Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(2); 2017 > Article
-
Original Article
GLUT1 as a Prognostic Factor for Classical Hodgkin’s Lymphoma: Correlation with PD-L1 and PD-L2 Expression - Young Wha Koh, Jae-Ho Han, Seong Yong Park1, Dok Hyun Yoon2, Cheolwon Suh2, Jooryung Huh3
-
Journal of Pathology and Translational Medicine 2017;51(2):152-158.
DOI: https://doi.org/10.4132/jptm.2016.11.03
Published online: February 21, 2017
Department of Pathology, Ajou University School of Medicine, Suwon, Korea
1Department of Thoracic and Cardiovascular Surgery, Ajou University School of Medicine, Suwon, Korea
2Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- Corresponding Author Jooryung Huh, MD Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea Tel: +82-2-3010-4553 Fax: +82-2-472-7898 E-mail: jrhuh@amc.seoul.kr
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Glucose transporter type 1 (GLUT1) expression is linked to glucose metabolism and tissue hypoxia. A recent study reported that GLUT1 was significantly associated with programmed death ligand 1 (PD-L1) as a therapeutic target in relapsed or refractory classical Hodgkin’s lymphoma (cHL). The purpose of this study was to measure the expression of GLUT1 and assess its prognostic significance and potential relationships with PD-L1, programmed death ligand 2 (PD-L2), and programmed death-1 (PD-1) expressions in cHL.
-
Methods
- Diagnostic tissues from 125 patients with cHL treated with doxorubicin, bleomycin, vinblastine, and dacarbazine were evaluated retrospectively via immunohistochemical analysis of GLUT1, PD-L1, PD-L2, and PD-1 expression.
-
Results
- The median follow-up time was 4.83 years (range, 0.08 to 17.33 years). GLUT1, PD-L1, PD-L2, and PD-1 were expressed in 44.8%, 63.2%, 9.6%, and 13.6% of the specimens, respectively. Positive correlations were found between GLUT1 and PD-L1 expression (p = .004) and between GLUT1 and PD-L2 expression (p = .031). GLUT1 expression in Hodgkin/Reed-Sternberg (HRS) cells was not associated with overall survival or event-free survival (EFS) in the entire cohort (p = .299 and p = .143, respectively). A subgroup analysis according to the Ann Arbor stage illustrated that GLUT1 expression in HRS cells was associated with better EFS in advanced-stage disease (p = .029). A multivariate analysis identified GLUT1 as a marginally significant prognostic factor for EFS (p = .068).
-
Conclusions
- This study suggests that GLUT1 expression is associated with better clinical outcomes in advanced-stage cHL and is significantly associated with PD-L1 and PD-L2 expressions.
- Patients
- A retrospective analysis of 125 consecutive patients diagnosed with cHL at Asan Medical Center between 1995 and 2012 and at Ajou University Hospital between 2008 and 2015 was performed. All patients were pathologically confirmed to have cHL and treated with the doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) therapy regimen, with or without radiation. The patients were ≥15 years of age at diagnosis, and they had no previous treatment or history of malignancy. Paraffin-embedded tumor tissues and follow-up data were available for all patients included in the study. The median follow-up time was 4.83 years (range, 0.08 to 17.33 years). Response criteria were based on standard guidelines. Routine follow-up imaging analyses were performed every 3 months for the first 2 years, every 6 months for the next 3 years, and annually (or whenever clinically indicated) thereafter. The results of PD-L1, PD-L2, and PD-1 expressions from a previously reported study were used [27]. The research was approved by the Institutional Review Board of Asan Medical Center.
- Histopathological analysis and immunohistochemistry
- All histological and immunophenotypic data were reviewed by two pathologists (J.H. and Y.W.K.). The cases of cHL were subtyped according to the World Health Organization criteria as nodular sclerosis, lymphocyte-rich, mixed cellularity, lymphocyte-depleted, or not otherwise specified cHL. Tissue microarrays (TMAs) were constructed using three 1-mm-diameter tumor cores from selected areas of formalin-fixed, paraffin-embedded tumor samples. TMA sections were stained using a Benchmark XT automatic immunohistochemistry staining device (Ventana Medical Systems, Tucson, AZ, USA). Anti-GLUT1 (Cell Marque, Rocklin, CA, USA), anti–PD-L1 (Cell Signaling Technology, Danvers, MA, USA), anti–PD-L2 (R&D Systems, Minneapolis, MN, USA), and anti–PD-1 antibodies (Cell Marque) were used. In the TMA, each case was represented by three tissue cores, and at least 10 CD30-positive Hodgkin/Reed-Sternberg (HRS) cells in at least one of the three core cylinders from each patient were read. To minimize the counting of cells, other than small lymphocytes, with nonspecific PD-1 staining we counted only the cells with staining that were morphologically compatible with small lymphocytes in the microenvironment (excluded fibroblasts, endothelial cells, HRS cells, and macrophages) based on their size, shape, and CD30 staining. We examined the protein expression of GLUT1, PD-L1, PD-L2, and PD-1 in 5% increments. The cut-off values of GLUT1, PD-L1, PD-L2, and PD-1 associated with the most significant differences in overall survival (OS) were selected. A sample was considered GLUT1-, PD-L1–, or PD-L2–positive if the expression of these markers was detected in ≥ 20% of HRS cells. A sample was considered PD-1–positive if PD-1 expression was detected in ≥20% of the peritumoral microenvironment. In situ hybridization analysis of Epstein-Barr virus–encoded RNA-1 and RNA-2 was performed and scored as previously described [28].
