Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 52(3); 2018 > Article
-
Original Article
The Major Role of NF-κB in the Depth of Invasion on Acral Melanoma by Decreasing CD8+ T Cells - Hermin Aminah Usman, Bethy S. Hernowo, Maringan Diapari Lumban Tobing1, Reti Hindritiani2
-
Journal of Pathology and Translational Medicine 2018;52(3):164-170.
DOI: https://doi.org/10.4132/jptm.2018.04.04
Published online: April 20, 2018
Department of Anatomical Pathology, Universitas Padjadjaran/Hasan Sadikin General Hospital, Bandung, Indonesia
1Department of Obstetrics and Gynecology, Universitas Padjadjaran/Hasan Sadikin General Hospital, Bandung, Indonesia
2Department of Dermatovenerology, Faculty of Medicine, Universitas Padjadjaran/Hasan Sadikin General Hospital, Bandung, Indonesia
- Corresponding Author Hermin Aminah Usman, MD Department of Anatomy Pathology, Faculty of Medicine, Universitas Padjadjaran, Jl. Eyckman No 38, Bandung, Indonesia Tel: +62-222551126 Fax: +62-222031447 E-mail: hermin@unpad.ac.id
© 2018 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- The tumor microenvironment including immune surveillance affects malignant melanoma (MM) behavior. Nuclear factor κB (NF-κB) stimulates the transcription of various genes in the nucleus and plays a role in the inflammatory process and in tumorigenesis. CD8+ T cells have cytotoxic properties important in the elimination of tumors. However, inhibitory receptors on the cell surface will bind to programmed death-ligand 1 (PD-L1), causing CD8+ T cells to lose their ability to initiate an immune response. This study analyzed the association of NF-κB and PD-L1 expression levels and CD8+ T-cell counts with depth of invasion of acral MM, which may be a predictor of aggressiveness related to an increased risk of metastasis.
-
Methods
- A retrospective cross-sectional study was conducted in the Department of Anatomical Pathology, Faculty of Medicine, Universitas Padjadjaran/Hasan Sadikin Hospital using 96 cases of acral melanoma. Immunohistochemical staining was performed on paraffin blocks using anti–NF-κB, –PD-L1, and -CD8 antibodies and invasion depth was measured using dotSlide-imaging software.
-
Results
- The study showed significant associations between the individual expression of NF-κB and PD-L1 and CD8+ T-cell number, with MM invasion depth. NF-κB was found to be a confounding variable of CD8+ T-cell number (p < .05), but not for PD-L1 expression (p = .154). Through multivariate analysis it was found that NF-κB had the greatest association with the depth of invasion (p < .001), whereas PD-L1 was unrelated to the depth of invasion because it depends on the number of CD8+ T cells (p = .870).
-
Conclusions
- NF-κB plays a major role in acral MM invasion, by decreasing the number of CD8+ T cells in acral MM.
- Paraffin blocks from patients who had undergone excisional and surgical biopsies and had been diagnosed histopathologically as having acral MM between 1 January 2011 and 31 December 2016 were used in this study. Ethical clearance was approved by the Health Research Ethics Committee of the Faculty of Medicine Universitas Padjadjaran with a waiver of informed consent (1155/UN6.C1.3.2/KEPK/PN/2016).
- Acral melanoma was defined as a melanoma located on the non-hair bearing skin of the palms and soles or under the nails, which has histopathological features of both acral lentiginous melanoma and subtypes such as superficial spreading melanoma and nodular melanoma. Clinicopathological parameters included in the analysis were: age, sex, Clark level, ulceration, and degree of lymphocytic infiltration. TILs were defined as lymphocytes infiltrating and disrupting tumor nests and/or in direct contact with tumor cells as observed by hematoxylin and eosin staining. The cases were classified into four grades (0–3) according to TIL density (mild, moderate, or marked) and distribution (focal, multifocal, or diffuse across the entire extent of the tumor). The invasion depth was measured from the epidermal surface to the deepest part of the invasion in the dermis using Olympus BX-51/22 dotSlide digital virtual microscope (Olympus, Center Valley, PA, USA).
