Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(1); 2017 > Article
-
Original Article
PD-L1 Expression and Combined Status of PD-L1/PD-1–Positive Tumor Infiltrating Mononuclear Cell Density Predict Prognosis in Glioblastoma Patients - Jiheun Han, Yongkil Hong1, Youn Soo Lee,
-
Journal of Pathology and Translational Medicine 2017;51(1):40-48.
DOI: https://doi.org/10.4132/jptm.2016.08.31
Published online: December 15, 2016
Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
1Department of Neurosurgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- Corresponding Author: Youn Soo Lee, MD, PhD Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 222 Banpo-daero, Seocho-gu, Seoul 06591, Korea Tel: +82-2-2258-1613 Fax: +82-2-2258-1628 E-mail: lys9908@catholic.ac.kr
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Expression features of targets for anti-glioma CAR-T cell immunotherapy
Peng Zhang, Chunzhao Li, Yi Wang, Xiaohan Chi, Tai Sun, Qianhe Zhang, Yang Zhang, Nan Ji
Journal of Neuro-Oncology.2025; 171(1): 179. CrossRef - Expression of Programmed Cell Death-Ligand 1 (PD-L1) in Astrocytic Tumors and Its Correlation With Histopathological Grade and Proliferative Index (Ki-67): A Cross-Sectional Study
Namita Singh, Ranjana Giri, Prita Pradhan, Diptiranjan Satapathy, Ipsita Debata
Cureus.2025;[Epub] CrossRef - Prognostic significance of PD-L1 and CD45RO+ cells in glioblastoma: The modulating role of MMR status
Yousef Mohammadi, Elina Kaviani, Simin Ahmadvand, Amirreza Dehghanian, Abbas Ghaderi
Journal of Neuroimmunology.2025; 406: 578669. CrossRef - PD-L1 Clones and Their Relevance in Glioblastoma, IDH-Wildtype: A Comparative Analysis
Michal Hendrych, Frantisek Vana, Marketa Hermanova, Radek Lakomy, Tomas Kazda, Kvetoslava Matulova, Alena Kopkova, Martina Jelinkova, Radim Jancalek, Martin Smrcka, Vaclav Vybihal, Jiri Sana
Bratislava Medical Journal.2025; 126(9): 2233. CrossRef - Tumor-associated microenvironment, PD-L1 expression and their relationship with immunotherapy in glioblastoma, IDH-wild type: A comprehensive review with emphasis on the implications for neuropathologists
Giuseppe Broggi, Giuseppe Angelico, Jessica Farina, Giordana Tinnirello, Valeria Barresi, Magda Zanelli, Andrea Palicelli, Francesco Certo, Giuseppe Barbagallo, Gaetano Magro, Rosario Caltabiano
Pathology - Research and Practice.2024; 254: 155144. CrossRef - Treatment advances in high-grade gliomas
Xi Chen, Yi Cui, Liqun Zou
Frontiers in Oncology.2024;[Epub] CrossRef - TRP-2 / gp100 DNA vaccine and PD-1 checkpoint blockade combination for the treatment of intracranial tumors
Joshua R. D. Pearson, Carles Puig-Saenz, Jubini E. Thomas, Lydia D. Hardowar, Murrium Ahmad, Louise C. Wainwright, Adam M. McVicar, Victoria A. Brentville, Chris J. Tinsley, A. Graham Pockley, Lindy G. Durrant, Stephanie E. B. McArdle
Cancer Immunology, Immunotherapy.2024;[Epub] CrossRef - Advanced immunotherapies for glioblastoma: tumor neoantigen vaccines in combination with immunomodulators
Berta Segura-Collar, Sara Hiller-Vallina, Olaya de Dios, Marta Caamaño-Moreno, Lucia Mondejar-Ruescas, Juan M. Sepulveda-Sanchez, Ricardo Gargini
