Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(4); 2017 > Article
-
Case Study
Mammary Carcinoma Arising in Microglandular Adenosis: A Report of Five Cases - Mimi Kim1, Milim Kim1,2, Yul Ri Chung1, So Yeon Park1,2
-
Journal of Pathology and Translational Medicine 2017;51(4):422-427.
DOI: https://doi.org/10.4132/jptm.2016.11.11
Published online: April 4, 2017
1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
2Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea
- Corresponding Author So Yeon Park, MD, PhD Department of Pathology, Seoul National University Bundang Hospital, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea Tel: +82-31-787-7712 Fax: +82-31787-4012 E-mail: sypmd@snu.ac.kr
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Mammary carcinoma arising in microglandular adenosis (MGA) is extremely rare, and MGA is regarded as a non-obligate precursor of triple-negative breast cancer. We report five cases of carcinoma arising in MGA of the breast. All cases showed a spectrum of proliferative lesions ranging from MGA to atypical MGA, ductal carcinoma in situ or invasive carcinoma. Immunohistochemically, all cases were triple-negative and expression of S-100 protein gradually decreased as the lesions progressed from MGA to atypical MGA and carcinoma. Three cases showed acinic cell differentiation with reactivity to α1-antitrypsin, and one case was metaplastic carcinoma. During clinical follow-up, one patient developed local recurrence. Carcinoma arising in MGA is a rare but distinct subset of triple-negative breast cancer with characteristic histologic and immunohistochemical findings.
- By searching the electronic database, we found five cases of MGACA (including an in situ lesion). The clinical characteristics of these five are summarized in Table 1. The median age of the patients at the time of the diagnosis was 47 years (range, 40 to 60 years). All patients underwent breast conserving surgery. Two patients had lymph node metastasis on pathologic examination and four received adjuvant chemo-radiation therapy. During follow-up, one patient (case 1) developed recurrence in the ipsilateral breast and axillary lymph nodes 1 year after the surgery. The rest of the patients had no evidence of disease and are being regularly followed up. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (protocol # B-1609-364-701), and informed consent was waived.
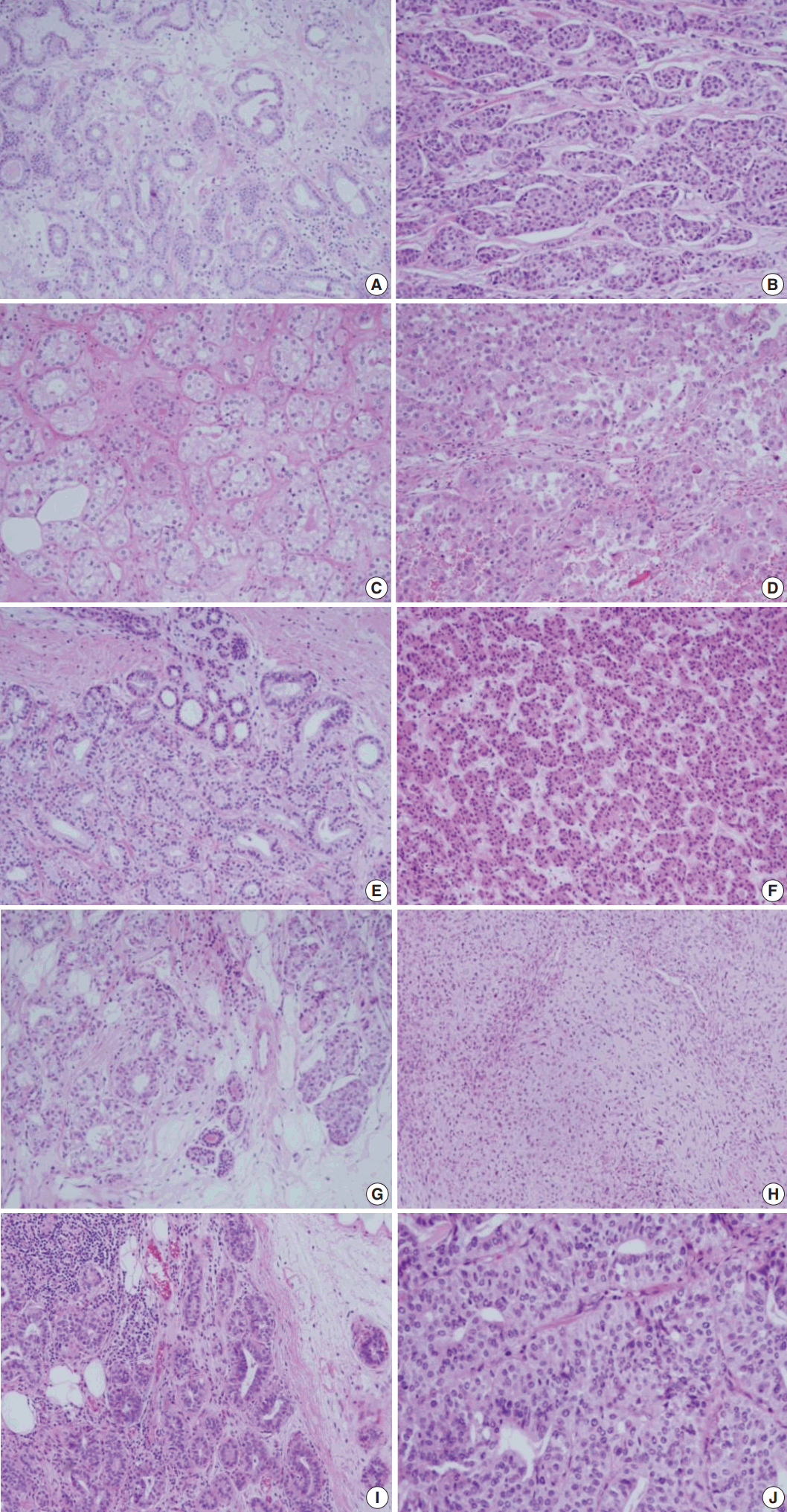
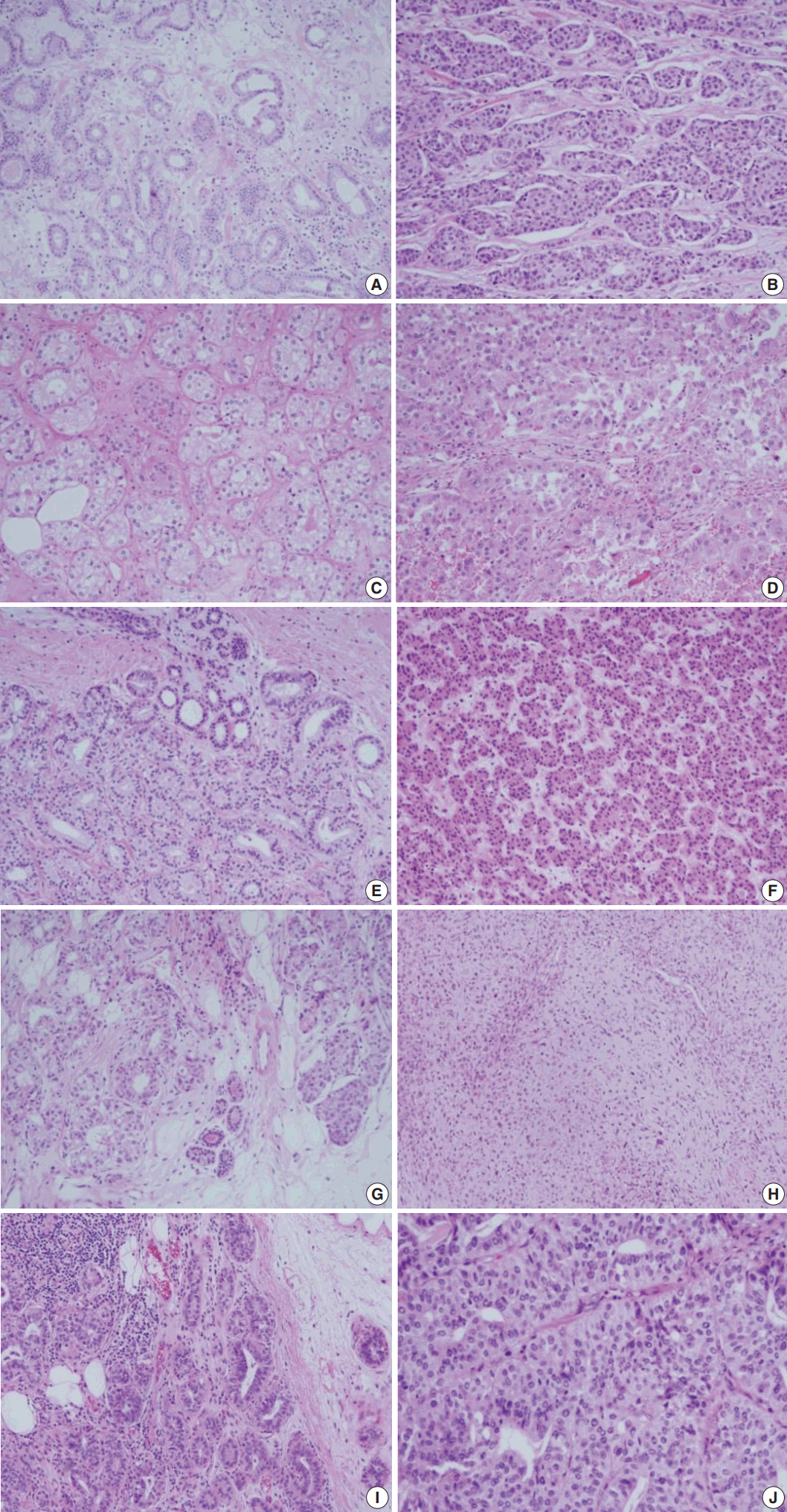
- The pathologic findings and the results of the immunohistochemical studies are shown in the Table 2. One patient (case 5) was diagnosed as ductal carcinoma in situ (DCIS) and the rest of the cases were all invasive carcinomas. The lesions in these five cases displayed the histologic spectrum ranging from MGA to AMGA and finally to MGACA (Fig. 1). The MGA areas had the typical structure: groups of small round glands infiltrating the stroma of the breast and adipose tissue. Glands were single layered and lined by an intact basement membrane, which can be highlighted by periodic acid–Schiff (PAS) and reticulin stains. However, myoepithelium was absent, as revealed by the absence of immunohistochemical reactivity for calponin. In the lumens of the glands, eosinophilic secretions were common.
- In areas diagnosed as AMGA, glands became irregular in shape and cytologically, the nuclear pleomorphism or atypism increased as the amount of eosinophilic secretions decreased. Furthermore, the proliferating atypical cells filled the lumens completely to form carcinoma in situ lesions and finally grew to break the basement membrane to invade the stroma which can be visualized as interrupted and discontinued strands of fibers on PAS and reticulin stains.
