Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 50(5); 2016 > Article
-
Original Article
Clinicopathologic Correlations of E-cadherin and Prrx-1 Expression Loss in Hepatocellular Carcinoma - Kijong Yi, Hyunsung Kim, Yumin Chung, Hyein Ahn, Jongmin Sim, Young Chan Wi, Ju Yeon Pyo, Young-Soo Song, Seung Sam Paik, Young-Ha Oh
-
Journal of Pathology and Translational Medicine 2016;50(5):327-336.
DOI: https://doi.org/10.4132/jptm.2016.06.22
Published online: August 31, 2016
Department of Pathology, Hanyang University College of Medicine, Seoul, Korea
- Corresponding Author Young-Ha Oh, MD Department of Pathology, Hanyang University College of Medicine, 222-1 Wangsimni-ro, Seongdong-gu, Seoul 04763, Korea Tel: +82-31-560-2498 Fax: +82-31-560-2339 E-mail: yhoh@hanyang.ac.kr
© 2016 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Developing predictive markers for hepatocellular carcinoma (HCC) is important, because many patients experience recurrence and metastasis. Epithelial to mesenchymal transition (EMT) is a developmental process that plays an important role during embryogenesis and also during cancer metastasis. Paired-related homeobox protein 1 (Prrx-1) is an EMT inducer that has recently been introduced, and its prognostic significance in HCC is largely unknown.
-
Methods
- Tissue microarray was constructed using surgically resected primary HCCs from 244 cases. Immunohistochemical staining of E-cadherin and Prrx-1 was performed. The correlation between E-cadherin loss and Prrx-1 expression, as well as other clinicopathologic factors, was evaluated.
-
Results
- E-cadherin expression was decreased in 96 cases (39.4%). Loss of E-cadherin correlated with a higher recurrence rate (p < .001) but was not correlated with patient’s survival. Thirty-two cases (13.3%) showed at least focal nuclear Prrx-1 immunoreactivity while all non-neoplastic livers (n = 22) were negative. Prrx-1 expression was not associated with E-cadherin loss, survival or recurrence rates, pathologic factors, or the Ki-67 labeling index. Twenty tumors that were positive for E-cadherin and Prrx-1 had significantly higher nuclear grades than the rest of the cohort (p = .037). In Cox proportional hazard models, E-cadherin loss and large vessel invasion were independent prognostic factors for shorter disease-free survival. Cirrhosis and high Ki-67 index (> 40%) were independent prognostic factors for shorter overall survival.
-
Conclusions
- Prrx-1 was expressed in small portions of HCCs but not in normal livers. Additional studies with a large number of Prrx-1-positive cases are required to confirm the results of this study.
- Patients and specimens
- A total of 244 patients who underwent surgical resection for HCC from 1991 to 2013 at Hanyang University Medical Center, Seoul and Guri hospitals were selected as subjects for this study. Medical records were reviewed to identify baseline clinical information, clinical course, underlying etiology, and liver function status at the diagnosis. Histological review was performed to determine stage, grade (Edmondson and Steiner’s grading system), vascular invasion, size of tumor, and multiplicity. This study was conducted under review of the Institutional Review Board (IRB) of Hanyang University Guri Hospital (Code, 2015-11-012).
- TMA, immunohistochemistry, and interpretation
- Representative tissue blocks were selected for TMA construction after histological review. For each case, a 2-mm single core was punched from the tumor but not from normal tissue. All immunostainings were performed using the automated system, Leica Bond III (Leica Biosystems, Nusslock, Germany) and Bond Polymer Refine Detection Kit (Leica Biosystems). E-cadherin antibody (mouse monoclonal, NCL-L-E-Cad, Novocastra, Newcastle upon Tyne, UK) was diluted at 1:25. Prrx-1 antibody (rabbit polyclonal, NBP2-13816, Novus Biologicals, Littleton, CO, USA) was diluted at 1:20. Ki-67 antibody (mouse monoclonal, NCL-L-Ki67-MM1, Novocastra) was diluted at 1:200. TMA sections (4-μm-thick) were de-waxed, and the antigen was retrieved with Bond Epitope Retrieval Solution 2 (Leica Biosystems), then incubated with primary antibodies for 60 minutes.
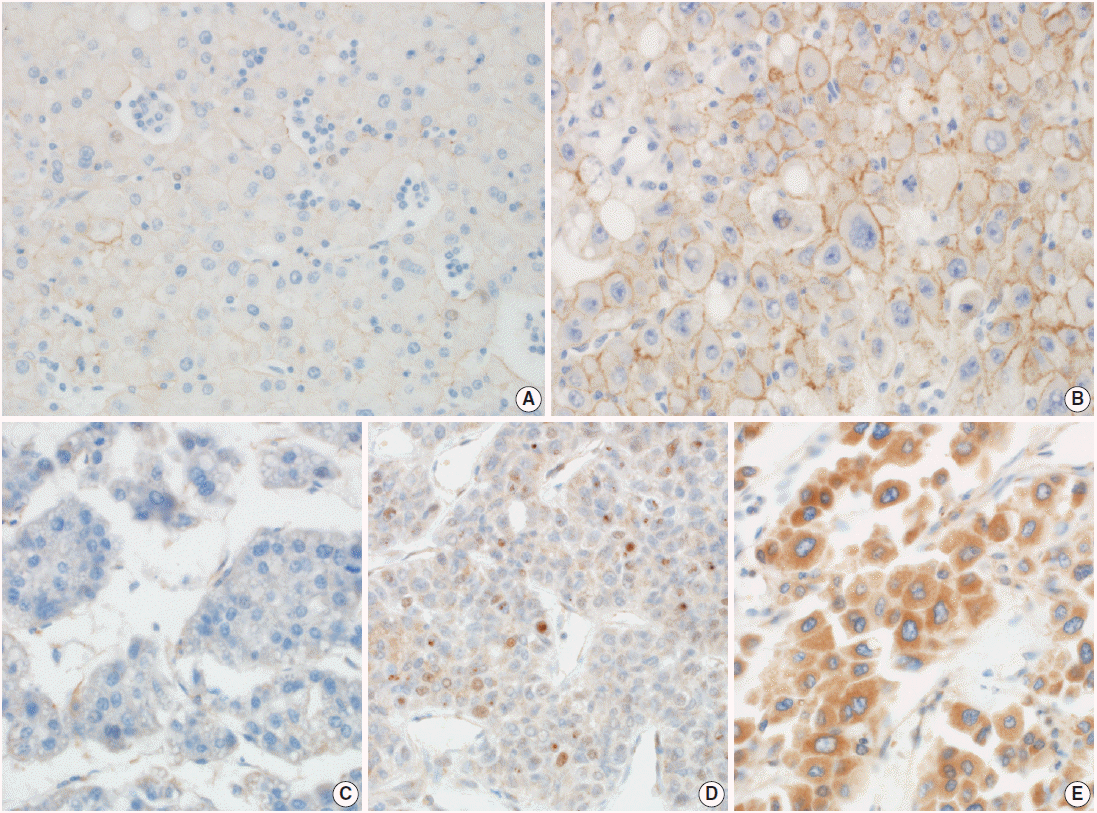
- Two pathologists assessed E-cadherin staining into two categories as described previously [9]; negative or faint membranous stained and moderate to strong membranous stained. The average E-cadherin stainability of HCC cells in each core was evaluated. Prrx-1 staining was evaluated by its nuclear positivity as previously described [4]. Cytoplasmic stained cases without nuclear positivity for Prrx-1 were interpreted as negative because they were considered to be nonspecific cytoplasmic antibody entrapping. Representative images of immunohistochemical staining are presented in Fig. 1. To determine whether the immunoexpression of Prrx-1 in HCCs was upregulated or lost, comparing to normal or cirrhotic liver, we performed Prrx-1 immunostain to two whole block sections and one TMA section that contained 20 cores of normal liver tissue block obtained that were obtained from liver resections from traumatic liver laceration, or metastatic colon cancer. We stained one whole section from each of the three Prrx-1–positive HCCs and three Prrx-1–negative HCCs, to determine if the single TMA core was representative of the entire tumor’s Prrx-1 expression status. The cases were randomly selected from the cohort and all sections included adjacent cirrhotic liver. Ki-67 immunostain was performed on TMA sections. The Ki-67 labeling index was calculated by a free image analyzing software, TMARKER v2.21625 [10].