Acta Neuropathologica Communications.2023;[Epub] CrossRef - Immunohistochemical Analysis of PD-1 and FOXP3 in Tumor-Infiltrating Lymphocytes in Human Gliomas
Priyanka Kanagaraj, Archana Balasubramanian, Raveena Suresh, Bhargavi Somasundaram, Sandhya Sundaram, Priyathersini Nagarajan
Cureus.2023;[Epub] CrossRef - Expression, prognostic significance and therapeutic implications of PD‐L1 in gliomas
Gayaththri Vimalathas, Bjarne Winther Kristensen
Neuropathology and Applied Neurobiology.2022;[Epub] CrossRef - PD-L1 tumor expression is associated with poor prognosis and systemic immunosuppression in glioblastoma
Carolina Noronha, Ana Sofia Ribeiro, Ricardo Taipa, Dina Leitão, Fernando Schmitt, Joaquim Reis, Cláudia Faria, Joana Paredes
Journal of Neuro-Oncology.2022; 156(3): 453. CrossRef - Assessment of radiographic and prognostic characteristics of programmed death-ligand 1 expression in high-grade gliomas
Makoto Ohno, Shigehisa Kitano, Kaishi Satomi, Akihiko Yoshida, Yasuji Miyakita, Masamichi Takahashi, Shunsuke Yanagisawa, Yukie Tamura, Koichi Ichimura, Yoshitaka Narita
Journal of Neuro-Oncology.2022; 160(2): 463. CrossRef - The prognostic significance of PD-L1 expression in patients with glioblastoma: A meta-analysis
Xin Guo, Yuelin Zhang, Hengxing Jiao, Xingyu Miao
Frontiers in Oncology.2022;[Epub] CrossRef - LncRNA UCA1 attenuated the killing effect of cytotoxic CD8 + T cells on anaplastic thyroid carcinoma via miR-148a/PD-L1 pathway
Xiaoming Wang, Yan Zhang, Jian Zheng, Cuixian Yao, Xiubo Lu
Cancer Immunology, Immunotherapy.2021; 70(8): 2235. CrossRef - Low tumour-infiltrating lymphocyte density in primary and recurrent glioblastoma
Kelsey Maddison, Moira C. Graves, Nikola A. Bowden, Michael Fay, Ricardo E. Vilain, Sam Faulkner, Paul A. Tooney
Oncotarget.2021; 12(21): 2177. CrossRef - A Systematic Review of the Tumor-Infiltrating CD8+ T-Cells/PD-L1 Axis in High-Grade Glial Tumors: Toward Personalized Immuno-Oncology
Mahdi Abdoli Shadbad, Zahra Asadzadeh, Negar Hosseinkhani, Afshin Derakhshani, Nazila Alizadeh, Oronzo Brunetti, Nicola Silvestris, Behzad Baradaran
Frontiers in Immunology.2021;[Epub] CrossRef - Prognostic value of programmed death ligand 1 (PD-L1) in glioblastoma: a systematic review, meta-analysis and validation based on dataset
Huan Wang, Youchao Xiao, Xingguang Ren, Dahai Wan
Bioengineered.2021; 12(2): 10366. CrossRef - Expression of Programmed Cell Death Ligand 1 and Associated Lymphocyte Infiltration in Olfactory Neuroblastoma
Nyall R. London, Lisa M. Rooper, Justin A. Bishop, Haiying Xu, Lydia J. Bernhardt, Masaru Ishii, Christine L. Hann, Janis M. Taube, Evgeny Izumchenko, Daria A. Gaykalova, Gary L. Gallia
World Neurosurgery.2020; 135: e187. CrossRef - CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas
Joseph A. Flores-Toro, Defang Luo, Adithya Gopinath, Matthew R. Sarkisian, James J. Campbell, Israel F. Charo, Rajinder Singh, Thomas J. Schall, Meenal Datta, Rakesh K. Jain, Duane A. Mitchell, Jeffrey K. Harrison
Proceedings of the National Academy of Sciences.2020; 117(2): 1129. CrossRef - Treatment Results for Recurrent Glioblastoma and Alteration of Programmed Death-Ligand 1 Expression After Recurrence
Kyoung Su Sung, Tae Hoon Roh, Ju Hyung Moon, Eui Hyun Kim, Seok-Gu Kang, Se Hoon Kim, Jong Hee Chang