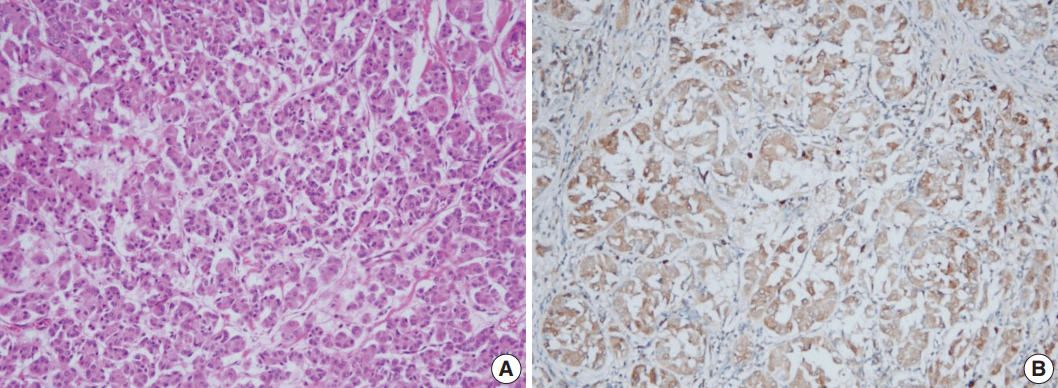
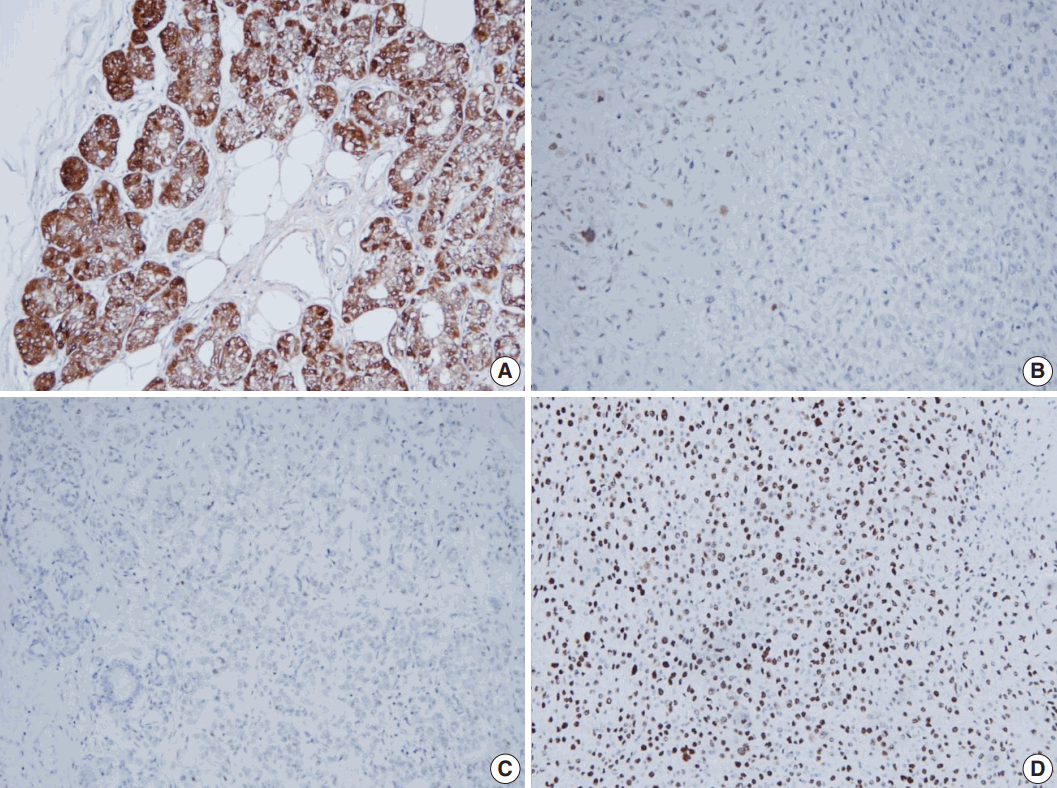
- Even though this histological spectrum occurred in all four cases of the invasive cancers, the invasive components of some cases had unique morphologic features to be sub-classified separately. Cases 2 and 3 had some characteristic features in the glands, which were similar to those of the pancreas or of the salivary gland. These specific glands tend to make acinar structures with abundant eosinophilic cytoplasmic granules. Case 5, the DCIS lesion, also showed acinic cell differentiation. The acinic cell differentiation in these three cases was confirmed by the immunohistochemical staining for α1-antitrypsin and chymotrypsin (Table 2, Fig. 2). Case 4 was diagnosed as metaplastic carcinoma, specifically a matrix-producing carcinoma showing an abrupt transition from the typical invasive carcinoma to spindle cell carcinoma with chondromyxoid matrix.