- Statistical analyses
- Event-free survival (EFS) was defined as the interval between the date of diagnosis and that of disease progression, relapse, or death from any cause. OS was defined as the interval between the date of diagnosis and that of death from any cause. The follow-up of patients was censored at their last follow-up date. Cumulative OS or EFS was analyzed using the Kaplan-Meier method, and comparisons were performed via log-rank testing. Multivariate prognostic analyses was performed with the Cox proportional hazards regression model using the enter method. Categorical variables were compared using the chi-squared test. All statistical analyses were performed using the SPSS statistical software program ver. 18.0 (SPSS Inc., Chicago, IL, USA). All p-values were two-sided associations, and p<.05 was considered statistically significant.
MATERIALS AND METHODS
- Patient characteristics
- The clinical characteristics of the 125 patients are summarized in Table 1. The median age of the patients was 39 years (range, 15 to 78 years). Twenty-five patients died. The median OS was not achieved, and the median EFS was 11 years. The estimated 5-year OS and EFS rates were 84% and 61%, respectively.
- GLUT1 expression in cHL tissues
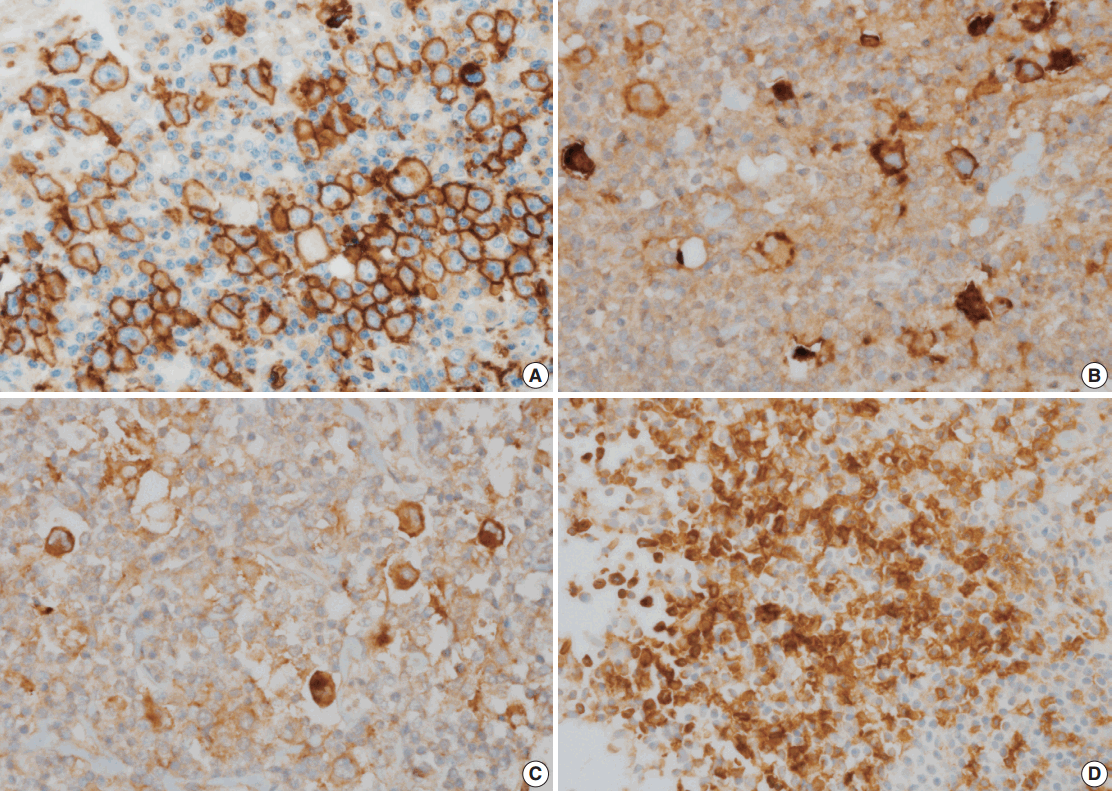
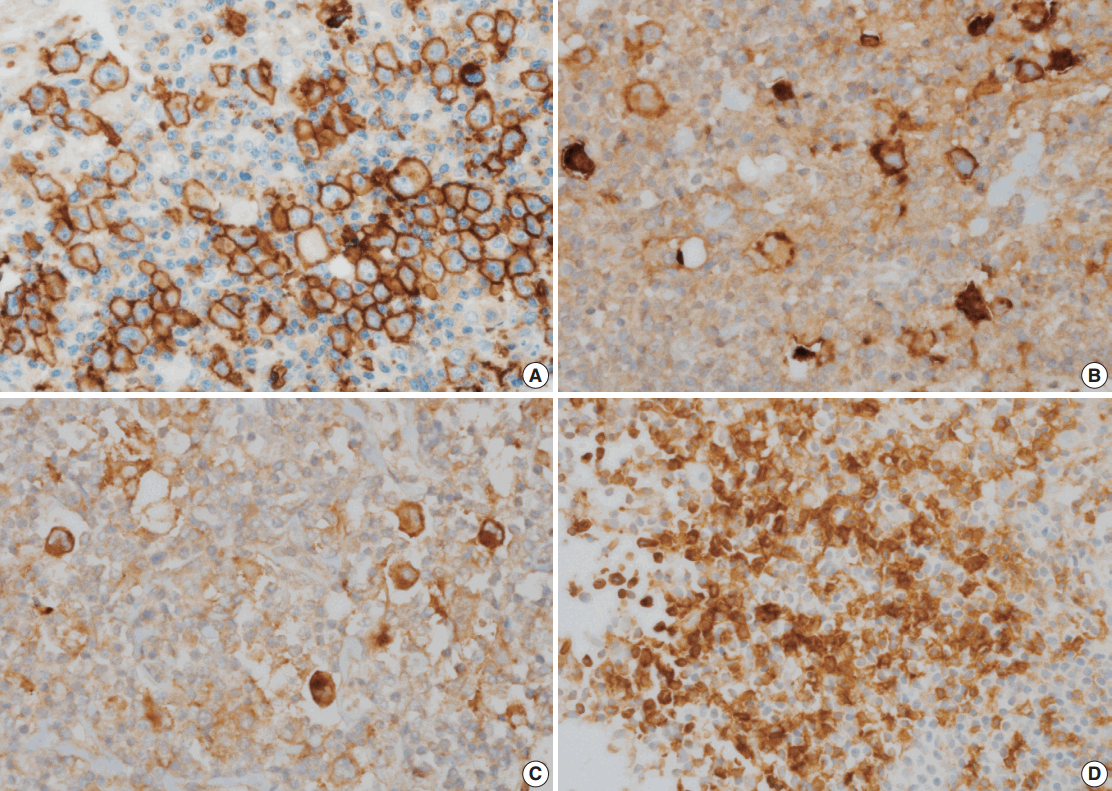
- The correlations between GLUT1 and clinical variables are summarized in Table 2. Fifty-six patients (44.8%) displayed membranous positivity for GLUT1 (Fig. 1A). There was no correlation between GLUT1 expression and clinical variables.