World Neurosurgery.2020; 135: e459. CrossRef - Current advances in PD-1/PD-L1 axis-related tumour-infiltrating immune cells and therapeutic regimens in glioblastoma
Chang Shu, Qingguo Li
Critical Reviews in Oncology/Hematology.2020; 151: 102965. CrossRef - PD-L1 Expression in Glioblastoma, the Clinical and Prognostic Significance: A Systematic Literature Review and Meta-Analysis
Chengcheng Hao, Gang Chen, Huishan Zhao, Yan Li, Jianxin Chen, Hongmei Zhang, Shan Li, Yuze Zhao, Feng Chen, Wenbin Li, Wen G. Jiang
Frontiers in Oncology.2020;[Epub] CrossRef - Checkpoint inhibitor immunotherapy for glioblastoma: current progress, challenges and future outlook
Patrick C. Gedeon, Cosette D. Champion, Kristen E. Rhodin, Karolina Woroniecka, Hanna R. Kemeny, Alexa N. Bramall, Joshua D. Bernstock, Bryan D. Choi, John H. Sampson
Expert Review of Clinical Pharmacology.2020; 13(10): 1147. CrossRef - Current clinical management of elderly patients with glioma
Alessia Pellerino, Francesco Bruno, Valeria Internò, Roberta Rudà, Riccardo Soffietti
Expert Review of Anticancer Therapy.2020; 20(12): 1037. CrossRef - The Prognostic and Therapeutic Value of PD-L1 in Glioma
Ruo Qiao Chen, Feng Liu, Xin Yao Qiu, Xiao Qian Chen
Frontiers in Pharmacology.2019;[Epub] CrossRef - Challenges and potential of PD-1/PD-L1 checkpoint blockade immunotherapy for glioblastoma
Xin Wang, Gaochao Guo, Hui Guan, Yang Yu, Jie Lu, Jinming Yu
Journal of Experimental & Clinical Cancer Research.2019;[Epub] CrossRef - Association Between Programmed Death-Ligand 1 Expression and Clinicopathological Characteristics, Structural Recurrence, and Biochemical Recurrence/Persistent Disease in Medullary Thyroid Carcinoma
Xiao Shi, Peng-Cheng Yu, Bo-Wen Lei, Cui-Wei Li, Yan Zhang, Li-Cheng Tan, Rong-Liang Shi, Jie Wang, Ben Ma, Wei-Bo Xu, Xiao Wang, Jia-Qian Hu, Nai-Si Huang, Wen-Jun Wei, Yu Wang, Tong-Zhen Chen, Yu-Long Wang, Qing-Hai Ji
Thyroid.2019; 29(9): 1269. CrossRef - The Binding of PD-L1 and Akt Facilitates Glioma Cell Invasion Upon Starvation via Akt/Autophagy/F-Actin Signaling
Ruo Qiao Chen, Xiao Hong Xu, Feng Liu, Chun Yang Li, Yuan Jun Li, Xiang Rui Li, Guo Yong Jiang, Feng Hu, Di Liu, Feng Pan, Xin Yao Qiu, Xiao Qian Chen
Frontiers in Oncology.2019;[Epub] CrossRef - Analysis of PD-L1 expression in salivary duct carcinoma with its efficacy as a tumor marker
Yong Ju Lee, Yoon Woo Koh, Sun Och Yoon, Hyang Joo Ryu, Hye Ryun Kim, Hyang Ae Shin
Korean Society for Head and Neck Oncology.2019; 35(1): 13. CrossRef - Prognostic relevance of programmed cell death ligand 1 expression in glioblastoma
Kyu Sang Lee, Kyoungyul Lee, Sumi Yun, Seyoung Moon, Yujun Park, Jung Ho Han, Chae-Yong Kim, Hye Seung Lee, Gheeyoung Choe
Journal of Neuro-Oncology.2018; 136(3): 453. CrossRef - Programmed Death-Ligand 1 Expression and Its Correlation with Lymph Node Metastasis in Papillary Thyroid Carcinoma
Hyo Jung An, Gyung Hyuck Ko, Jeong-Hee Lee, Jong Sil Lee, Dong Chul Kim, Jung Wook Yang, Min Hye Kim, Jin Pyeong Kim, Eun Jung Jung, Dae Hyun Song
Journal of Pathology and Translational Medicine.2018; 52(1): 9. CrossRef - Radiological evaluation of response to immunotherapy in brain tumors: Where are we now and where are we going?