- Immunohistochemical examination
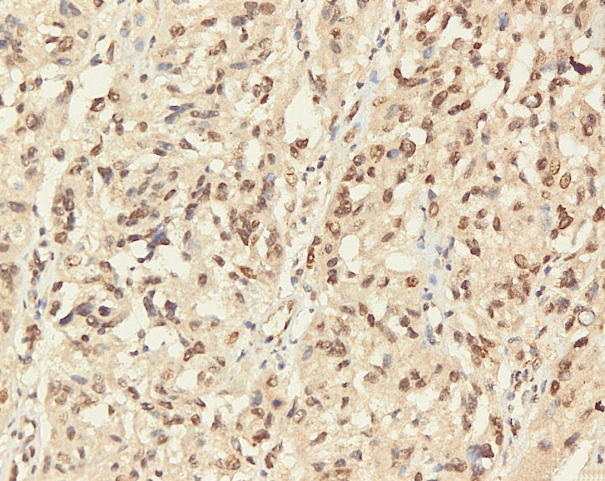
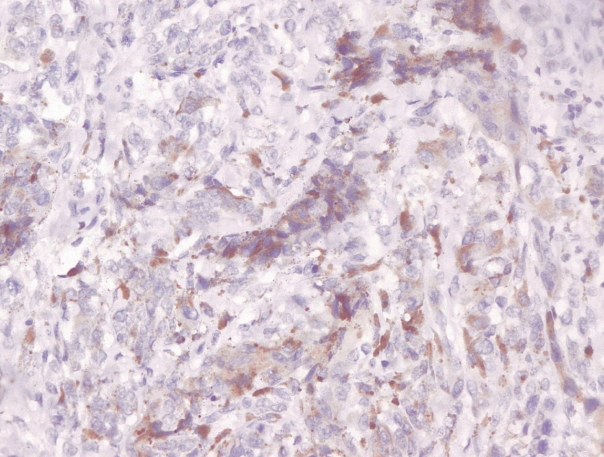
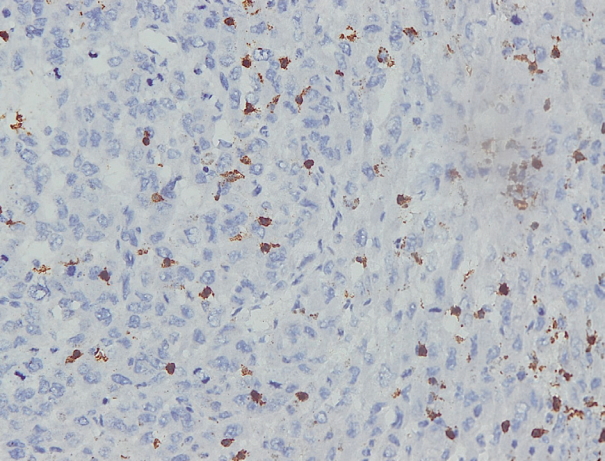
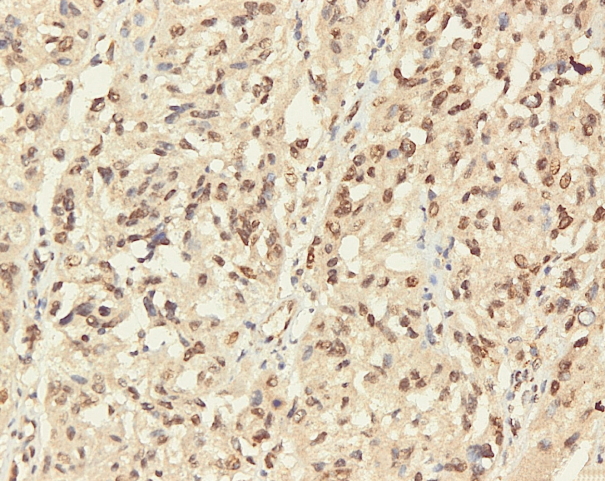
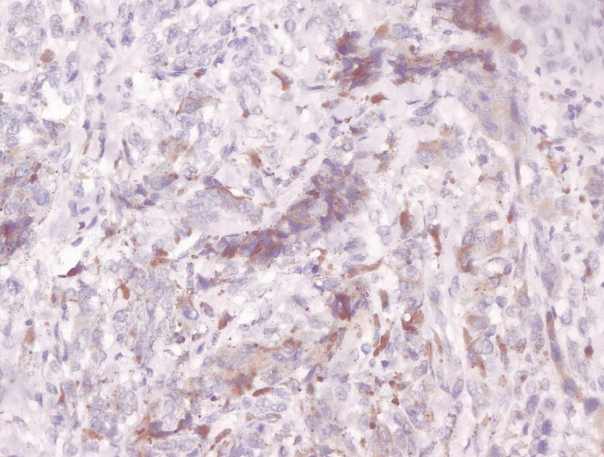
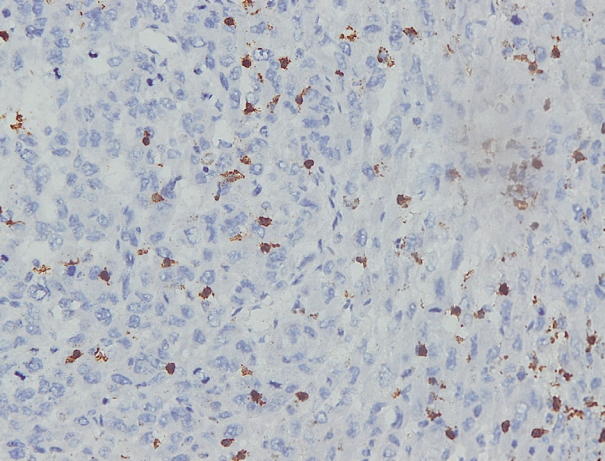
- Immunohistochemical staining on the samples was performed manually using a labeled streptavidin biotin immunoperoxide complex method, using the Starr Trek Universal HRP Detection system (Biocare Medical, Concord, CA, USA). Samples were sectioned to 4-μm thicknesses, deparaffinized using xylene and rehydrated using an alcohol solution. Antigen retrieval used a decloaking tool for 45–60 minutes at a temperature of 98°C. The primary antibodies were NF-κB, CD8 (clobe SP16), and PDL1/CD274 (clone SP142) purchased from Spring Bioscience (Pleasanton, CA, USA). Immunoexpression of NF-κB in nuclei was assessed using semi-quantitative scores based on the intensity and distribution of the positive cells. Intensity scores were negative (0), weak (1), moderate (2), and strong (3), and the percentage of positive cells were grades as follows: 0, 0%; 1, < 25%; 2, 26%–50%; 3, 51%–75%; and 4, 76%–100%. The final score was calculated using Histoscore, namely the intensity × distribution with scores of 0–6 regarded as negative, scores of 8–12 were stated as positive (Fig. 1) [15]. PD-L1 staining in membranes and in the cytoplasm was assessed on a semi-quantitative scale: positive when stained area was ≥ 5% and negative when < 5% (Fig. 2) [16]. The number of CD8+ T cells was assessed by counting the number lymphocytes stained brown on the tumor cell membrane by an anti-CD8 antibody. Results were divided into < 25 and ≥ 25 lymphocytes (Fig. 3).