- Correlations of PD-L1, PD-L2, and PD-1 expression with GLUT1 expression
- PD-L1, PD-L2, and PD-1 were expressed in 63.2%, 9.6%, and 13.6% of the specimens, respectively (Fig. 1B–D). The correlations of PD-L1, PD-L2, and PD-1 expression with GLUT1 expression are summarized in Table 3. GLUT1 protein expression was associated with high PD-L1 (p=.004) and high PD-L2 protein expression in HRS cells (p=.031). However, there was no correlation between GLUT1 levels in HRS cells and PD-1 expression in the peritumoral microenvironment (p=.198).
- Prognostic significance of GLUT1 expression
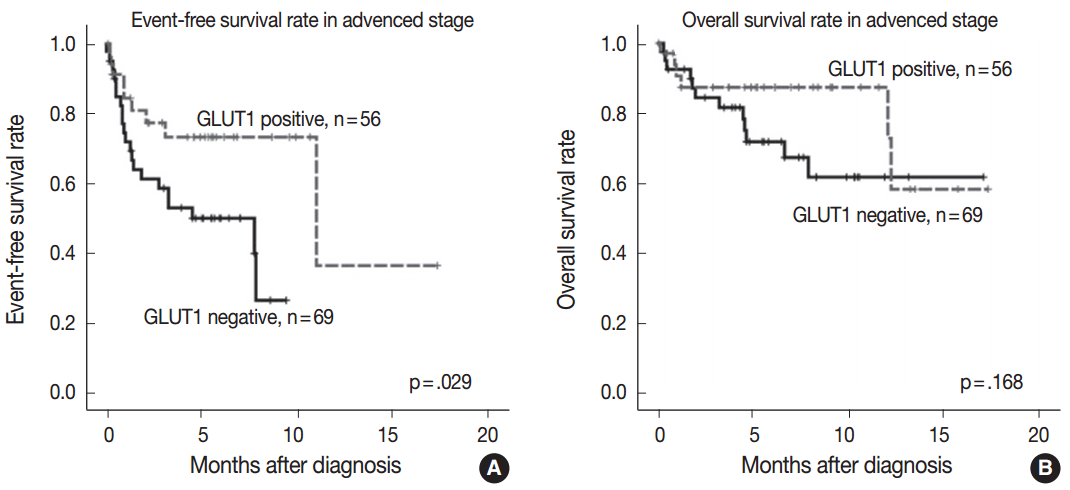
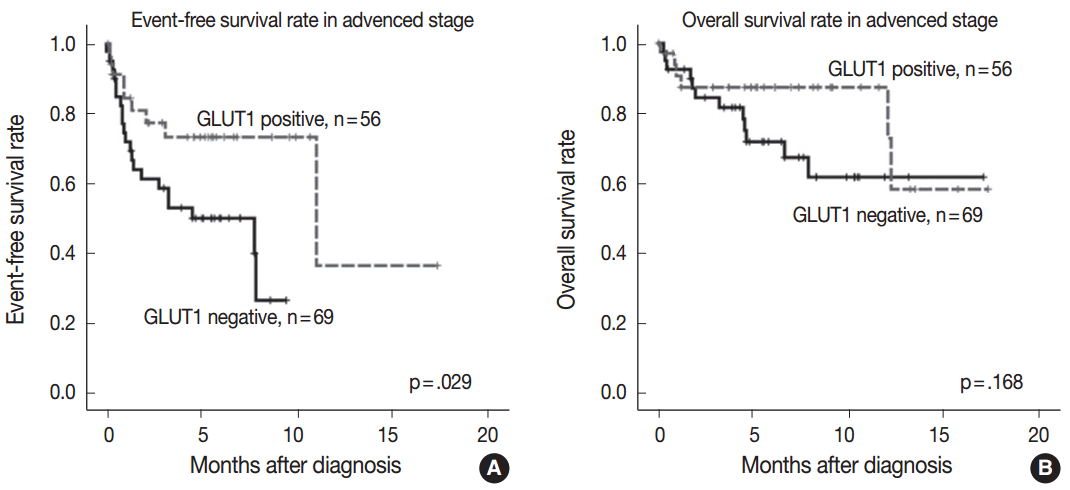
- GLUT1 expression was not associated with EFS and OS rates (p=.143 and p=.299, respectively). As the prognostic significance of the IPS is limited in advanced-stage disease [3], we performed a subgroup analysis according to the disease stage to determine whether GLUT1 expression has prognostic significance in certain stages. In limited-stage cHL, GLUT1 expression was not associated with EFS and OS rates (p=.906 and p=.833, respectively). In advanced-stage cHL, GLUT1 positivity was associated with better EFS (p=.029) (Fig. 2A). However, there was no association between GLUT1 expression and OS in advanced-stage cHL (p=.168) (Fig. 2B).