Michele Porcu, Cinzia Solinas, Paolo Garofalo, Evandro de Azambuja, Mario Scartozzi, Karen Willard-Gallo, Matthias Preusser, Luca Saba
Critical Reviews in Oncology/Hematology.2018; 126: 135. CrossRef - The expression of programed death ligand‐1 could be related with unfavorable prognosis in salivary duct carcinoma
Fumihiko Sato, Jun Akiba, Akihiko Kawahara, Yoshiki Naito, Takeharu Ono, Yorihiko Takase, Kazuya Murata, Hideyuki Abe, Tomohiko Yamaguchi, Hiroaki Miyoshi, Yushi Abe, Yutaro Mihara, Masahiko Tanikawa, Momoko Akashi, Hirofumi Kurose, Hirohito Umeno, Hirohi
Journal of Oral Pathology & Medicine.2018; 47(7): 683. CrossRef - Expression Patterns, Prognostic Value, and Intratumoral Heterogeneity of PD-L1 and PD-1 in Thymoma and Thymic Carcinoma
Dwight Owen, Benjamin Chu, Amy M. Lehman, Lakshmanan Annamalai, Jennifer H. Yearley, Konstantin Shilo, Gregory A. Otterson
Journal of Thoracic Oncology.2018; 13(8): 1204. CrossRef - PD-L1 and immune escape: insights from melanoma and other lineage-unrelated malignancies