- Statistical analysis
- We investigated the relationship between the loss of E-cadherin and clinicopathologic factors as well as survival time and recurrence. Expression of Prrx-1 was evaluated with the same method. Concordance of loss of E-cadherin and Prrx-1 expression was analyzed. We also correlated the expression with other markers to investigate whether those markers reflect aggressive pathologic features. The association between immunohistochemical stain results and clinicopathologic parameters was analyzed using the chi-square test, Fisher exact test, and Mann-Whitney U test. Recurrence and survival rate was calculated by the Kaplan-Meier method, and the comparisons were made using the log-rank test. The Cox proportional hazard regression model was adopted to determine the prognostic impact of immunohistochemical results and other clinicopathologic parameters. For the Cox regression, high Ki-67 was defined as Ki-67 index over 40, by considering the cutoff values of previous studies [11,12]. For all calculations, differences at p<.05 were considered statistically significant. All statistical calculations were performed using the R software ver. 3.1.0 (R Core Team 2014).
MATERIALS AND METHODS
- Clinicopathological characteristics
- The clinicopathologic features are summarized in Table 1. The median age of the 244 patients was 56 years (range, 24 to 87 years). One hundred eighty-three patients were male (75%), and 61 were female (25%). One hundred seventy-one patients had hepatitis B virus (HBV, 70.1%), and 16 had hepatitis C virus (HCV, 6.6%). Two patients had both HCV and HBV coinfection (0.8%). Twenty-five patients had no viral hepatitis but had a history of chronic alcohol intake without any apparent viral etiology (10.2%). Thirty patients had neither viral hepatitis nor history of chronic alcoholic intake (12.3%). Cirrhosis was detected in 200 patients (82%). Child-Pugh classes were stage B in 14 patients (5.7%), while the others are stage A (94.3%). Regarding pathologic stage, 132 patients were stage I (54.1%), 79 were II (33.4%), eight were IIIA (3.3%), 24 were IIIB (9.8%), and one was IIIC (0.4%). Median tumor size was 3.5 cm (range, 0.6 to 19.5 cm). Two hundred one cases presented as a solitary tumor (82.4%) while the others presented as multiple tumors. Portal vein, hepatic vein, or their major branch involvement was detected in 45 patients (18.4%).
- One hundred and six patients died during follow-up. Median survival time was 70.8 months (range, 1.3 to 287.7 months) and 5-year survival rate was 55.6%. In addition, 35% of cases showed a tumor recurrence within 1 year, and 65.3% underwent recurrence within 5 years. The Kaplan-Meier method showed a significant difference in overall survival and disease-free survival between stages I and II (p=.006, and p<.001, respectively). However, those between stage II and stage III were not statistically significant (p=.450 and p=.106, respectively). Additionally, 3.9% of stage I patients experienced tumor recurrence within 1 year (n=19), compared with 20.6% of stage II (n=19), and 29.8% of stage III (n=11).
- E-cadherin and Prrx-1 immunohistochemical staining results
- E-cadherin expression was decreased in 96 cases (39.3%), whereas others showed moderate to strong membranous immunoreactivity. Thirty-two cases (13.3%) showed at least focal nuclear Prrx-1 immunoreactivity. Loss of E-cadherin and Prrx-1 expression was not concordant (p=.972 on the chi-square test). One hundred twenty cases showed minimally weak cytoplasmic stainability (49.2%). Cytoplasmic staining of Prrx-1 was not correlated with loss of E-cadherin expression (p=.217 on the chi-square test).
- All normal livers, including two whole section and one TMA section, of which there were twenty cases, were negative for Prrx-1 staining. All Prrx-1 stainings with whole sections of three Prrx-1–positive HCCs and three Prrx-1–negative HCCs showed consistent results with the TMA stainings. All adjacent cirrhotic livers from the six sections were negative for Prrx-1.
- HCC biomarker relationships
- We examined the correlation between Prrx-1 and E-cadherin immunohistochemical staining results and various clinicopathological factors. HCCs with decreased E-cadherin tend to occur more frequently in the background of cirrhosis, compared to E-cadherin maintained HCCs (88.5% vs 77.7%, p=.048 on the chi-square test). Both large vessel (portal vein, hepatic vein, or their major branches) invasion and small vessel invasion were more frequent in the E-cadherin reduced group compared with the maintained group; however, they were not statistically significant (large vessel invasion: 11.5% vs 8.8%, p=.642 on chi-square test; small vessel invasion: 42.7% vs 35.8%, p=.344 on chi-square test). Hepatitis B, hepatitis C, history of chronic alcohol intake, age, sex, pathologic stage, multiplicity of the tumor, tumor size, and histologic grade were not significantly associated with E-cadherin expression (Table 2).
- None of the factors were statistically significantly correlated with Prrx-1 expression and clinicopathological factors, although both large vessel invasion and small vessel invasion were more frequent in Prrx-1–positive HCCs (large vessel invasion: 12.5% vs 9.4%, p=.533; small vessel invasion: 40.6% vs 38.2%, p=.946) (Table 2). Prrx-1–positive HCCs showed more frequent p53 overexpression (25% vs 13.7%, p=.114 on chi-square test), although this result was not statistically significant.