- Statistical analysis
- Statistical analysis used the non-parametric Mann-Whitney test. A p ≤ .05 was considered significant. The data obtained were recorded on a special form and then processed using SPSS program ver. 22.0 for Windows (IBM Corp., Armonk, NY, USA).
MATERIALS AND METHODS
- In this study, 135 total samples were available but only 96 were eligible for inclusion in the study.
- Table 1 shows that the mean age of acral MM patients was 61.73 years old. Males and females accounted for 52.1% and 47.9% of patients, respectively, and the mean of depth of invasion was 8.074 mm. There were no samples that has a Clark level I and samples with a Clark level (II–V) and TILs grade (0–IV) were divide into two groups as shown below.
- Table 2 shows that the Clark level and ulceration have statistically significant associations with the depth of invasion of acral melanoma (p < .001). The association of pathological characteristics (NF-κB expression, CD8+ T-cell number, and PD-L1 expression) with the depth of invasion of acral melanoma was also statistically significant.
- NF-κB expression had a positive association with the depth of invasion (p < .001). When cells were positive for NF-κB expression, the invasion of tumor cells in acral melanoma was deeper.
- Table 2 demonstrates that when the invasion of tumor cells in acral melanoma was deeper, fewer CD8+ T cells were observed (< 25). Thus, the association was negative and statistically significant statistic (p < .001). The association between PD-L1 expression and the depth of invasion was also a negative association. When the depth of invasion was deeper, the percentage of cells expressing PD-L1 was < 5% and was a statistically significant result (p = .001).