Noah Frydenlund, Meera Mahalingam
Human Pathology.2017; 66: 13. CrossRef - Clinical Trials Investigating Immune Checkpoint Blockade in Glioblastoma
Russell Maxwell, Christopher M. Jackson, Michael Lim
Current Treatment Options in Oncology.2017;[Epub] CrossRef - Topotecan Decreases the Expression of Programmed Death-Ligand 1 in Glioblastoma Cell Lines; Implications for Immunotherapy
Joshua Bernstock, Daniel Ye, Florian Gessler, Luca Peruzzotti-Jametti, Mark Gilbert, Yves Pommier, Stefano Pluchino, Ichiro Nakano, John Hallenbeck
Matters.2017;[Epub] CrossRef - Relationship between expression of PD-L1 and tumor angiogenesis, proliferation, and invasion in glioma
Song Xue, Man Hu, Peifeng Li, Ji Ma, Li Xie, Feifei Teng, Yufang Zhu, Bingjie Fan, Dianbin Mu, Jinming Yu
Oncotarget.2017; 8(30): 49702. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



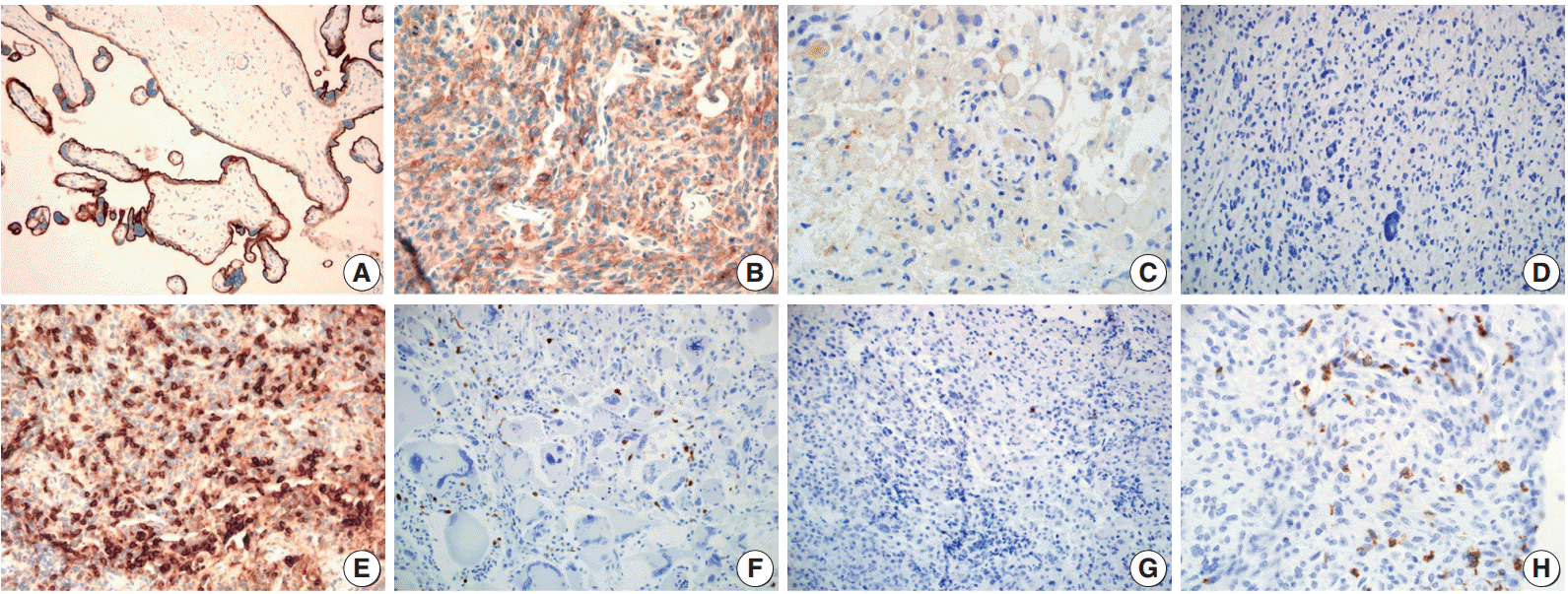
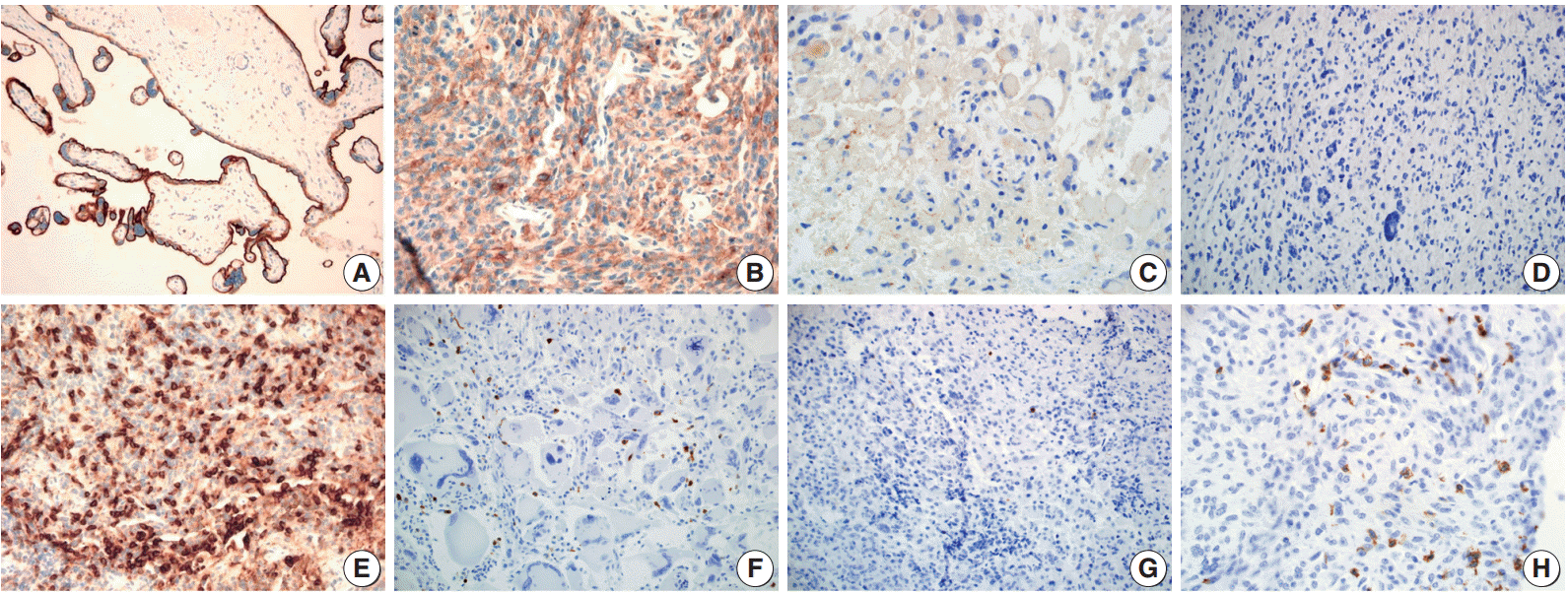
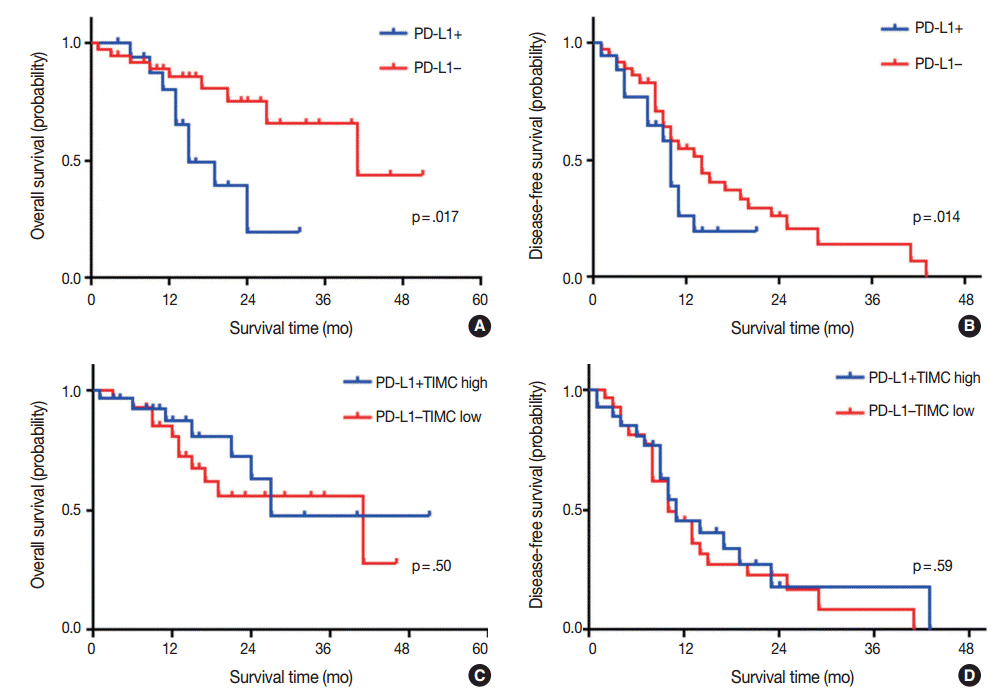
Fig. 1.