- Univariate analysis revealed that EFS was associated with advanced age and anemia (Table 4). Multivariate analysis identified GLUT1 as a marginally significant prognostic factor for EFS, together with advanced age and anemia (hazard ratio, 0.462; p=.068) (Table 4).
RESULTS
- In the present study, the expression of GLUT1 was significantly correlated with PD-L1 and PD-L2 expression, supporting the hypothesis that GLUT1-related signaling pathways play an important role in the PD-L1 or PD-L2 pathway. In particular, GLUT1 expression was marginally associated with better EFS in advanced-stage cHL.
- There are several explanations for the relationships between PD-1 pathways and GLUT1 expression. HIF-2α overexpression stabilized by hypoxia increased PD-L1 mRNA and protein levels in a kidney clear cell carcinoma cell line [19]. A previous study also revealed the direct binding of HIF-2α to a transcriptionally active hypoxia-response element in the human PD-L1 proximal promoter [19]. GLUT1 is an important downstream target of HIF-2α [19,22]. Therefore, a significant correlation between GLUT1 and PD-L1 expression was identified in clear cell renal cell carcinoma [24]. In our study, 91% of patients with GLUT1-positive status had PD-L1 positive status, supporting the HIF-2α–Glut1–PD-L1 pathway identified in a previous study. However, 48% of patients with PD-L1–positive status had GLUT1 negative status. Further studies are needed to confirm the precise mechanism of the PD-L1 pathway, regardless of GLUT1 expression, in patients with cHL.
- In the present study, GLUT1 expression was associated with better EFS in advanced-stage cHL, although the result was of borderline statistical significance. GLUT1 expression was not associated with EFS or OS rates in the entire patient cohort. Hartmann et al. [9] reported no prognostic significance for GLUT1 expression in cHL. However, Hartmann et al. [9] did not perform subgroup analysis according to the Ann Arbor stage. Some prognostic biomarkers have limited importance in certain clinical stages. The IPS also has prognostic significance in advanced-stage cHL. Moreover, further research is needed to confirm the prognostic impact of GLUT1 expression.
- Although our results revealed a favorable clinical outcome in patients with GLUT1-positive advanced-stage cHL, previous studies revealed correlations between high GLUT1 expression and poor survival in other solid malignancies [5-8]. In the present study, all patients received the ABVD regimen. Doxorubicin and vinblastine are associated with DNA damage. Doxorubicin inhibits the progression of the enzyme topoisomerase II, which relaxes supercoils in DNA for transcription [29]. Vinblastine can suppress microtubule dynamics, resulting in mitotic block and apoptosis [30]. Therefore, HRS cells with higher proliferation rates would be more susceptible to chemotherapy-associated DNA damage. GLUT1 overexpression increases glucose metabolism in tumors to enhance cellular proliferation in melanoma and gastric cancer [31,32]. Some studies ascribed the superior survival of patients with diffuse large B-cell lymphoma or cHL to increased sensitivity to chemotherapy due to higher cell proliferation rates [33,34]. Although we did not examine the proliferation rates of HRS cells in our series, the superior survival of cHL patients with GLUT1 expression in our study may imply higher proliferation rates of HRS cells.
- Differential expression of GLUT1 was detected according to the histologic type of cHL in a previous study [9]. GLUT1 expression was more rarely observed in HRS cells in the lymphocyterich classical cHL subtype (30%) than in the nodular sclerosis (56%), mixed cellularity (41%), or lymphocyte-depleted subtype (100%). Our series also identified lower expression of GLUT1 in the lymphocyte-rich classical cHL subtype (0%) than in other subtypes.
- A GLUT1-specific inhibitor (STF-31) induces apoptosis and sensitizes multiple myeloma cells to conventional chemotherapeutic agents [26]. A recent study found that the GLUT1-specific inhibitor apigenin induces growth retardation and apoptosis through metabolic and oxidative stress caused by the suppression of glucose utilization in lung cancer and adenoid cystic carcinoma [35]. WZB117 also exerts inhibitory effects on cancer cell growth in breast and lung cancer cell lines [36,37]. Nivolumab has been tested in patients with advanced cHL, and impressive results were obtained in phase I trial [25]. Pembrolizumab, an IgG4 monoclonal antibody directed against PD-1, also induced favorable responses in a heavily pretreated patient with cHL [38]. Although an objective response was reported in 87% of patients with advanced cHL treated with nivolumab, only 17% of patients exhibited complete responses [25]. Based on the significant correlations of GLUT1 with PD-L1 and PD-L2 in this study and a previous study [24], the HIF-2α–GLUT1–PD-L1 pathway may be efficiently inhibited by GLUT1-specific inhibitor and PD-1–specific inhibitor.
- In summary, our results suggest that GLUT1 expression is significantly associated with the expression of PD-L1 and PD-L2. GLUT1 expression has prognostic significance in advanced-stage cHL. Further efforts to understand the mechanisms of the correlations of GLUT1 with PD-L1 and PD-L2 may help the development of effective therapeutic agents for the treatment of cHL.