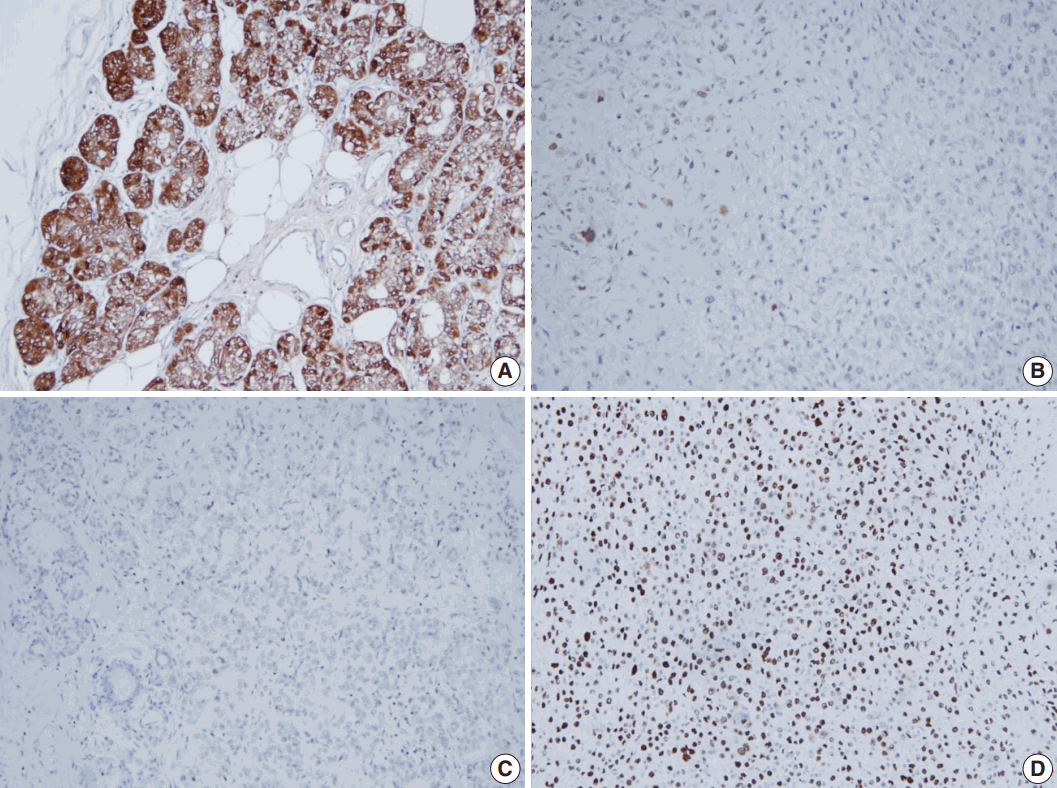
- All tumor cells of MGACA in our cases were negative to estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2, that is, triple-negative. For the immunohistochemical study with S-100 protein, which is the most well-known immunohistochemical marker for MGA lesions, we used a polyclonal antibody which is mainly reactive to S-100b subunit. The reactivity gradually decreased as the lesions progressed from MGA to AMGA and MGACA, showing near negativity in some areas of invasive carcinoma (Fig. 3). On the other hand, Ki-67 index increased form MGA to AMGA and finally to MGACA, as expected. p53 reactivity was negative in four cases except for case 4. For cytokeratin 5/6, four cases including one DCIS case demonstrated at least focal positivity (Table 2).
CASE REPORT
- The MGA differs substantially from other adenosis lesions in many aspects including the immunohistochemical properties. The myoepithelial linings, which disappear in MGA, resulted in negative reactivity to the myoepithelial markers such as calponin and p63. In our case, calponin was all negative in the MGA lesions as well as in the associated carcinoma. The PAS and reticulin staining instead revealed the intact and regular linings of the basement membranes of MGA. These staining patterns therefore could be used to distinguish the invasive portion from the DCIS or other AMGA lesions. The invasive cancers showed the irregular and discontinuous stromal fibers around tumor cell nests.