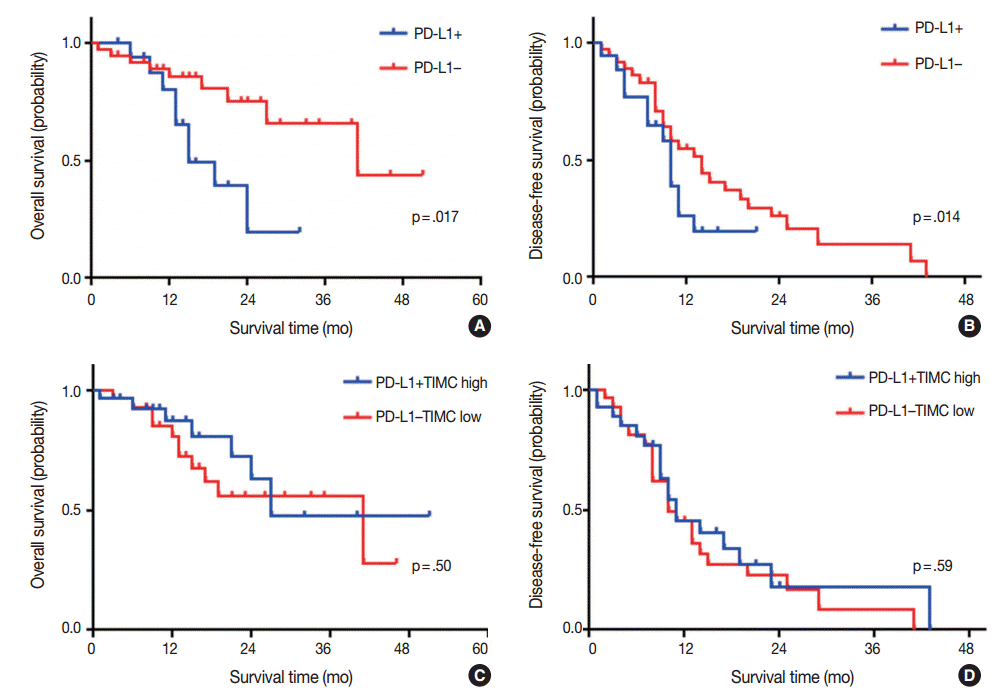
Fig. 2.