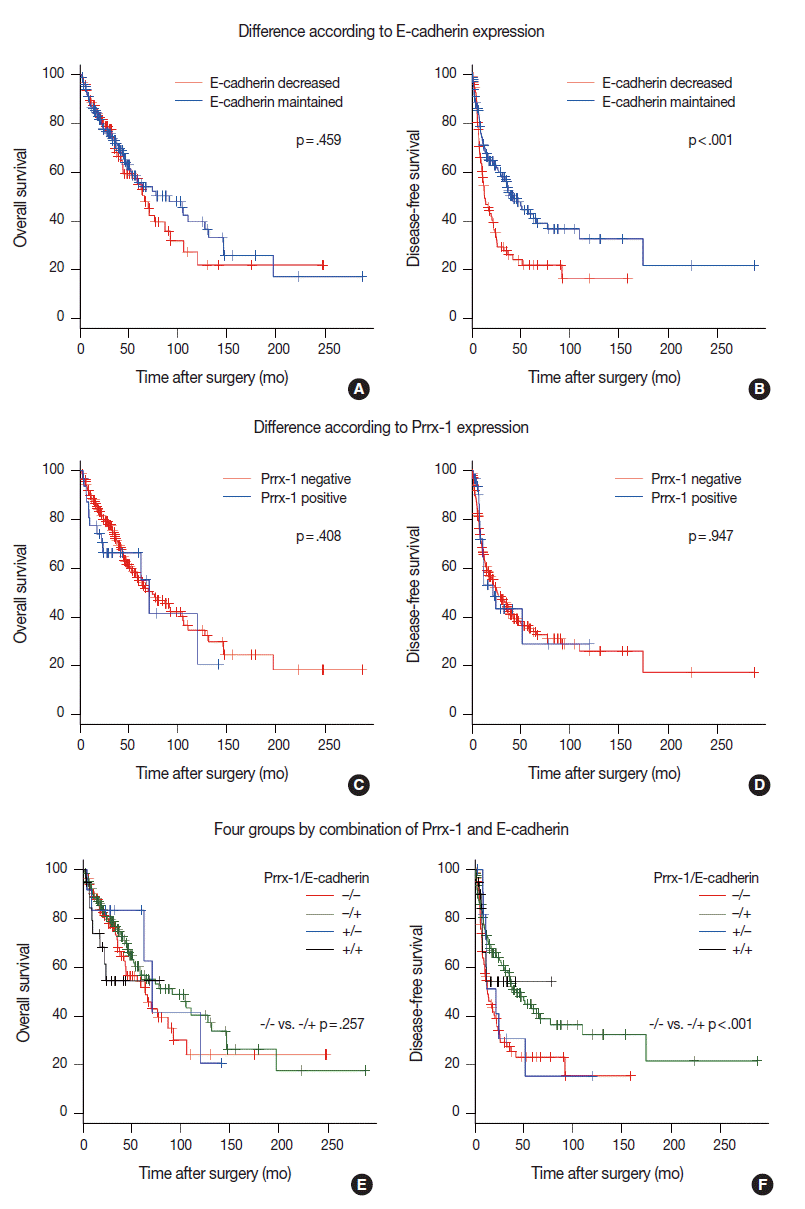
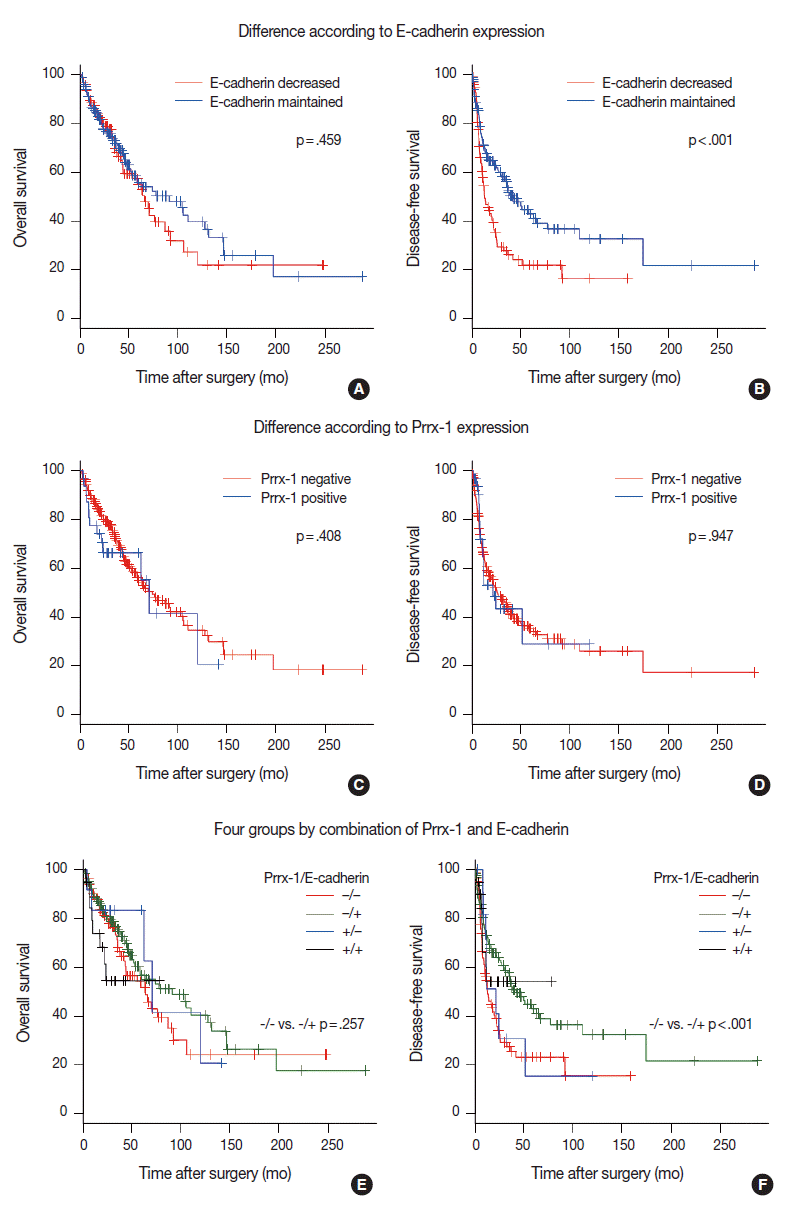
- Survival analysis indicated that E-cadherin decreased HCCs that were identified in earlier recurrences than in E-cadherin maintained HCCs (mean, 1.08 years vs 3.51 years; p<.001 on the log-rank test) (Fig. 2B). Within one year, 41 cases (45.8%) of E-cadherin decreased HCCs experienced recurrence, while 40 cases of E-cadherin (28.5%) maintained HCCs did not. The overall survival rate was not significantly different on the log-rank test (p=.459) (Fig. 2A). We also examined the correlation between patient’s survival/recurrence and Prrx-1 expression. Although 1-year recurrence rate (43.1% vs 34.2%) and 5-year recurrence rate (71.2% vs 64.8%) were higher in Prrx-1 that expressed HCCs than in Prrx-1–negative HCCs, the difference was not statistically significant on the log-rank test (p=.947) (Fig. 2D). Overall survival was also not significantly different (p=.408) (Fig. 2C).
- We categorized HCCs into four groups, depending on the E-cadherin and Prrx-1 expression. The clinicopathologic parameters, recurrence rate, and survival rate were compared between Prrx-1–positive and Prrx-1–negative groups, and among E-cadherin–positive cases, in addition to the same comparison among E-cadherin–negative cases. Each of the four groups were also compared with the other three groups. The E-cadherin–positive and Prrx-1–positive group (n=20) showed significantly higher nuclear grades than the rest of the group (n =224) (nuclear grade 3 or 4 proportion: 80% vs 53.1%, p=.037 on chi-squared test). Other parameters in the various group comparison were not significantly different (Table 3, Fig. 2E and F).