- Through the immunohistochemical studies, we could detect some phenotypic changes from MGA to MGACA. Ki-67 index increased in a stepwise manner as the diseases progressed. Especially in case 4, p53 overexpression gradually increased during transition from AMGA to MGACA. In contrast, the positivity of S-100 protein, the most unique immunohistochemical property of MGA, diminished gradually as the disease progressed to MGACA.
- However, some studies reported positive results with S-100 protein in carcinoma portions as strong as in the pure MGA lesions [5,6]. This discordance may have resulted from the use of different members of S-100 protein, which is known to have at least 21 types currently [7]. In a study of MGACA, Koenig et al. [3] used two separate antibodies of S-100 protein, S-100a and S-100b for immunohistochemical characterization of MGACA, and reported that S-100b protein showed decreased reactivity, which is similar to our result. They suggested that this finding might be derived from the different localizations of the S-100a and S-100b, which are ductal cytoplasm and myoepithelium, respectively [3]. However, as MGA shows immunoreactivity to S-100b, it is plausible that epithelial cells of MGA pose myoepithelial cell features and this characteristic may be lost during the progression from MGA to MGACA.
- The various histologic and immunohistochemical features of MGACA in our cases have some points of interest. Among the various molecular subtypes of the triple-negative breast cancer [8], the metaplastic carcinoma case resembles mainly the mesenchymal subtype because it had chondromyxoid and spindle cell areas and was highly proliferative (Ki-67 index, 80%). Several MGACAs of our cases demonstrated acinic cell differentiation. Interestingly, two invasive cancers with acinic cell differentiation were all positive for α1-antitrypsin and totally negative for chymotrypsin. The DCIS case with acinic cell differentiation showed weak positivity for α1-antitrypsin. These findings are reminiscent of acinic cell carcinoma of the salivary gland rather than that of the pancreas, which is usually positive for chymotrypsin and α1-antichymotrypsin and almost always negative for α1-antitrypsin. It is also consistent with the fact that the traditional acinic cell carcinomas of the breast look similar to that of the salivary gland. Large proportions of the acinic cell carcinomas of the breast reported in articles share many aspects in common with our cases both histologically and immunohistochemically; this suggests that most acinic cell carcinomas originated in breast may arise from MGA lesions.
- The molecular characteristics of MGA and MGACA are currently being investigated [9-12] and one study reported some shared genetic mutations between MGA and MGACA in four out of 12 cases [11]. The overlapped sequences included recurrent gains on 1q, 2q, and 8q and losses in 14q. They suggested that MGA might be a non-obligate precursor to a specific subgroup of high grade triple-negative breast carcinomas [11].
- As the histologic spectrum of the MGA associated lesions (from the MGA to AMGA and MGACA) becomes a frequent finding, some researchers suggested a new term, “microglandular adenoma” instead of “microglandular adenosis” for its potential to become a cancer [9]. According to their opinions, MGA seems to be more similar to the adenoma of the gastrointestinal tract rather than other adenosis lesions of the breast in terms of the possibility to progress to malignancies.
- In conclusion, MGACA is one of the rare breast cancers and has many unique features that are worth classifying. Further studies will be needed to find the association between the genetic backgrounds and the characteristics of the lesions.
DISCUSSION
- 1. Rosen PP. Rosen’s breast pathology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2001.