DISCUSSION
Acknowledgments
| Characteristic |
GLUT1 expression |
p-value | |
|---|---|---|---|
| Low (< 20%) (n = 69) | High (≥ 20%) (n = 56) | ||
| Age (yr) | .858a | ||
| <45 | 38 (55.1) | 32 (57.1) | |
| ≥45 | 31 (44.9) | 24 (42.9) | |
| Gender | .276a | ||
| Male | 44 (63.8) | 30 (53.6) | |
| Female | 25 (36.2) | 26 (46.4) | |
| Disease subtype | .182b | ||
| Nodular sclerosis | 43 (62.3) | 40 (71.4) | |
| Mixed cellularity | 14 (20.3) | 13 (23.2) | |
| Lymphocyte-rich | 6 (8.7) | 0 | |
| Lymphocyte-depleted | 2 (2.9) | 1 (1.8) | |
| Not classifiable | 4 (5.8) | 2 (3.6) | |
| B symptom | .184a | ||
| Absent | 50 (72.5) | 34 (60.7) | |
| Present | 19 (27.5) | 22 (39.3) | |
| Ann Arbor stage | .586a | ||
| Limited | 28 (40.6) | 20 (35.7) | |
| Advanced | 41 (59.4) | 36 (64.3) | |
| IPS | .467a | ||
| < 3 | 44 (63.8) | 32 (57.1) | |
| ≥ 3 | 25 (36.2) | 24 (42.9) | |
| EBER | .467a | ||
| Negative | 44 (63.8) | 32 (57.1) | |
| Positive | 25 (36.2) | 24 (42.9) | |
| Primary treatment | .674a | ||
| Chemotherapy | 51 (73.9) | 44 (78.6) | |
| Chemoradiotherapy | 18 (26.1) | 12 (21.4) | |
| Characteristic |
GLUT1 expression |
p-value | |
|---|---|---|---|
| Low (< 20%) (n = 69) | High (≥ 20%) (n = 56) | ||
| PD-L1 expression in HRS cells | .004a | ||
| Low (< 20%) | 19 (33.3) | 4 (8.9) | |
| High (≥ 20%) | 38 (66.7) | 41 (91.1) | |
| PD-L2 expression in HRS cells | .299a | ||
| Low (< 20%) | 54 (94.7) | 36 (80.0) | |
| High (≥ 20%) | 3 (5.3) | 9 (20.0) | |
| PD-1 expression in peritumoral microenvironment | .198a | ||
| Low (< 20%) | 57 (82.6) | 51 (91.1) | |
| High (≥ 20%) | 12 (17.4) | 5 (8.9) | |
| Covariate | HR | 95% CI | p-valuea | |
|---|---|---|---|---|
| Univariate analysis | ||||
| Age | < 45 yr vs ≥ 45 yr | 3.22 | 1.47–7.05 | .003 |
| Sex | Female vs male | 1.52 | 0.72–3.20 | .271 |
| B symptoms | (–) vs (+) | 1.85 | 0.86–3.96 | .112 |
| EBER | (–) vs (+) | 1.64 | 0.94–2.85 | .081 |
| Lymphopenia | (–) vs (+) | 1.32 | 0.31–5.55 | .706 |
| Leukocytosis | (–) vs (+) | 1.38 | 0.46–4.14 | .563 |
| Hypoalbuminemia | (–) vs (+) | 1.65 | 0.62–4.31 | .311 |
| Anemia | (–) vs (+) | 2.31 | 1.07–4.94 | .032 |
| GLUT1 expression | (-) vs (+) | 0.42 | 0.18–0.94 | .035 |
| Multivariate analysis | ||||
| Age | < 45 yr vs ≥ 45 yr | 2.47 | 1.08–5.61 | .031 |
| Anemia | (–) vs (+) | 1.92 | 0.86–4.24 | .107 |
| GLUT1 expression | (–) vs (+) | 0.46 | 0.20–1.05 | .068 |
- 1. Björkholm M, Axdorph U, Grimfors G, et al. Fixed versus response-adapted MOPP/ABVD chemotherapy in Hodgkin’s disease: a prospective randomized trial. Ann Oncol 1995; 6: 895-9. PubMed
- 2. Quddus F, Armitage JO. Salvage therapy for Hodgkin’s lymphoma. Cancer J 2009; 15: 161-3. ArticlePubMed
- 3. Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease: international prognostic factors project on advanced Hodgkin’s disease. N Engl J Med 1998; 339: 1506-14. PubMed
- 4. Barron CC, Bilan PJ, Tsakiridis T, Tsiani E. Facilitative glucose transporters: implications for cancer detection, prognosis and treatment. Metabolism 2016; 65: 124-39. ArticlePubMed
- 5. Osugi J, Yamaura T, Muto S, et al. Prognostic impact of the combination of glucose transporter 1 and ATP citrate lyase in node-negative patients with non-small lung cancer. Lung Cancer 2015; 88: 310-8. ArticlePubMed
- 6. Pinheiro C, Sousa B, Albergaria A, et al. GLUT1 and CAIX expression profiles in breast cancer correlate with adverse prognostic factors and MCT1 overexpression. Histol Histopathol 2011; 26: 1279-86. PubMed
- 7. Haber RS, Rathan A, Weiser KR, et al. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer 1998; 83: 34-40. ArticlePubMed
- 8. Kawamura T, Kusakabe T, Sugino T, et al. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer 2001; 92: 634-41. ArticlePubMed
- 9. Hartmann S, Agostinelli C, Diener J, et al. GLUT1 expression patterns in different Hodgkin lymphoma subtypes and progressively transformed germinal centers. BMC Cancer 2012; 12: 586.ArticlePubMedPMCPDF
- 10. Shim HK, Lee WW, Park SY, Kim H, Kim SE. Relationship between FDG uptake and expressions of glucose transporter type 1, type 3, and hexokinase-II in Reed-Sternberg cells of Hodgkin lymphoma. Oncol Res 2009; 17: 331-7. ArticlePubMed
- 11. Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer: preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 2010; 37: 430-9. ArticlePubMed
- 12. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26: 677-704. ArticlePubMedPMC
- 13. Zeng Z, Shi F, Zhou L, et al. Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS One 2011; 6: e23621. Article