- Cox proportional hazard regression model
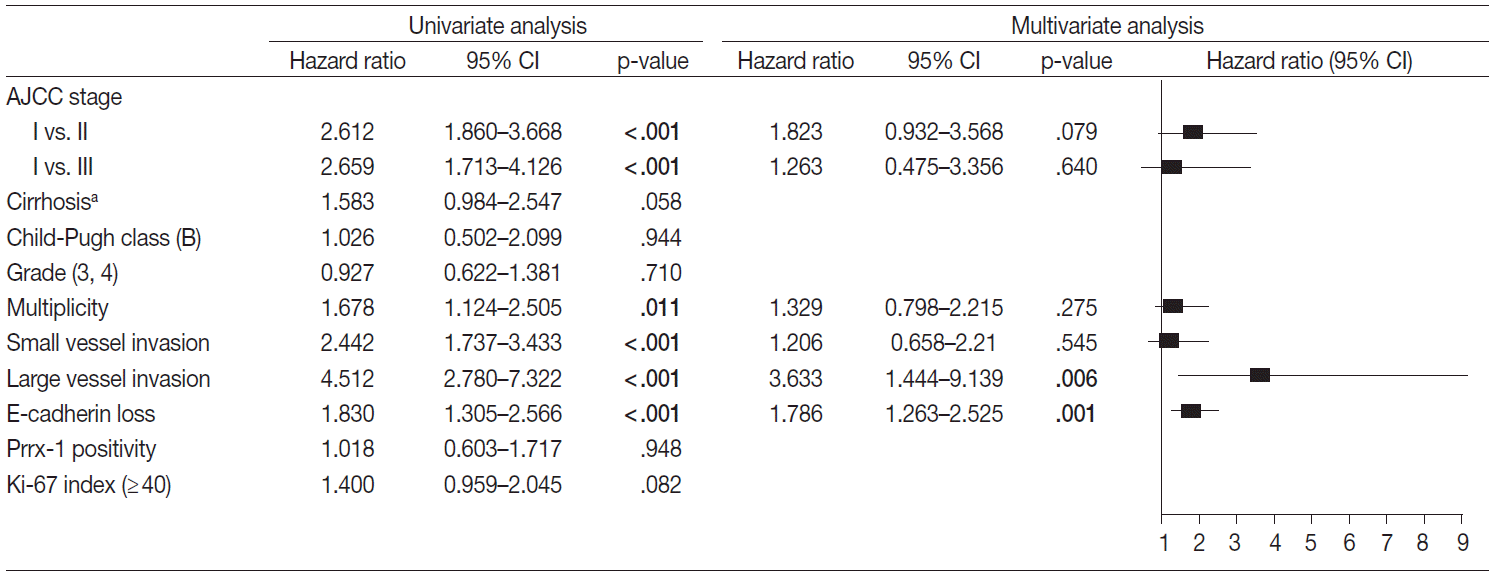
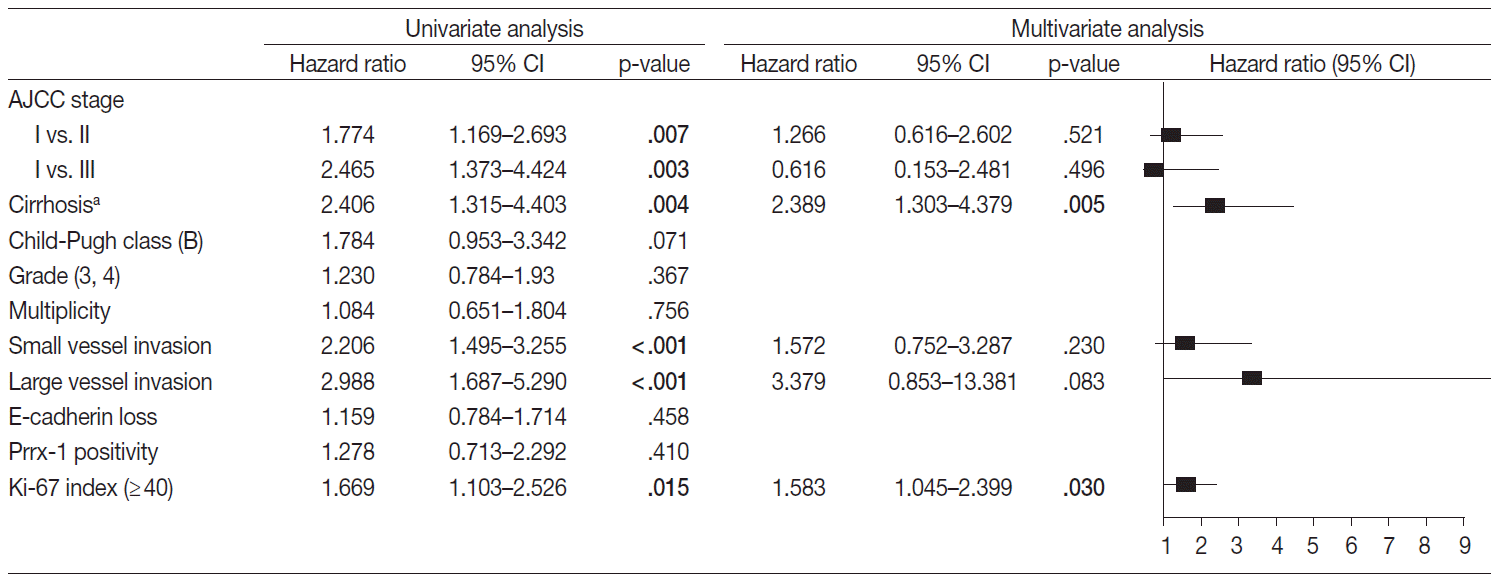
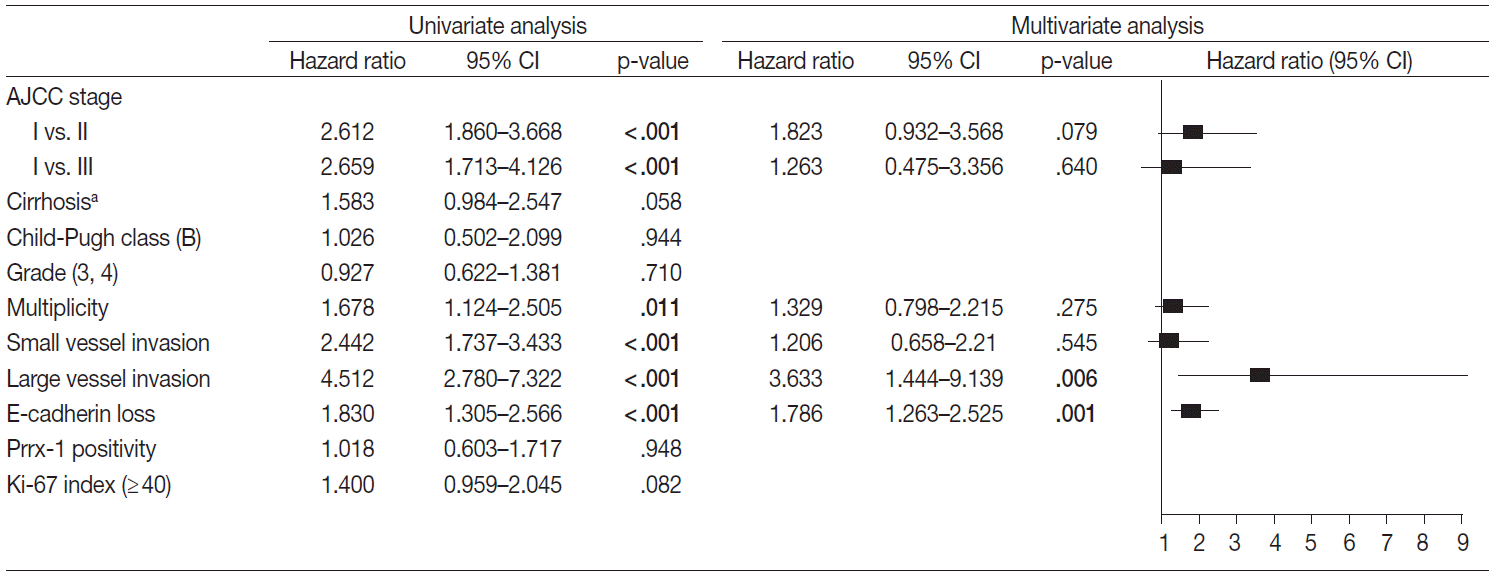
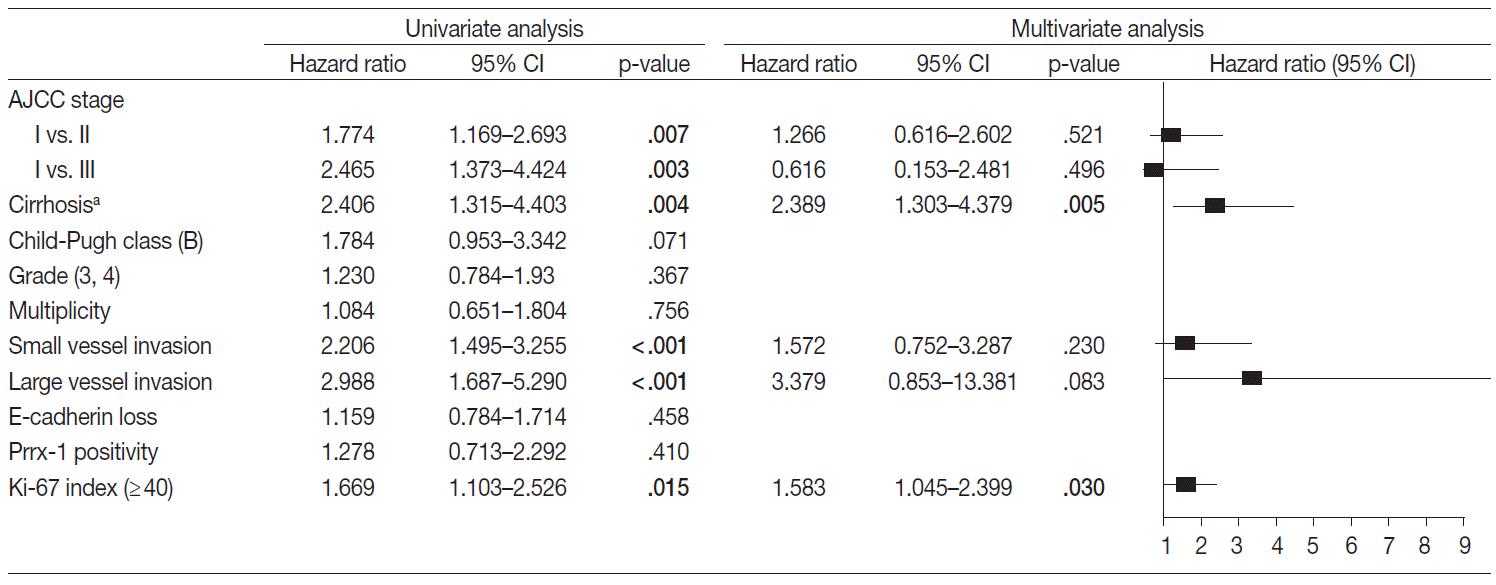
- Factors that may affect the prognosis of HCC were filtered for the multivariate Cox proportional hazard regression model (p<.05 in univariate Cox proportional hazard regression model). The disease-free and overall survival were separately processed and are in Figs. 3 and 4, respectively. The stage, multiplicity, small and large vessel invasion, and decrease of E-cadherin were selected for factors in disease-free survival. Among them, the large vessel invasion (p=.006) and loss of E-cadherin (p=.001) were independent risk factors. The stage, cirrhosis, small and large vessel invasion, and high Ki-67 labeling index were selected for overall survival. Cirrhosis (p=.005) and high Ki-67 labeling index (p=.030) were independent risk factors for overall survival.