- The data in Table 2 demonstrate that all pathological characteristics examined (NF-κB, PD-L1, and CD8+ T-cell number) had individual association with the depth of invasion. The data show a positive association between NF-κB expression and the depth of invasion, while PD-L1 expression and CD8+ T-cell number had negative associations in acral melanoma.
- The data in Table 3 demonstrate that the association of NF-κB expression with PD-L1 expression and CD8+ T-cell number has different statistical significance. The statistical test for CD8+ T-cell number was significant (p = .001) while PD-L1 was not significant (p = .154). This proves that NF-κB is a confounding variable for CD8+ T-cell numbers but not for PD-L1 expression.
- Data on the association of PD-L1 with CD8+ T cells is shown in the Table 4. The number of CD8+ T cells affects the level of PD-L1 expression; this result was statistically significant (p < .001).
- The multivariate analysis results presented in Table 5 show that the most influential factor on the invasion depth in acral MM is NF-κB (p < .001). No significant results were found in the analysis of Clark level (p = .185), ulceration (p = .156), TILS (p = .935), CD8+ T-cell number (p = .870) or PD-L1 (p = .495).
RESULTS
- NF-κB immunoexpression is strongly associated with the depth of invasion in acral MM. An association between immunopositivity for NF-κB and the in depth of invasion was found, as shown in Table 2. The NF-κB pathway is important for tumor cell survival, as NF-κB serves as a transcription factor to regulate the expression of anti-apoptotic, pro-proliferative, and pro-metastatic genes. The NF-κB pathway is activated through IκB kinase (IKK). IKK stimulates phosphorylation and degradation of the kB inhibitor (IκB) through the proteasome, causing translocation of NF-κB into the cell nucleus. One of the genes upregulated by NF-κB in the nucleus is Snail, an inducer of metastasis through the epithelial mesenchymal transition (EMT) process of initiating tumor invasion [17]. Snail is a transcription factor in the zinc-finger protein family that plays a role in metastasis and in the antiapoptotic processes. Snail stimulates the metastasis through repression of E-cadherin, an adhesion molecule in cells. Loss of cell adhesion resulting from decreased E-cadherin will lead to the EMT process. Snail also affects EMT through down-regulation of claudin and occludin. Claudin and occludin maintain cell polarity. When claudin is down-regulated, cell polarity is lost, which increases the potential for EMT and invasion. In addition, Snail may induce matrix metalloproteinase (MMP) to increase tumor cell invasion capabilities [17,18]. Another possible explanation for the association of NF-κB with the invasion depth of acral MM is that NF-κB translocation to the cell nucleus also directly stimulates the expression of MMPs, especially MMP-9. Overexpression of MMP-9 increases the ability of cells to degrade extracellular matrix, making it easier for cells to invade [19]. Song et al. [15] reported a similar event where EMT process was stimulated through the NF-κB activation pathway in hepatic carcinoma.
- According to Table 2, the depth of invasion of acral MM is also associated with the number of CD8+ T cells: that is low CD8+ T-cell counts (< 25) more commonly found at invasion depths of > 4 mm (mean, 9.078 mm). This is in accordance with the research conducted by Castaneda et al. [16] In that study, it was stated that TILs in acral MM were fewer in number compared to in other MM types and this corresponded to the depth of invasion, progression rate and survival rate.
- In this study, NF-κB was a confounding variable for the emergence of CD8+ T cells, as is supported by the data presented in Table 3. Thus, the association can be explained through various other data as described below.
- NF-κB is associated with various inflammatory factors including tumor necrosis factor α (TNF-α), interleukin 1, interleukin 6, reactive oxygen species, and cyclooxygenase 2 (COX-2). The imbalance of these inflammatory factors can lead to various conditions such as DNA damage, suppressor genes inactivity, increased invasion and metastatic capability of cancer cells, immune escapes, or other tumorigenic mechanisms [20].
- In some studies, the increased activity of NF-κB stimulates expression of COX-2 inflammatory factor [20,21]. COX-2, also known as prostaglandin endoperoxidase 2 (PTGS2), is an enzyme that converts arachidonic acid metabolism into prostaglandins, especially prostaglandin E2 (PGE2), which is a main mediator of the inflammatory and angiogenic processes. COX-2 functions by inhibiting apoptosis and immune surveillance, promoting angiogenesis and improving cancer cell invasion and metastasis as well as influencing cell differentiation [20,21]. COX-2 can also suppress antigen presentation and immune activation in cancer. COX-2 suppresses interferon-gamma secretion from T cells and induces immunosuppressive factor of regulatory T cells, which in turn plays a role in causing tumor resistance to immunotherapy [22].