- 14. Bellmunt J, Mullane SA, Werner L, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol 2015; 26: 812-7. ArticlePubMed
- 15. Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2014; 146: 15-24. ArticlePubMedPMCPDF
- 16. Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther 2014; 7: 567-73. ArticlePubMedPMC
- 17. Droeser RA, Hirt C, Viehl CT, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 2013; 49: 2233-42. ArticlePubMed
- 18. Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology 2014; 143: 512-9. ArticlePubMedPMC
- 19. Messai Y, Gad S, Noman MZ, et al. Renal cell carcinoma programmed death-ligand 1, a new direct target of hypoxia-inducible factor-2 alpha, is regulated by von Hippel-Lindau gene mutation status. Eur Urol 2016; 70: 623-32. ArticlePubMed
- 20. Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211: 781-90. PubMedPMC
- 21. Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 2014; 74: 665-74. ArticlePubMedPDF
- 22. Li QQ, Sun YP, Ruan CP, et al. Cellular prion protein promotes glucose uptake through the Fyn-HIF-2alpha-Glut1 pathway to support colorectal cancer cell survival. Cancer Sci 2011; 102: 400-6. PubMed
- 23. Rapisarda A, Uranchimeg B, Scudiero DA, et al. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res 2002; 62: 4316-24. PubMed
- 24. Ruf M, Moch H, Schraml P. PD-L1 expression is regulated by hypoxia inducible factor in clear cell renal cell carcinoma. Int J Cancer 2016; 139: 396-403. ArticlePubMedPDF
- 25. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015; 372: 311-9. ArticlePubMed
- 26. Matsumoto T, Jimi S, Migita K, Takamatsu Y, Hara S. Inhibition of glucose transporter 1 induces apoptosis and sensitizes multiple myeloma cells to conventional chemotherapeutic agents. Leuk Res 2016; 41: 103-10. ArticlePubMed
- 27. Koh YW, Jeon YK, Yoon DH, Suh C, Huh J. Programmed death 1 expression in the peritumoral microenvironment is associated with a poorer prognosis in classical Hodgkin lymphoma. Tumour Biol 2016; 37: 7507-14. PubMedPDF
- 28. Huh J, Cho K, Heo DS, Kim JE, Kim CW. Detection of Epstein-Barr virus in Korean peripheral T-cell lymphoma. Am J Hematol 1999; 60: 205-14. ArticlePubMed
- 29. Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol 2010; 17: 421-33. ArticlePubMedPMC
- 30. Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer 2004; 4: 253-65. ArticlePubMedPDF
- 31. Koch A, Lang SA, Wild PJ, et al. Glucose transporter isoform 1 expression enhances metastasis of malignant melanoma cells. Oncotarget 2015; 6: 32748-60. PubMedPMC
- 32. Yan S, Wang Y, Chen M, Li G, Fan J. Deregulated SLC2A1 promotes tumor cell proliferation and metastasis in gastric cancer. Int J Mol Sci 2015; 16: 16144-57. ArticlePubMedPMC
- 33. Uddin S, Hussain AR, Ahmed M, et al. Inhibition of c-MET is a potential therapeutic strategy for treatment of diffuse large B-cell lymphoma. Lab Invest 2010; 90: 1346-56. ArticlePubMedPDF
- 34. Dinand V, Malik A, Unni R, Arya LS, Pandey RM, Dawar R. Proliferative index and CD15 expression in pediatric classical Hodgkin lymphoma. Pediatr Blood Cancer 2008; 50: 280-3. ArticlePubMed
- 35. Lee YM, Lee G, Oh TI, et al. Inhibition of glutamine utilization sensitizes lung cancer cells to apigenin-induced apoptosis resulting from metabolic and oxidative stress. Int J Oncol 2016; 48: 399-408. PubMed
- 36. Liu Y, Cao Y, Zhang W, et al. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther 2012; 11: 1672-82. ArticlePubMedPDF
- 37. Zhao F, Ming J, Zhou Y, Fan L. Inhibition of Glut1 by WZB117 sensitizes radioresistant breast cancer cells to irradiation. Cancer Chemother Pharmacol 2016; 77: 963-72. PubMedPDF
- 38. Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol 2016 Jun 27 [Epub]. https://doi.org/10.1200/JCO.2016.67.3467. Article
REFERENCES
Figure & Data
References
Citations

- Transcriptomic and miRNA Signatures of ChAdOx1 nCoV-19 Vaccine Response Using Machine Learning
Jinting Lin, Qinglan Ma, Lei Chen, Wei Guo, Kaiyan Feng, Tao Huang, Yu-Dong Cai
Life.2025; 15(6): 981. CrossRef - Metabolic Reprogramming and Potential Therapeutic Targets in Lymphoma
Yuyang Pang, Tingxun Lu, Zijun Y. Xu-Monette, Ken H. Young
International Journal of Molecular Sciences.2023; 24(6): 5493. CrossRef - Peptide-based PROTAC degrader of FOXM1 suppresses cancer and decreases GLUT1 and PD-L1 expression
Kun Wang, Xiaoyong Dai, Albert Yu, Chunyan Feng, Kewei Liu, Laiqiang Huang