RESULTS
- EMT has an important role in cancer progression because it influences tumor cell migration, allows local tissue invasion, and into the blood vessel, which is a necessary step in cancer metastasis [3]. In colorectal cancer, tumor budding is one of the most important prognostic factors and reflects aggressive biologic behavior. The tumor budding is also explained as an EMT process, consistently with its morphologic change to fibroblast-like appearance [13]. E-cadherin is a type of cadherins, which is a type 1 transmembrane protein, that plays an important role in cell adhesion. Loss of the E-cadherin is a hallmark of EMT and it is related to poor prognosis in various human cancers [14]. In addition, several studies based on immunohistochemical staining of E-cadherin have reported the prognostic significance of HCC [15-17].
- Loss of E-cadherin is clearly associated with hematogenous tumor spread, lymph node metastasis, and local recurrence in various types of cancer, including breast cancer, colorectal cancer, and liver cancer [18-20]. For HCC, Chen et al. [18] performed a meta-analysis to access the prognostic value of E-cadherin in HCC, and revealed that loss of E-cadherin was strongly associated with poor overall survival, recurrence-free survival, metastasis, vascular invasion, higher grade, and advanced TNM stage [18]. As mentioned earlier, several other EMT inducers were evaluated as prognostic markers. SNAI1, an EMT inducer, was expressed in an invasive portion of HCC and was associated with the higher grade. Twist-1, another EMT inducer, was found to be associated with distant metastasis in HCC [21].
- As previously mentioned, either upregulation or downregulation of Prrx-1 has been reported to be associated with poor prognosis, depending on the type of cancer. In colorectal cancer, overexpression of PRRX1 mRNA was involved in metastasis and poor prognosis [7]. In thyroid cancer, 57.7% of anaplastic thyroid carcinomas and two of five hobnail variants of papillary carcinoma, which are very aggressive and associated with high mortality rate, were positive for Prrx-1 on immunohistochemical staining, while all conventional papillary carcinomas were negative [4]. In comparison, Ocana et al. [5] found that loss of Prrx-1 contributes to metastatic colonization and poor prognosis in breast cancer. They performed an experiment with a xenograft model, using human breast cancer cells that expressed both Prrx-1 and Twist1. A knockdown of both Prrx-1 and Twist1 increased lung metastasis but not in a setting of only one knockdown. This indicated that downregulation of Prrx-1 is necessary to develop metastatic nodules and gain proliferative activity [5]. Similarly, Hirata et al. [8] studied mRNA expression level of Prrx-1 in HCC and found that the Prrx-1 downregulated group showed a poorer prognosis than the upregulated group. However, this study showed that normal livers did not express Prrx-1 protein immunohistochemically, and evokes a question that the decreased level of the PRRX1 mRNA has biological implications, as only a few (in our case, 13%) of the HCCs showed nuclear immunoreactivity on Prrx-1.
- Ki-67 is a marker that reflects proliferative activity, which increases during carcinogenesis. Poorly differentiated HCCs show a higher index of Ki-67 than in well- to moderately-differentiated HCCs [22]. In general, the proliferating activity decreases when tumor cells undergo EMT [23]. We expected that loss of E-cadherin and expression of Prrx-1 would inversely correlate with the Ki-67 index. However, there was no significant difference, which was likely due to the limitation of a small number of Prrx-1–positive cases and many other biological confounding variables that effect on the proliferation.
- Through this study, we attempted to elucidate whether Prrx-1–positive or Prrx-1–negative HCCs are associated with poor prognosis. As EMT is a dynamic process and its timely regulation is necessary to metastasis, it is necessary to develop new markers to know whether the tumor will re-gain proliferative and epithelial property after hematogenous spread, when we observe E-cadherin loss in the resected primary tumor. However, we only validated E-cadherin as a useful prognostic factor to predict recurrence, and Prrx-1 was not correlated with patient’s prognosis and recurrence. Our experiment had some limitations, which were attributable, in part to the role of Prrx-1 on the prognosis of HCCs. The design of the present experiment did not implicate the complex mechanism of EMT–reverse EMT process during cancer metastasis. Cross-sectional studies using immunohistochemical staining on paraffin block have a limitation to dealing dynamic processes. Especially, as Prrx-1 is a transcription factor, two assumptions are required for this immunohistochemical study: (1) the expression of Prrx-1 should be consistent through the time until E-cadherin molecules disappear in the cell membrane, and (2) the functional expression range and threshold should be consistent with detection range and interpretation cutoff. However, many cellular signals work in a stochastic manner and show oscillatory responses, which give an uncertainty to our assumption [24,25]. Prrx-1 overexpression was observed in 15.1% of HCCs in this study by immunohistochemistry. It is quite a small percentage comparing with 45.2% (28/62), which is the fraction of high-PRRX1 mRNA that expressed HCCs in the experiment by Hirata et al. [8]. We believe that this also gives an uncertainty to our second assumption.
- In summary, Prrx-1 was expressed in small portion (13.3%) of HCCs but not in non-neoplastic livers. We validated that E-cadherin loss had the prognostic value to predict recurrence in HCCs. Prrx-1 was not correlated with EMT nor prognosis nor recurrence by immunohistochemical staining study. Further study with a larger series of HCCs is required to elucidate the significance of Prrx-1 in HCCs.