- Cancer immunology is a dynamic process involving the immune system and tumor cells consisting of three phases: elimination, equilibrium, and escape. During the elimination phase, the immune system successfully eliminates tumor cells through the immune surveillance network. Equilibrium is a latent period of the immune system after elimination phase failure causes tumor cells to become less-immunogenic. The escape phase is when tumor cells become less immunogenic, resulting in a tumor mass that is clinically detectable as a result of immune evasion [22]. The study by Jang [22] showed a strong association between COX-2 expression and the number of regulatory T cells. Reg T cells induce apoptosis in CD8+ T cells via perforin, FasL, and granzyme B, resulting in an inverse relationship between the number of Reg T cells and CD8+ T cells [22]. In addition, other studies have described that CD8+ T cells will decrease in number as a result of PGE2 expressed from COX-2. This happens because PGE2 removes CD127 on the surface of CD8+ T cells so as to decrease the function of CD8+ T cells in immune surveillance and decrease their proliferative ability [23]. Thus, based on this explanation and data from the results of this study (Tables 2, 3), the decreased number of CD8+ T cells may be affected by NF-κB via COX2 and Reg T cells and this corresponds to the depth of invasion in acral MM.
- In this study, we found an association between PD-L1 immunoexpression and the depth of invasion. Specifically, as shown in Table 2, expression of PD-L1 in < 5% of cells was found at invasion depths of > 4 mm (mean, 9.002 mm). PD-L1 expression in various types of malignancies including MM correlates with different prognostic outcomes. The results of this study were consistent with studies of squamous carcinoma [24], melanocytic lesions [25] and hepatic carcinoma [26]. In these prior studies, it was stated that low PD-L1 expression was associated with larger tumor size, increased depth of invasion, disease progression and lower survival rates. However, results of this study differed from other studies of desmoplastic melanoma [27], breast carcinoma [28], gastric carcinoma [29], renal carcinoma [30], and glioma [31]. In these studies, although stated that there is an association of PD-L1 expression with disease progressivity and a worse prognosis, the association was negative such that the higher the PD-L1 expression the worse the prognosis. In renal carcinoma, PD-L1 expression has been reported to induce EMT by up-regulating the sterol regulatory element-binding protein 1 (SREBP-1c) gene [30]. In glioma studies, PD-L1 expression has been reported to stimulate tumor cell proliferation and induce vascular endothelial growth factor expression causing angiogenesis and tumor progression [31]. However, in this study low PD-L1 expression was actually associated with the depth of invasion.
- T cells have inhibitory receptors called PD-1 on their surfaces which are bound by PD-L1 as their ligand. The PD-1/PD-L1 bonding axis inhibits proliferation, inhibit anti-apoptotic, decreases T-cell survival and suppresses signaling by gamma interferon, interleukin 2 and TNF-α against T cells [12]. PD-L1 is expressed by tumor cells. The presence of a bond between PD-1 and PD-L1 results in a decrease in anti-tumor activity from effector T cells [32].
- PD-L1 expression is consistent with the number of CD8+ T cells in the adjacent tumor and is associated with disease progression [27,28,30,31]. The association between PD-L1 expression and the number of CD8+ T cells was demonstrated in this study, based on the data in Table 4. Specifically, when PD-L1 was expressed at < 5%, the number of CD8+ T cells counted was < 25. Based on the data in Table 2, there is a proven association between PD-L1 expression and invasion depth in acral melanoma. However, the data in Table 3 suggests that NF-κB is apparently not a confounding variable for the emergence of PD-L1 expression. Thus, we concluded that the emergence of PD-L1 expression in acral MM depends on the presence of CD8+ T cells (Table 4). Multivariate analysis data (Table 5) revealed that NF-κB has a major role in invasion of acral malignant melanoma. Its expression affects the depth of invasion while decreasing the number of CD8+ cells at the same time.