Journal of Experimental & Clinical Cancer Research.2022;[Epub] CrossRef - TIMP-1 Dependent Modulation of Metabolic Profiles Impacts Chemoresistance in NSCLC
Wei Xiao, Pankaj Ahluwalia, Lan Wang, John Howard, Ravindra Kolhe, Amyn M. Rojiani, Mumtaz V. Rojiani
Cells.2022; 11(19): 3036. CrossRef - Hypoxia-related tumor environment correlated with immune infiltration and therapeutic sensitivity in diffuse large B-cell lymphoma
Chen Liu, Lin Liu
Frontiers in Genetics.2022;[Epub] CrossRef - Non-invasive measurement of PD-L1 status and prediction of immunotherapy response using deep learning of PET/CT images
Wei Mu, Lei Jiang, Yu Shi, Ilke Tunali, Jhanelle E Gray, Evangelia Katsoulakis, Jie Tian, Robert J Gillies, Matthew B Schabath
Journal for ImmunoTherapy of Cancer.2021; 9(6): e002118. CrossRef - Tumor immunity is related to 18F‐FDG uptake in thymic epithelial tumor
Hisao Imai, Kyoichi Kaira, Kosuke Hashimoto, Hiroyuki Nitanda, Ryo Taguchi, Akitoshi Yanagihara, Tetsuya Umesaki, Ou Yamaguchi, Atsuto Mouri, Tomonori Kawasaki, Masanori Yasuda, Kunihiko Kobayashi, Hirozo Sakaguchi, Ichiei Kuji, Hiroshi Kagamu
Cancer Medicine.2021; 10(18): 6317. CrossRef - Transcutaneous Carbon Dioxide Decreases Immunosuppressive Factors in Squamous Cell Carcinoma In Vivo
Nanae Yatagai, Takumi Hasegawa, Rika Amano, Izumi Saito, Satomi Arimoto, Daisuke Takeda, Yasumasa Kakei, Masaya Akashi, Peter J. Oefner
BioMed Research International.2021;[Epub] CrossRef - Current Role of Functional Imaging in the Management of Lymphoma
Bruce D. Cheson, Michel Meignan
Current Oncology Reports.2021;[Epub] CrossRef - Diagnostic value of 18F-FDG-PET to predict the tumour immune status defined by tumoural PD-L1 and CD8+tumour-infiltrating lymphocytes in oral squamous cell carcinoma
Maria Togo, Takehiko Yokobori, Kimihiro Shimizu, Tadashi Handa, Kyoichi Kaira, Takaaki Sano, Mariko Tsukagoshi, Tetsuya Higuchi, Satoshi Yokoo, Ken Shirabe, Tetsunari Oyama
British Journal of Cancer.2020; 122(11): 1686. CrossRef - Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape
Xianjie Jiang, Jie Wang, Xiangying Deng, Fang Xiong, Junshang Ge, Bo Xiang, Xu Wu, Jian Ma, Ming Zhou, Xiaoling Li, Yong Li, Guiyuan Li, Wei Xiong, Can Guo, Zhaoyang Zeng
Molecular Cancer.2019;[Epub] CrossRef - Fluorodeoxyglucose uptake is associated with low tumor-infiltrating lymphocyte levels in patients with small cell lung cancer
Norimitsu Kasahara, Kyoichi Kaira, Koichi Yamaguchi, Hiroaki Masubuchi, Hiroaki Tsurumaki, Kenichiro Hara, Yasuhiko Koga, Reiko Sakurai, Tetsuya Higuchi, Tadashi Handa, Tetsunari Oyama, Takehiko Yokobori, Kimihiro Shimizu, Takayuki Asao, Takeshi Hisada
Lung Cancer.2019; 134: 180. CrossRef - MYC Expression and Metabolic Redox Changes in Cancer Cells: A Synergy Able to Induce Chemoresistance
Barbara Marengo, Ombretta Garbarino, Andrea Speciale, Lorenzo Monteleone, Nicola Traverso, Cinzia Domenicotti
Oxidative Medicine and Cellular Longevity.2019; 2019: 1. CrossRef - Sustain, Adapt, and Overcome—Hypoxia Associated Changes in the Progression of Lymphatic Neoplasia
Orsolya Matolay, Gábor Méhes
Frontiers in Oncology.2019;[Epub] CrossRef - Correlation of tumor-related immunity with 18F-FDG-PET in pulmonary squamous-cell carcinoma
Norimitsu Kasahara, Kyoichi Kaira, Pinjie Bao, Tetsuya Higuchi, Yukiko Arisaka, Bilguun Erkhem-Ochir, Noriaki Sunaga, Yoichi Ohtaki, Toshiki Yajima, Takayuki Kosaka, Tetsunari Oyama, Takehiko Yokobori, Takayuki Asao, Masahiko Nishiyama, Yoshito Tsushima,
Lung Cancer.2018; 119: 71. CrossRef - High Serum Level of Soluble Programmed Death Ligand 1 is Associated With a Poor Prognosis in Hodgkin Lymphoma
Xiaofang Guo, Juan Wang, Jietian Jin, Hao Chen, Zijun Zhen, Wenqi Jiang, Tongyu Lin, Huiqiang Huang, Zhongjun Xia, Xiaofei Sun
Translational Oncology.2018; 11(3): 779. CrossRef - Hodgkin lymphoma and imaging in the era of anti-PD-1/PD-L1 therapy
Margarita Kirienko, Martina Sollini, Arturo Chiti
Clinical and Translational Imaging.2018; 6(6): 417. CrossRef - New developments in the pathology of malignant lymphoma: a review of the literature published from January to April 2017
J. Han van Krieken
Journal of Hematopathology.2017; 10(1): 25. CrossRef - Programmed cell death ligands expression in phaeochromocytomas and paragangliomas: Relationship with the hypoxic response, immune evasion and malignant behavior
David J. Pinato, James R. Black, Sebastian Trousil, Roberto E. Dina, Pritesh Trivedi, Francesco A. Mauri, Rohini Sharma
OncoImmunology.2017; 6(11): e1358332. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


Fig. 1.