DISCUSSION
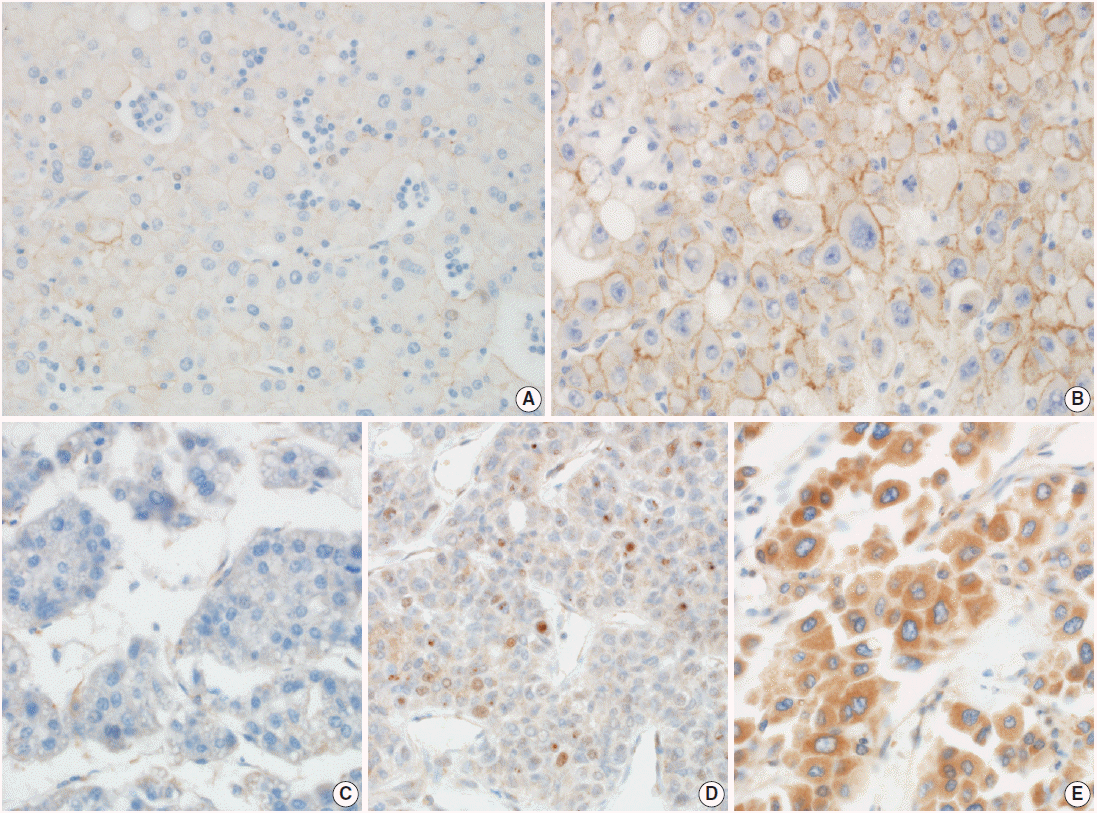
| Parameter | No. (%) |
|---|---|
| Age (yr)a | 56 (24–87) |
| Sex (male:female) | 183:61 (3:1) |
| Etiological factor | |
| HBV | 171 (70.1) |
| HCV | 16 (6.6) |
| HBV + HCV | 2 (0.8) |
| Alcohol | 25 (10.2) |
| Unknown | 30 (12.3) |
| Underlying disease | |
| Chronic hepatitis | 44 (18) |
| Cirrhosis | 200 (82) |
| Child-Pugh class | |
| A | 230 (94.3) |
| B | 14 (5.7) |
| AJCC stage | |
| I | 132 (54.1) |
| II | 79 (32.4) |
| III | 33 (13.5) |
| Tumor size (cm)a | 3.5 (0.6–19.5) |
| Multiplicity (solitary/multiple) | |
| Solitary | 201 (82.4) |
| Multiple | 43 (17.6) |
| Histologic grade | |
| 1, 2 | 109 (44.7) |
| 3, 4 | 135 (55.3) |
| Small vessel invasion | 94 (38.5) |
| Large vessel invasion | 24 (9.8) |
| Recurrence rate (%) | |
| 1 Year | 35.0 |
| 5 Years | 65.3 |
| Survival rate (%) | |
| 1 Year | 87.4 |
| 5 Years | 55.6 |
| Parameter |
E-cadherin |
p-value |
Prrx-1 |
p-value | ||
|---|---|---|---|---|---|---|
| Decreased (n = 96) | Maintained (n = 148) | Positive (n = 32) | Negative (n = 212) | |||
| Age (yr)a | 57 (31–77) | 55 (24–87) | .135b | 60 (24–74) | 55.5 (28–87) | .161b |
| Sex (male:female) | 74:22 (3.4:1) | 109:39 (2.8:1) | .650c | 24:8 (3:1) | 159:53 (3:1) | >.990c |
| Etiology (%) | .090d | |||||
| HBV | 59 (61.5) | 112 (75.7) | 20 (62.5) | 151 (71.2) | .501d | |
| HCV | 10 (10.4) | 6 (4.1) | 2 (6.2) | 14 (6.6) | ||
| HBV + HCV | 1 (1) | 1 (0.7) | 0 | 2 (0.8) | ||
| Alcohol | 13 (13.5) | 12 (8.1) | 3 (9.4) | 22 (10.8) | ||
| Unknown | 13 (13.5) | 17 (11.5) | 7 (21.9) | 23 (10.8) | ||
| Underlying disease (%) | .048c | .893d | ||||
| Chronic hepatitis | 11 (11.5) | 33 (22.3) | 5 (18.4) | 39 (15.6) | ||
| Cirrhosis | 85 (88.5) | 115 (77.7) | 27 (81.6) | 173 (81.6) | ||
| Child-Pugh class | .996c | >.990d | ||||
| A | 91 (94.8) | 139 (93.9) | 30 (93.8) | 200 (94.3) | ||
| B | 5 (5.2) | 9 (6.1) | 2 (5.7) | 12 (5.7) | ||
| AJCC stage | .707c | .399d | ||||
| I | 50 (52.1) | 82 (55.4) | 20 (62.5) | 112 (52.8) | ||
| II | 34 (35.4) | 45 (30.4) | 7 (21.9) | 72 (34.0) | ||
| III | 12 (12.5) | 21 (14.2) | 5 (15.6) | 28 (13.2) | ||
| Multiplicity | .406c | .571c | ||||
| Single | 82 (85.4) | 119 (80.4) | 28 (87.5) | 173 (81.6) | ||
| Multiple | 14 (14.6) | 29 (19.6) | 4 (12.5) | 39 (18.4) | ||
| Tumor sizea | 3.3 (0.6–19.5) | 3.5 (0.7–17) | .877b | 3.15 (1.2–15) | 3.5 (0.6–19.5) | |
| Histologic grade | .224c | .286c | ||||
| 1, 2 | 48 (50) | 61 (41.2) | 11 (34.4) | 98 (46.2) | ||
| 3, 4 | 48 (50) | 87 (58.8) | 21 (65.6) | 114 (53.8) | ||
| Large vessel invasion | 11 (11.5) | 13 (8.8) | .642c | 4 (12.5) | 20 (9.4) | .533d |
| Small vessel invasion | 41 (42.7) | 53 (35.8) | .344c | 13 (40.6) | 81 (38.2) | .946c |
| Prrx-1 positivity | 12 (12.5) | 20 (13.5) | .972c | 12 (37.5) | 84 (39.6) | .972c |
| Ki-67 indexe | 21 (9–37.5) | 22 (10–41) | .754b | 21 (9–35.5) | 22 (9.5–40.5) | .533b |
| Recurrence rate (%) | < .001f | .947f | ||||
| 1 Year | 45.8 | 28.5 | 43.1 | 34.2 | ||
| 5 Years | 78.1 | 57.1 | 71.2 | 64.8 | ||
| Survival rate (%) | .459f | .409f | ||||
| 1 Year | 88.3 | 86.8 | 77.5 | 88.9 | ||
| 5 Years | 55.2 | 55.6 | 66.3 | 55.1 | ||
Values are presented as number (%) unless otherwise indicated.