- Our data demonstrates that the depth of invasion of acral melanoma is affected by NF-κB, PD-L1 and the number of CD8+ T cells. We conclude that NF-κB is the major factor associated with the depth of invasion and is negatively associated with the number of CD8+ T cells in acral melanoma. Thus, positive NF-κB immunoexpression can be a predictive factor of acral melanoma aggressiveness related to an increasing risk of metastasis. Further studies on the microenvironment of tumors emphasizing various inflammatory factors and immune response are needed to find an appropriate, inexpensive, easily available targeted therapy for MM cases.
DISCUSSION
| Variable |
NF-κB |
p-value | |
|---|---|---|---|
| Negative (n = 44) | Positive (n = 52) | ||
| PD-L1 | .154 | ||
| < 5% | 28 (63.6) | 40 (76.9) | |
| ≥ 5% | 16 (36.4) | 12 (23.1) | |
| CD8+ T cells | .001 | ||
| < 25 | 21 (47.7) | 41 (78.8) | |
| ≥ 25 | 23 (52.3) | 11 (21.2) | |
| PD-L1 |
CD8+ T cells |
p-value | |
|---|---|---|---|
| < 25 (n = 62) | ≥ 25 (n = 34) | ||
| < 5% | 60 (96.8) | 8 (23.5) | < .001 |
| ≥ 5% | 2 (3.2) | 26 (76.5) | |
- 1. Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 2009; 1: a000034.ArticlePubMedPMC
- 2. Bommarito A, Richiusa P, Carissimi E, et al. BRAFV600E mutation, TIMP-1 upregulation, and NF-κB activation: closing the loop on the papillary thyroid cancer trilogy. Endocr Relat Cancer 2011; 18: 669-85. ArticlePubMed
- 3. Yang G, Xiao X, Rosen DG, et al. The biphasic role of NF-κB in progression and chemoresistance of ovarian cancer. Clin Cancer Res 2011; 17: 2181-94. ArticlePubMedPMCPDF
- 4. Mao Y, Qu Q, Zhang Y, Liu J, Chen X, Shen K. The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS One 2014; 9: e115103. ArticlePubMedPMC
- 5. Bogunovic D, O'Neill DW, Belitskaya-Levy I, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci U S A 2009; 106: 20429-34. ArticlePubMedPMC
- 6. Kluger HM, Zito CR, Barr ML, et al. Characterization of PD-L1 expression and associated T-cell infiltrates in metastatic melanoma samples from variable anatomic sites. Clin Cancer Res 2015; 21: 3052-60. ArticlePubMedPMCPDF
- 7. Thomas NE, Busam KJ, From L, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol 2013; 31: 4252-9. ArticlePubMedPMC
- 8. DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev 2012; 246: 379-400. ArticlePubMed
- 9. Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol 2011; 12: 715-23. ArticlePubMedPDF
- 10. Kakavand H, Wilmott JS, Menzies AM, et al. PD-L1 expression and tumor-infiltrating lymphocytes define different subsets of MAPK inhibitor-treated melanoma patients. Clin Cancer Res 2015; 21: 3140-8. ArticlePubMedPDF
- 11. Tjin EP, Krebbers G, Meijlink KJ, et al. Immune-escape markers in relation to clinical outcome of advanced melanoma patients following immunotherapy. Cancer Immunol Res 2014; 2: 538-46. ArticlePubMedPDF
- 12. Dolan DE, Gupta S. PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control 2014; 21: 231-7. ArticlePubMedPDF
- 13. Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res 2013; 19: 5300-9. ArticlePubMedPDF
- 14. Momtaz P, Postow MA. Immunologic checkpoints in cancer therapy: focus on the programmed death-1 (PD-1) receptor pathway. Pharmgenomics Pers Med 2014; 7: 357-65. PubMedPMC
- 15. Song FN, Duan M, Liu LZ, et al. RANKL promotes migration and invasion of hepatocellular carcinoma cells via NF-κB-mediated epithelial-mesenchymal transition. PLoS One 2014; 9: e108507. ArticlePubMedPMC
- 16. Castaneda CA, Torres-Cabala C, Castillo M, et al. Tumor infiltrating lymphocytes in acral lentiginous melanoma: a study of a large cohort of cases from Latin America. Clin Transl Oncol 2017; 19: 1478-88. ArticlePubMedPDF