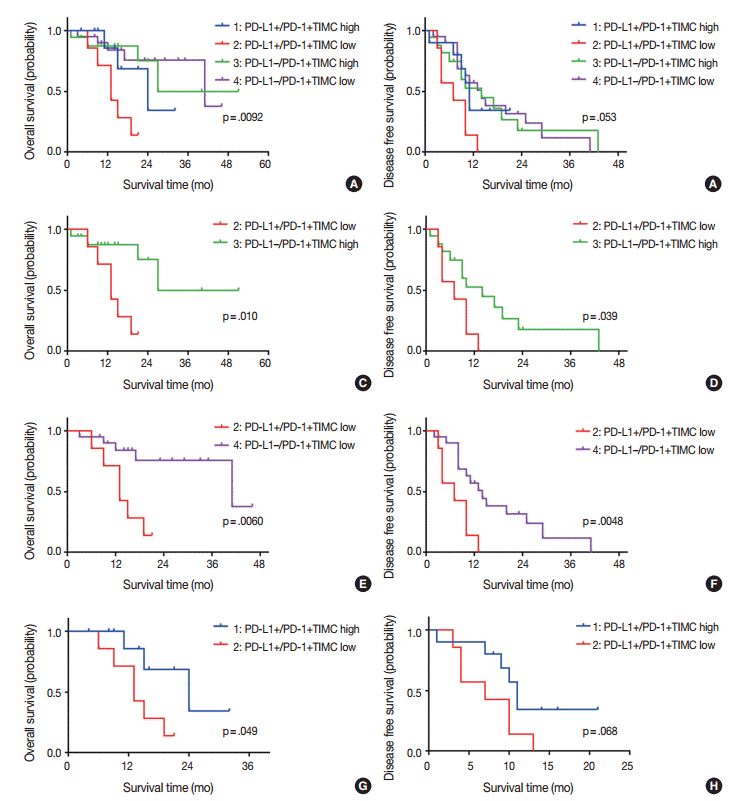
Fig. 3.
| Characteristic | No. (%) |
|---|---|
| Gender | |
| Male | 26 (48.1) |
| Female | 28 (51.9) |
| Primary/Secondary | |
| Primary | 43 (79.6) |
| Secondary | 11 (20.4) |
| Surgical treatment | |
| Total resection | 40 (74.1) |
| Subtotal resection | 11 (20.4) |
| Biopsy and others | 3 (5.6) |
| Adjuvant treatment | |
| CCRT | 43 (79.6) |
| CTx or RTx alone | 9 (16.7) |
| No treatment | 2 (3.7) |
| No. of lesions | |
| Single | 32 (59.3) |
| Multiple (multifocal, multicentric) | 22 (40.7) |
| Alive at last follow-up | |
| Yes | 36 (66.7) |
| No | 18 (33.3) |
| Progress/Recurrence | |
| Yes | 40 (74.1) |
| No | 14 (25.9) |
| Overall survival time, mean (range, mo) | 17.57 (1.0–51.0) |
| Disease free survival, mean (range, mo) | 12.13 (1.0–43.0) |
| All cases (n=54) | PD-L1 |
PD-1+TIMC |
|||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | p-value | Low | High | p-value | ||
| All cases | 37 (68.5) | 17 (31.5) | 27 (50) | 27 (50) | |||
| Gender | .914 | .586 | |||||
| Male | 26 | 18 (69.2) | 8 (30.8) | 14 (53.8) | 12 (46.2) | ||
| Female | 28 | 19 (67.9) | 9 (32.1) | 13 (48.1) | 15 (55.6) | ||
| Age at diagnosis (yr) Mean (min-max) | 57.62 (31–85) | 56.18 (36–78) | .814 | 54.26 (32–77) | 60.07 (31–85) | .115 | |
| Primary/Secondary | .47 | .311 | |||||
| Primary | 43 | 28 (65.1) | 15 (34.9) | 23 (53.5) | 20 (46.5) | ||
| Secondary | 11 | 9 (81.8) | 2 (18.2) | 4 (36.4) | 7 (63.6) | ||
| Surgical treatment | .672 | .804 | |||||
| Total resection | 40 | 26 (65.0) | 14 (35.0) | 19 (47.5) | 21 (52.5) | ||
| Subtotal resection | 11 | 9 (81.8) | 2 (18.2) | 6 (54.5) | 5 (45.5) | ||
| Biopsy and others | 3 | 2 (66.7) | 1 (33.3) | 2 (66.7) | 1 (33.3) | ||
| Adjuvant treatment | .257 | .082 | |||||
| CCRT | 43 | 27 (62.8) | 16 (37.2) | 23 (53.5) | 20 (46.5) | ||
| CTx or RTx alone | 9 | 8 (88.9) | 1 (11.1) | 2 (22.2) | 7 (77.8) | ||
| No treatment | 2 | 2 (100) | 0 | 2 (100) | 0 | ||
| No. of lesions | .713 | .78 | |||||
| Single | 33 | 22 (66.7) | 11 (33.3) | 16 (48.5) | 17 (51.5) | ||
| Multiple | 21 | 15 (71.4) | 6 (28.6) | 11 (52.4) | 10 (47.6) | ||
| Alive at last follow-up | .038 | .248 | |||||
| Yes | 36 | 28 (77.8) | 8 (22.2) | 16 (44.4) | 20 (55.6) | ||
| No | 18 | 9 (50.0) | 9 (50.0) | 11 (61.1) | 7 (38.9) | ||
| Progress/Recurrence | 1 | .214 | |||||
| Yes | 40 | 27 (67.5) | 13 (32.5) | 22 (55.0) | 18 (45.0) | ||
| No | 14 | 10 (71.4) | 4 (28.6) | 5 (35.7) | 9 (64.3) | ||
| Variable | OS |
DFS |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | Hazard ratio (95% CI) | p-value | HR (95% CI) | p-value | |
| PD-L1 expression | ||||||||
| Positive | 3.058 (1.160–8.060) | .024 | 4.958 (1.557–15.79) | .007 | 1.651 (0.821–3.319) | .16 | ||