Prrx-1, paired-related homeobox protein 1; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; AJCC, American Joint Committee on Cancer.
aMedian (range);
bMann-Whitney U test;
cChi-square test;
dFisher exact test;
eMedian (first quantile–third quantile);
fLog-rank test.
| Parameter |
E-cadherin positive |
E-cadherin negative |
||
|---|---|---|---|---|
| Prrx-1 positive (n = 20) | Prrx-1 negative (n = 128) | Prrx-1 positive (n = 12) | Prrx-1 negative (n = 84) | |
| AJCC stage | ||||
| I | 11 (55) | 73 (57) | 9 (75) | 41 (48.8) |
| II | 6 (30) | 40 (31.2) | 1 (8.3) | 33 (39.3) |
| III | 3 (15) | 15 (11.7) | 2 (16.7) | 10 (11.9) |
| Multiplicity | ||||
| Single | 18 (90) | 101 (78.9) | 10 (83.3) | 72 (85.7) |
| Multiple | 2 (10) | 27 (21.1) | 2 (16.7) | 12 (14.3) |
| Histologic gradea | ||||
| 1, 2 | 4 (20) | 57 (44.5) | 7 (58.3) | 41 (48.8) |
| 3, 4 | 16 (80) | 71 (55.5) | 5 (41.7) | 43 (51.2) |
| Large vessel invasion | 18 (90) | 117 (91.4) | 10 (83.3) | 75 (89.3) |
| Small vessel invasion | 10 (50) | 85 (66.4) | 9 (75) | 46 (54.8) |
| Ki-67 indexb | 26 (7.5–43.5) | 21 (10–40.5) | 21 (12.5–39) | 21 (9–34.5) |
| Recurrence rate (%) | ||||
| 1 Year | 60.1 | 73 | 51.1 | 54.6 |
| 5 Years | 54.1 | 43 | 15.3 | 23.2 |
| Survival rate (%) | ||||
| 1 Year | 73.9 | 88.8 | 83.3 | 89 |
| 5 Years | 54.6 | 56.9 | 83.3 | 51.7 |
Values are presented as number (%) unless otherwise indicated.
Prrx-1, paired-related homeobox protein 1; HCC, hepatocellular carcinoma; AJCC, American Joint Committee on Cancer.
aComparing both E-cadherin and Prxx-1 positive-group (the first column) to the others, the former showed significantly higher histologic grade (p = .037 on chi-squared test);
bMedian (first quantile–third quantile).
- 1. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat 2013; 45: 1-14. ArticlePubMedPMCPDF
- 2. Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg 2000; 232: 10-24. ArticlePubMedPMC
- 3. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871-90. ArticlePubMed
- 4. Hardin H, Guo Z, Shan W, et al. The roles of the epithelial-mesenchymal transition marker PRRX1 and miR-146b-5p in papillary thyroid carcinoma progression. Am J Pathol 2014; 184: 2342-54. ArticlePubMedPMC
- 5. Ocana OH, Corcoles R, Fabra A, et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 2012; 22: 709-24. ArticlePubMed
- 6. Reichert M, Takano S, von Burstin J, et al. The Prrx1 homeodomain transcription factor plays a central role in pancreatic regeneration and carcinogenesis. Genes Dev 2013; 27: 288-300. ArticlePubMedPMC
- 7. Takahashi Y, Sawada G, Kurashige J, et al. Paired related homoeobox 1, a new EMT inducer, is involved in metastasis and poor prognosis in colorectal cancer. Br J Cancer 2013; 109: 307-11. ArticlePubMedPMCPDF
- 8. Hirata H, Sugimachi K, Takahashi Y, et al. Downregulation of PRRX1 confers cancer stem cell-like properties and predicts poor prognosis in hepatocellular carcinoma. Ann Surg Oncol 2015; 22 Suppl 3: S1402-9. ArticlePubMedPDF
- 9. Woo HY, Min AL, Choi JY, Bae SH, Yoon SK, Jung CK. Clinicopathologic significance of the expression of Snail in hepatocellular carcinoma. Korean J Hepatol 2011; 17: 12-8. ArticlePubMedPMC
- 10. Schuffler PJ, Fuchs TJ, Ong CS, Wild PJ, Rupp NJ, Buhmann JM. TMARKER: A free software toolkit for histopathological cell counting and staining estimation. J Pathol Inform 2013; 4(Suppl):S2.PubMedPMC
- 11. Nakanishi K, Sakamoto M, Yamasaki S, Todo S, Hirohashi S. Akt phosphorylation is a risk factor for early disease recurrence and poor prognosis in hepatocellular carcinoma. Cancer 2005; 103: 307-12. ArticlePubMed
- 12. Ito Y, Matsuura N, Sakon M, et al. Both cell proliferation and apoptosis significantly predict shortened disease-free survival in hepatocellular carcinoma. Br J Cancer 1999; 81: 747-51. ArticlePubMedPMCPDF
- 13. Prall F. Tumour budding in colorectal carcinoma. Histopathology 2007; 50: 151-62. ArticlePubMed
- 14. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007; 7: 415-28. ArticlePubMedPDF
- 15. Cho SB, Lee KH, Lee JH, et al. Expression of E- and N-cadherin and clinicopathology in hepatocellular carcinoma. Pathol Int 2008; 58: 635-42. ArticlePubMed
- 16. Zhang L, Huang G, Li X, et al. Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor -1alpha in hepatocellular carcinoma. BMC Cancer 2013; 13: 108.PubMedPMC
- 17. Kwon GY, Yoo BC, Koh KC, Cho JW, Park WS, Park CK. Promoter methylation of E-cadherin in hepatocellular carcinomas and dysplastic nodules. J Korean Med Sci 2005; 20: 242-7. ArticlePubMedPMC
- 18. Chen J, Zhao J, Ma R, Lin H, Liang X, Cai X. Prognostic significance of E-cadherin expression in hepatocellular carcinoma: a meta-analysis. PLoS One 2014; 9: e103952. Article
- 19. Aoki S, Shimamura T, Shibata T, et al. Prognostic significance of dysadherin expression in advanced colorectal carcinoma. Br J Cancer 2003; 88: 726-32. ArticlePubMedPMCPDF
- 20. Asgeirsson KS, Jonasson JG, Tryggvadóttir L, et al. Altered expression of E-cadherin in breast cancer. patterns, mechanisms and clinical significance. Eur J Cancer 2000; 36: 1098-106. PubMed
- 21. Zhao XL, Sun T, Che N, et al. Promotion of hepatocellular carcinoma metastasis through matrix metalloproteinase activation by epithelial-mesenchymal transition regulator Twist1. J Cell Mol Med 2011; 15: 691-700. ArticlePubMed
- 22. Tannapfel A, Geissler F, Köckerling F, Katalinic A, Hauss J, Wittekind C. Apoptosis and proliferation in relation to histopathological variables and prognosis in hepatocellular carcinoma. J Pathol 1999; 187: 439-45. ArticlePubMed
- 23. Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 2012; 22: 725-36. ArticlePubMedPMC
- 24. Ferrell JE Jr, Machleder EM. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science 1998; 280: 895-8. ArticlePubMed
- 25. Batchelor E, Loewer A, Lahav G. The ups and downs of p53: understanding protein dynamics in single cells. Nat Rev Cancer 2009; 9: 371-7. ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