- 2. Rosenblum MK, Purrazzella R, Rosen PP. Is microglandular adenosis a precancerous disease? A study of carcinoma arising therein. Am J Surg Pathol 1986; 10: 237-45. PubMed
- 3. Koenig C, Dadmanesh F, Bratthauer GL, Tavassoli FA. Carcinoma arising in microglandular adenosis: an immunohistochemical analysis of 20 intraepithelial and invasive neoplasms. Int J Surg Pathol 2000; 8: 303-15. ArticlePubMedPDF
- 4. Khalifeh IM, Albarracin C, Diaz LK, et al. Clinical, histopathologic, and immunohistochemical features of microglandular adenosis and transition into in situ and invasive carcinoma. Am J Surg Pathol 2008; 32: 544-52. ArticlePubMed
- 5. Shui R, Bi R, Cheng Y, Lu H, Wang J, Yang W. Matrix-producing carcinoma of the breast in the Chinese population: a clinicopathological study of 13 cases. Pathol Int 2011; 61: 415-22. ArticlePubMed
- 6. Shui R, Yang W. Invasive breast carcinoma arising in microglandular adenosis: a case report and review of the literature. Breast J 2009; 15: 653-6. ArticlePubMed
- 7. Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun 2004; 322: 1111-22. ArticlePubMed
- 8. Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011; 121: 2750-67. ArticlePubMedPMC
- 9. Geyer FC, Kushner YB, Lambros MB, et al. Microglandular adenosis or microglandular adenoma? A molecular genetic analysis of a case associated with atypia and invasive carcinoma. Histopathology 2009; 55: 732-43. ArticlePubMed
- 10. Shin SJ, Simpson PT, Da Silva L, et al. Molecular evidence for progression of microglandular adenosis (MGA) to invasive carcinoma. Am J Surg Pathol 2009; 33: 496-504. ArticlePubMed
- 11. Geyer FC, Lacroix-Triki M, Colombo PE, et al. Molecular evidence in support of the neoplastic and precursor nature of microglandular adenosis. Histopathology 2012; 60: E115-30. ArticlePubMed
- 12. Tsang JY, Tse GM. Microglandular adenosis: a prime suspect in triple-negative breast cancer development. J Pathol 2016; 239: 129-32. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- Microglandular adenosis associated with invasive breast carcinoma: Tertiary care oncology centre experience of an under-recognized entity
Ayushi Sahay, Asawari Patil, Trupti Pai, Poonam Panjwani, Shalaka Joshi, Palak Popat Thakkar, Sangeeta B. Desai, Tanuja M. Shet
Annals of Diagnostic Pathology.2026; 80: 152576. CrossRef - Two similar but distinct types of breast acinar cell carcinoma: evidence from histological, immunohistochemical and molecular features
Mingfang Sun, Lin Fu, Hongjiu Ren, Jian Wang, Xuyong Lin, Qingfu Zhang
Histopathology.2025; 87(6): 904. CrossRef - Acinic Cell Carcinoma of the Breast: A Population‐Based Clinicopathologic Study
Faruk Skenderi, Giridhara Rathnaiah Babu, Una Glamoclija, Emir Veledar, Zoran Gatalica, Janez Lamovec, Semir Vranic
Cancer Reports.2025;[Epub] CrossRef - Acinic Cell Carcinoma of the Breast: A Case Report and Review of Literature
Ihab S Atta
Cureus.2024;[Epub] CrossRef - Histological and electron microscopic features of the extracellular matrix of invasive breast ductal carcinoma of no special type. Report of 5 cases and literature review
M.V. Mnikhovich, A.V. Romanov, T.M. Nguyen, T.V. Bezuglova, D.A. Pastukhova
Ultrastructural Pathology.2023; 47(4): 261. CrossRef - Ductal carcinoma in situ arises from microglandular adenosis and atypical microglandular adenosis in a young woman
Nguyen Thu Huong, Tran-Thi Hue, Nguyen Duy Hung, Nguyen Minh Duc
Journal of Clinical Imaging Science.2023; 13: 15. CrossRef - Primary acinic cell carcinoma of the breast: A case report and literature review
Zhi-Min Deng, Yi-Ping Gong, Feng Yao, Ma-Li Wu, Zi-Tao Wang, Jing-Ping Yuan, Yan-Xiang Cheng