Fig. 2.
| Characteristics at diagnosis | No. of patients (%) |
|---|---|
| Age, median (range, yr) | 39 (15–78) |
| Male gender | 74 (59.2) |
| Histologic subtype | |
| Nodular sclerosis | 83 (66.4) |
| Mixed cellularity | 27 (21.6) |
| Lymphocyte-rich | 6 (4.8) |
| Lymphocyte-depleted | 3 (2.4) |
| Not classifiable | 6 (4.8) |
| Ann Arbor stage | |
| I | 26 (20.8) |
| II | 41 (32.8) |
| III | 29 (23.2) |
| IV | 29 (23.2) |
| Stage (limited vs advanced) | |
| Limited | 48 (38.4) |
| Advanced | 77 (61.6) |
| B symptoms present | 41 (32.8) |
| International Prognostic Score ≥ 3 (high-risk) | 49 (39.2) |
| EBER positivity | 49 (39.2) |
| Primary treatment | |
| Chemotherapy | 95 (76.0) |
| Chemoradiotherapy | 30 (24.0) |
| Characteristic | GLUT1 expression |
p-value | |
|---|---|---|---|
| Low (< 20%) (n = 69) | High (≥ 20%) (n = 56) | ||
| Age (yr) | .858 |
||
| <45 | 38 (55.1) | 32 (57.1) | |
| ≥45 | 31 (44.9) | 24 (42.9) | |
| Gender | .276 |
||
| Male | 44 (63.8) | 30 (53.6) | |
| Female | 25 (36.2) | 26 (46.4) | |
| Disease subtype | .182 |
||
| Nodular sclerosis | 43 (62.3) | 40 (71.4) | |
| Mixed cellularity | 14 (20.3) | 13 (23.2) | |
| Lymphocyte-rich | 6 (8.7) | 0 | |
| Lymphocyte-depleted | 2 (2.9) | 1 (1.8) | |
| Not classifiable | 4 (5.8) | 2 (3.6) | |
| B symptom | .184 |
||
| Absent | 50 (72.5) | 34 (60.7) | |
| Present | 19 (27.5) | 22 (39.3) | |
| Ann Arbor stage | .586 |
||
| Limited | 28 (40.6) | 20 (35.7) | |
| Advanced | 41 (59.4) | 36 (64.3) | |
| IPS | .467 |
||
| < 3 | 44 (63.8) | 32 (57.1) | |
| ≥ 3 | 25 (36.2) | 24 (42.9) | |
| EBER | .467 |
||
| Negative | 44 (63.8) | 32 (57.1) | |
| Positive | 25 (36.2) | 24 (42.9) | |
| Primary treatment | .674 |
||
| Chemotherapy | 51 (73.9) | 44 (78.6) | |
| Chemoradiotherapy | 18 (26.1) | 12 (21.4) | |
| Characteristic | GLUT1 expression |
p-value | |
|---|---|---|---|
| Low (< 20%) (n = 69) | High (≥ 20%) (n = 56) | ||
| PD-L1 expression in HRS cells | .004 |
||
| Low (< 20%) | 19 (33.3) | 4 (8.9) | |
| High (≥ 20%) | 38 (66.7) | 41 (91.1) | |
| PD-L2 expression in HRS cells | .299 |
||
| Low (< 20%) | 54 (94.7) | 36 (80.0) | |
| High (≥ 20%) | 3 (5.3) | 9 (20.0) | |
| PD-1 expression in peritumoral microenvironment | .198 |
||
| Low (< 20%) | 57 (82.6) | 51 (91.1) | |
| High (≥ 20%) | 12 (17.4) | 5 (8.9) | |
| Covariate | HR | 95% CI | p-value |
|
|---|---|---|---|---|
| Univariate analysis | ||||
| Age | < 45 yr vs ≥ 45 yr | 3.22 | 1.47–7.05 | .003 |
| Sex | Female vs male | 1.52 | 0.72–3.20 | .271 |
| B symptoms | (–) vs (+) | 1.85 | 0.86–3.96 | .112 |
| EBER | (–) vs (+) | 1.64 | 0.94–2.85 | .081 |
| Lymphopenia | (–) vs (+) | 1.32 | 0.31–5.55 | .706 |
| Leukocytosis | (–) vs (+) | 1.38 | 0.46–4.14 | .563 |
| Hypoalbuminemia | (–) vs (+) | 1.65 | 0.62–4.31 | .311 |
| Anemia | (–) vs (+) | 2.31 | 1.07–4.94 | .032 |
| GLUT1 expression | (-) vs (+) | 0.42 | 0.18–0.94 | .035 |
| Multivariate analysis | ||||
| Age | < 45 yr vs ≥ 45 yr | 2.47 | 1.08–5.61 | .031 |
| Anemia | (–) vs (+) | 1.92 | 0.86–4.24 | .107 |
| GLUT1 expression | (–) vs (+) | 0.46 | 0.20–1.05 | .068 |
EBER, Epstein-Barr virus–encoded RNA-1 and RNA-2 assessed by
Values are presented as number (%). GLUT1, glucose transporter 1; IPS, International Prognostic Score; EBER, Epstein-Barr virus-encoded RNA-1 and RNA-2 assessed by Chi-squared test by two-sided Pearson’s test; Chi-squared test by two-sided Fisher test.
Values are presented as number (%). GLUT1, glucose transporter type 1; PD-L1, programmed death ligand 1; PD-L2, programmed death ligand 2; PD-1, programmed death-1; HRS, Hodgkin/Reed–Sternberg. Chi-squared test by two-sided Pearson’s test.
HR, hazard ratio; CI, confidence interval; EBER, Epstein-Barr virus–encoded RNA-1 and RNA-2 assessed by Cox univariate analysis.

 E-submission
E-submission