- The Prognostic Importance of Ki-67 in Gastrointestinal Carcinomas: A Meta-analysis and Multi-omics Approach
Mahdieh Razmi, Fatemeh Tajik, Farideh Hashemi, Ayna Yazdanpanah, Fatemeh Hashemi-Niasari, Adeleh Divsalar
Journal of Gastrointestinal Cancer.2024; 55(2): 599. CrossRef - Homotypic cell-in-cell structures as an adverse prognostic predictor of hepatocellular carcinoma
Ruizhi Wang, Yichao Zhu, Hao Zhong, Xinyue Gao, Qiang Sun, Meifang He
Frontiers in Oncology.2022;[Epub] CrossRef - Dysregulated paired related homeobox 1 impacts on hepatocellular carcinoma phenotypes
Weronika Piorońska, Zeribe Chike Nwosu, Mei Han, Michael Büttner, Matthias Philip Ebert, Steven Dooley, Christoph Meyer
BMC Cancer.2021;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure




Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
| Parameter | No. (%) |
|---|---|
| Age (yr) |
56 (24–87) |
| Sex (male:female) | 183:61 (3:1) |
| Etiological factor | |
| HBV | 171 (70.1) |
| HCV | 16 (6.6) |
| HBV + HCV | 2 (0.8) |
| Alcohol | 25 (10.2) |
| Unknown | 30 (12.3) |
| Underlying disease | |
| Chronic hepatitis | 44 (18) |
| Cirrhosis | 200 (82) |
| Child-Pugh class | |
| A | 230 (94.3) |
| B | 14 (5.7) |
| AJCC stage | |
| I | 132 (54.1) |
| II | 79 (32.4) |
| III | 33 (13.5) |
| Tumor size (cm) |
3.5 (0.6–19.5) |
| Multiplicity (solitary/multiple) | |
| Solitary | 201 (82.4) |
| Multiple | 43 (17.6) |
| Histologic grade | |
| 1, 2 | 109 (44.7) |
| 3, 4 | 135 (55.3) |
| Small vessel invasion | 94 (38.5) |
| Large vessel invasion | 24 (9.8) |
| Recurrence rate (%) | |
| 1 Year | 35.0 |
| 5 Years | 65.3 |
| Survival rate (%) | |
| 1 Year | 87.4 |
| 5 Years | 55.6 |
| Parameter | E-cadherin |
p-value | Prrx-1 |
p-value | ||
|---|---|---|---|---|---|---|
| Decreased (n = 96) | Maintained (n = 148) | Positive (n = 32) | Negative (n = 212) | |||
| Age (yr) |
57 (31–77) | 55 (24–87) | .135 |
60 (24–74) | 55.5 (28–87) | .161 |
| Sex (male:female) | 74:22 (3.4:1) | 109:39 (2.8:1) | .650 |
24:8 (3:1) | 159:53 (3:1) | >.990 |
| Etiology (%) | .090 |
|||||
| HBV | 59 (61.5) | 112 (75.7) | 20 (62.5) | 151 (71.2) | .501 |
|
| HCV | 10 (10.4) | 6 (4.1) | 2 (6.2) | 14 (6.6) | ||
| HBV + HCV | 1 (1) | 1 (0.7) | 0 | 2 (0.8) | ||
| Alcohol | 13 (13.5) | 12 (8.1) | 3 (9.4) | 22 (10.8) | ||
| Unknown | 13 (13.5) | 17 (11.5) | 7 (21.9) | 23 (10.8) | ||
| Underlying disease (%) | .048 |
.893 |
||||
| Chronic hepatitis | 11 (11.5) | 33 (22.3) | 5 (18.4) | 39 (15.6) | ||
| Cirrhosis | 85 (88.5) | 115 (77.7) | 27 (81.6) | 173 (81.6) | ||
| Child-Pugh class | .996 |
>.990 |
||||
| A | 91 (94.8) | 139 (93.9) | 30 (93.8) | 200 (94.3) | ||
| B | 5 (5.2) | 9 (6.1) | 2 (5.7) | 12 (5.7) | ||
| AJCC stage | .707 |
.399 |
||||
| I | 50 (52.1) | 82 (55.4) | 20 (62.5) | 112 (52.8) | ||
| II | 34 (35.4) | 45 (30.4) | 7 (21.9) | 72 (34.0) | ||
| III | 12 (12.5) | 21 (14.2) | 5 (15.6) | 28 (13.2) | ||
| Multiplicity | .406 |
.571 |
||||
| Single | 82 (85.4) | 119 (80.4) | 28 (87.5) | 173 (81.6) | ||
| Multiple | 14 (14.6) | 29 (19.6) | 4 (12.5) | 39 (18.4) | ||
| Tumor size |
3.3 (0.6–19.5) | 3.5 (0.7–17) | .877 |
3.15 (1.2–15) | 3.5 (0.6–19.5) | |
| Histologic grade | .224 |
.286 |
||||
| 1, 2 | 48 (50) | 61 (41.2) | 11 (34.4) | 98 (46.2) | ||
| 3, 4 | 48 (50) | 87 (58.8) | 21 (65.6) | 114 (53.8) | ||
| Large vessel invasion | 11 (11.5) | 13 (8.8) | .642 |
4 (12.5) | 20 (9.4) | .533 |
| Small vessel invasion | 41 (42.7) | 53 (35.8) | .344 |
13 (40.6) | 81 (38.2) | .946 |
| Prrx-1 positivity | 12 (12.5) | 20 (13.5) | .972 |
12 (37.5) | 84 (39.6) | .972 |
| Ki-67 index |
21 (9–37.5) | 22 (10–41) | .754 |
21 (9–35.5) | 22 (9.5–40.5) | .533 |
| Recurrence rate (%) | < .001 |
.947 |
||||
| 1 Year | 45.8 | 28.5 | 43.1 | 34.2 | ||
| 5 Years | 78.1 | 57.1 | 71.2 | 64.8 | ||
| Survival rate (%) | .459 |
.409 |
||||
| 1 Year | 88.3 | 86.8 | 77.5 | 88.9 | ||
| 5 Years | 55.2 | 55.6 | 66.3 | 55.1 | ||
| Parameter | E-cadherin positive |
E-cadherin negative |
||
|---|---|---|---|---|
| Prrx-1 positive (n = 20) | Prrx-1 negative (n = 128) | Prrx-1 positive (n = 12) | Prrx-1 negative (n = 84) | |
| AJCC stage | ||||
| I | 11 (55) | 73 (57) | 9 (75) | 41 (48.8) |
| II | 6 (30) | 40 (31.2) | 1 (8.3) | 33 (39.3) |
| III | 3 (15) | 15 (11.7) | 2 (16.7) | 10 (11.9) |
| Multiplicity | ||||
| Single | 18 (90) | 101 (78.9) | 10 (83.3) | 72 (85.7) |
| Multiple | 2 (10) | 27 (21.1) | 2 (16.7) | 12 (14.3) |
| Histologic grade |
||||
| 1, 2 | 4 (20) | 57 (44.5) | 7 (58.3) | 41 (48.8) |
| 3, 4 | 16 (80) | 71 (55.5) | 5 (41.7) | 43 (51.2) |
| Large vessel invasion | 18 (90) | 117 (91.4) | 10 (83.3) | 75 (89.3) |
| Small vessel invasion | 10 (50) | 85 (66.4) | 9 (75) | 46 (54.8) |
| Ki-67 index |
26 (7.5–43.5) | 21 (10–40.5) | 21 (12.5–39) | 21 (9–34.5) |
| Recurrence rate (%) | ||||
| 1 Year | 60.1 | 73 | 51.1 | 54.6 |
| 5 Years | 54.1 | 43 | 15.3 | 23.2 |
| Survival rate (%) | ||||
| 1 Year | 73.9 | 88.8 | 83.3 | 89 |
| 5 Years | 54.6 | 56.9 | 83.3 | 51.7 |
HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; AJCC, American Joint Committee on Cancer. Median (range).
Values are presented as number (%) unless otherwise indicated. Prrx-1, paired-related homeobox protein 1; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; AJCC, American Joint Committee on Cancer. Median (range); Mann-Whitney U test; Chi-square test; Fisher exact test; Median (first quantile–third quantile); Log-rank test.
Values are presented as number (%) unless otherwise indicated. Prrx-1, paired-related homeobox protein 1; HCC, hepatocellular carcinoma; AJCC, American Joint Committee on Cancer. Comparing both E-cadherin and Prxx-1 positive-group (the first column) to the others, the former showed significantly higher histologic grade (p = .037 on chi-squared test); Median (first quantile–third quantile).

 E-submission
E-submission