjogren's syndrome | 62 | F | 8,478 | 647 | ANA (1:10,240, homogeneous), anti-SS-A (+), anti-SS-B (+) | 0.92 | 0.82 g/gCr | General malaise, dry mouth, Raynaud’s phenomenon, anemia, lower extremity weakness, hypergammaglobulinemia | 15 | - | No immunoglobulin or complement deposition | - | Chronic plasma cell-rich TIN | Kawano et al. [45] |
| lgG4-RD and Churg-Strauss Syndrome | 68 | F | 1,997 | 275 | ANCA (–), anti–SS-A (–), RF (+) | 0.9 | 1.2 g/day | Asthma, multifocal pulmonary infiltrates, marked eosinophilia, a rash on feet, right median nerve paralysis, salivary gland swelling | - | 10 | IgG, C3 (granular, capillary), lgG1, lgG4 (+) | Electron-dense to electron-lucent subepithelial deposits in glomerular capillary walls | MGN (stage lll-IV) with eosinophil-rich TIN | Ayuzawa et al. [53] |
| IgG4-RD and Lupus nephritis | 71 | F | lgG1:1,230, lgG2:735, lgG3:418 | 37.1 | ANA (1.320 homogeneous) | 9.65 | 2.6 g/day | Abdominal pain, vomiting, diarrhea, epigastric tenderness, bilateral lower extremity pitting edema, marked leukocytosis, hypoalbuminemia, no skin changes | 13 | - | IgG, K, L (2+, granular, mesangial), IgM, IgA, C3 (1+, granular, mesangial) | Small paramesangial and scattered small electron dense to electron lucent subepithelial and intramembranous deposits | IgG4-related TIN with MGN, and/or lupus membranous nephritis with TIN | Zaarour et al. [54] |
| ANCA (–), anti–SS-A (–), anti–SS-B (–), anti-dsDNS (–), anti-Sm (–), anti-GBM (–) |
SCr, serum creatinine; Cr, creatinine; ANA, antinuclear antibodies; RF, rheumatoid factor; Sa, salivary gland; LN, lymph node; Pa, pancreas; La, lacrimal gland; Lu, lung; Li, liver; Pr, prostate; TIN, tubulointerstitial nephritis; MGN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis; EC, endocapillary hypercellularity; RP, retroperitoneum; Jo, joint; Ne, nerve; IgAN, IgA nephropathy; HSPN, Henoch-Schönlein purpura nephritis.
IgG4-TIN, tubulointerstitial nephritis with dominant IgG4-positive cell infiltrate; SCr, serum creatinine; HPF, high-power field; IF, immunofluorescence; EM, electron microscopy; IgG4-RD, IgG4-related disease; ANA, antinuclear antibodies; TIN, tubulointerstitial nephritis; ANCA, anti-neutrophil cytoplasmic antibody; RF, rheumatoid factor; MGN, membranous nephropathy; GBM, glomerular basement membrane; K. kappa light chain; L, lambda light chain.

 E-submission
E-submission