Heliyon.2023; 9(9): e20160. CrossRef - Two cases of mammary acinic cell carcinomas with microglandular structures mimicking microglandular adenosis
Fang Yu, Li Niu, Bicheng Wang, Wei Fan, Jian Xu, Qiongrong Chen
Pathology International.2022; 72(6): 343. CrossRef - Metaplastic Matrix-Producing Carcinoma and Apocrine Lobular Carcinoma In Situ Associated with Microglandular Adenosis: A Unique Case Report
Nektarios Koufopoulos, Dionysios Dimas, Foteini Antoniadou, Kyparissia Sitara, Dimitrios Balalis, Ioannis Boutas, Alina Roxana Gouloumis, Adamantia Kontogeorgi, Lubna Khaldi
Diagnostics.2022; 12(6): 1458. CrossRef - Salivary gland-like breast tumors: A review of diagnostic features and prognosis
Vicente Marco
Revista de Senología y Patología Mamaria.2022; 35: S43. CrossRef - Acinic cell carcinoma of the breast: A comprehensive review
Azra Ajkunic, Faruk Skenderi, Nada Shaker, Saghir Akhtar, Janez Lamovec, Zoran Gatalica, Semir Vranic
The Breast.2022; 66: 208. CrossRef - Unusual cerebrospinal fluid finding of intracytoplasmic granules in metaplastic carcinoma of the breast with acinar differentiation
Ruhani Sardana, Anil V Parwani, Xiaoyan Cui, Jayalakshmi Balakrishna
Diagnostic Cytopathology.2021;[Epub] CrossRef - Salivary gland-type mammary carcinoma arising in microglandular adenosis: A case report and clinicopathological review of the literature
Victoria Rico, Yukiko Shibahara, Marjorie Monteiro, Elzbieta Slodkowska, Samantha Tam, Pearl Zaki, Carlo De Angelis, Edward Chow, Katarzyna Joanna Jerzak
Cancer Treatment and Research Communications.2020; 24: 100178. CrossRef - Triple negative metaplastic breast carcinoma presenting in the background of atypical microglandular adenosis with candidacy for atezolizumab immunotherapy
Hector Chavarria, Sean Hacking, Cao Jin, Nidhi Kataria, Florin Glodan, Tawfiqul Bhuiya, Mansoor Nasim
Human Pathology: Case Reports.2020; 21: 200398. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1.
Fig. 2.
Fig. 3.
| Characteristic | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Age (yr) | 40 | 38 | 47 | 46 | 60 |
| Location | Left | Left | Left | Left | Left |
| T stage (size of tumor, cm) | T1c (2.0) | T2 (2.3) | T1c (1.2) | T2 (2.5) | Tis (4.5) |
| N stage | N1a | N0 | N1a | N0 | N0 |
| Type of surgery | BCS | BCS | BCS | BCS | BCS |
| Adjuvant CRTx | Received | Received | Received | Received | Not received |
| Disease status | Local recurrence | NED | NED | NED | NED |
| Characteristic | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Histologic subtype | Invasive carcinoma, NST | Invasive carcinoma, NST | Invasive carcinoma, NST | Metaplastic carcinoma | Ductal carcinoma in situ |
| Acinic cell differentiation | Absent | Present | Present | Absent | Present |
| Estrogen receptor | Negative | Negative | Negative | Negative | Negative |
| Progesterone receptor | Negative | Negative | Negative | Negative | Negative |
| HER-2 | Negative | Negative | Negative | Negative | Negative |
| S-100 protein | Decreased | Decreased | Decreased | Decreased | Intact |
| Cytokeratin 5/6 | Positive | Positive | Positive | Negative | Positive |
| p53 | Negative | Negative | Negative | Positive | Negative |
| Ki-67 index (%) | 50 | 20 | 30 | 80 | 5 |
| α1-Antitrypsin | Negative | Positive | Positive | Negative | Positive |
| Chymotrypsin | Negative | Negative | Negative | Negative | Negative |
MGACA, carcinoma arising in microglandular adenosis; BCS, breast-conserving surgery; CRTx, chemo-radiation therapy; NED, no evidence of disease.
MGACA, carcinoma arising in microglandular adenosis; NST, no special type; HER-2, human epidermal growth factor receptor 2.

 E-submission
E-submission