| Negative | Reference | Reference | Reference | |||||
| PD-1+TIMC | ||||||||
| High | 0.726 (0.280–1.879) | .509 | 0.842 (0.445–1.593) | .597 | ||||
| Low | Reference | Reference | ||||||
| Age (continuous) | 0.989 (0.953–1.026) | .541 | 0.997 (0.973–1.002) | .825 | ||||
| Gender | ||||||||
| Male | 2.000 (0.747–5.360) | .168 | 4.053 (1.230–13.35) | .021 | 1.806 (0.940–3.472) | .076 | 2.142 (1.077–4.260) | .03 |
| Female | Reference | Reference | Reference | Reference | ||||
| Primary vs secondary | ||||||||
| Primary | 1.830 (0.418–8.007) | .423 | 1.023 (0.447–2.340) | .957 | ||||
| Secondary | Reference | Reference | ||||||
| Numver of lesions | ||||||||
| Single | Reference | .133 | Reference | .078 | Reference | .278 | Reference | .092 |
| Multiple | 2.103 (0.797–5.547) | 2.715 (0.893–8.253) | 1.438 (0.746–2.772) | 1.814 (0.907–3.629) | ||||
| Surgical treatment | ||||||||
| Total resection | Reference | Reference | ||||||
| Subtotal resection | 1.134 (0.365–3.528) | .828 | 1.099 (0.497–2.433) | .815 | ||||
| Biopsy and others | 1.453 (0.186–11.34) | .721 | 2.807 (0.838–9.405) | .094 | ||||
| Adjuvant treatment | ||||||||
| CCRT | Reference | Reference | Reference | |||||
| CTx or RTx alone | 1.309 (0.369–4.640) | .677 | 2.369 (0.562–9.989) | .024 | 0.706 (0.245–2.030) | .518 | ||
| No treatment | 7.717 (0.880–67.674) | .065 | 5.760 (2.089–317.6) | .011 | 1.346 (0.181–10.011) | .771 | ||
| Recurrence or progression | 2.238 (0.511–9.795) | .285 | ||||||
| Variable | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| (PD-L1+/high PD-1+TIMC) | (PD-L1+/low PD-1+TIMC) | (PD-L1-/high PD-1+TIMC) | (PD-L1-/low PD-1+TIMC) | |
| No. of patients (%) | 10 (18.5) | 7 (13) | 17 (31.5) | 20 (37) |
| Age, mean (range, yr) | 59.6 (40–70) | 51.3 (36–69) | 60.3 (31–85) | 55.3 (32–68) |
| Ki-67 index, mean (range, %) | 28.3 (10–60) | 41.4 (5–80) | 29.9 (6–55) | 32.3 (4–95) |
| Tumor cellularity | Moderate–marked | Marked | Mild–moderate | Mild–moderate |
| TIMC density | High | Low | High | Low~high |
CCRT, concurrent chemoradiotherapy; CTx, chemotherapy; RTx, radiotherapy.
Values are presented as number (%) unless otherwise indicated. PD-L1, programmed death ligand 1; PD-1, programmed cell death 1; TIMC, tumor infiltrating mononuclear cell; GBM, glioblastoma; CCRT, concurrent chemoradiotheraphy; CTx, chemotherapy; RTx, radiation therapy.
OS, overall survival; DFS, disease free survival; HR, hazard ratio; CI, confidence interval; PD-L1, programmed death ligand 1; PD-1, programmed cell death 1; TIMC, tumor infiltrating mononuclear cell; CCRT, concurrent chemoradiation therapy; CTx, chemotherapy; RTx, radiation therapy.
PD-L1, programmed death ligand 1; PD-1, programmed cell death 1; TIMC, tumor infiltrating mononuclear cell.

 E-submission
E-submission