- 17. Lin K, Baritaki S, Militello L, Malaponte G, Bevelacqua Y, Bonavida B. The role of B-RAF mutations in melanoma and the induction of EMT via dysregulation of the NF-κB/Snail/RKIP/PTEN Circuit. Genes Cancer 2010; 1: 409-20. ArticlePubMedPMC
- 18. Wu Y, Zhou BP. TNF-α/NF-κB/Snail pathway in cancer cell migration and invasion. Br J Cancer 2010; 102: 639-44. ArticlePubMedPMCPDF
- 19. Guarneri C, Bevelacqua V, Polesel J, et al. NF-κB inhibition is associated with OPN/MMP9 downregulation in cutaneous melanoma. Oncol Rep 2017; 37: 737-46. ArticlePubMedPMC
- 20. Dai J, Wang H, Dong Y, Zhang Y, Wang J. Bile acids affect the growth of human cholangiocarcinoma via NF-κB pathway. Cancer Invest 2013; 31: 111-20. PubMed
- 21. Nguyen LK, Cavadas MA, Kholodenko BN, Frank TD, Cheong A. Species differential regulation of COX2 can be described by an NFκB-dependent logic AND gate. Cell Mol Life Sci 2015; 72: 2431-43. ArticlePubMedPMCPDF
- 22. Jang TJ. Progressive increase of regulatory T cells and decrease of CD8+ T cells and CD8+ T cells/regulatory T cells ratio during colorectal cancer development. Korean J Pathol 2013; 47: 443-51. ArticlePubMedPMC
- 23. Chou JP, Ramirez CM, Ryba DM, Koduri MP, Effros RB. Prostaglandin E2 promotes features of replicative senescence in chronically activated human CD8+ T cells. PLoS One 2014; 9: e99432. PubMedPMC
- 24. Oliveira-Costa JP, de Carvalho AF, da Silveira da GG, et al. Gene expression patterns through oral squamous cell carcinoma development: PD-L1 expression in primary tumor and circulating tumor cells. Oncotarget 2015; 6: 20902-20. PubMedPMC
- 25. Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4: 127ra37.PubMedPMC
- 26. Sideras K, Biermann K, Verheij J, et al. PD-L1, Galectin-9 and CD8+ tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. Oncoimmunology 2017; 6: e1273309.PubMedPMC
- 27. Frydenlund N, Leone D, Yang S, et al. Tumoral PD-L1 expression in desmoplastic melanoma is associated with depth of invasion, tumor-infiltrating CD8 cytotoxic lymphocytes and the mixed cytomorphological variant. Mod Pathol 2017; 30: 357-69. PubMedPDF
- 28. Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS One 2014; 9: e88557. PubMedPMC
- 29. Qing Y, Li Q, Ren T, et al. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des Devel Ther 2015; 9: 901-9. PubMedPMC
- 30. Wang Y, Wang H, Zhao Q, Xia Y, Hu X, Guo J. PD-L1 induces epithelial-to-mesenchymal transition via activating SREBP-1c in renal cell carcinoma. Med Oncol 2015; 32: 212.PubMed
- 31. Xue S, Hu M, Li P, et al. Relationship between expression of PD-L1 and tumor angiogenesis, proliferation, and invasion in glioma. Oncotarget 2017; 8: 49702-12. PubMedPMC
- 32. Hersey P, Gallagher S. A focus on PD-L1 in human melanoma. Clin Cancer Res 2013; 19: 514-6. PubMed
REFERENCES
Figure & Data
References
Citations

- The Roles of Vitamin D Receptor (VDR) and CD8+ T‐Lymphocytes in Acral and Mucosal Melanoma Invasion Depth
Hermin Aminah Usman, Fitria Sholihah, Birgitta M. Dewayani, Octavianus Giovani
Journal of Cutaneous Pathology.2025; 52(3): 227. CrossRef - Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols
Hui-Min Liu, Ming-Yan Cheng, Meng-Han Xun, Zhi-Wei Zhao, Yun Zhang, Wei Tang, Jun Cheng, Jia Ni, Wei Wang
International Journal of Molecular Sciences.2023; 24(4): 3755. CrossRef - Clinical features, molecular pathology, and immune microenvironmental characteristics of acral melanoma
Jianping Gui, Zhen Guo, Di Wu
Journal of Translational Medicine.2022;[Epub] CrossRef - High Expression of COX-2 Associated with the Depth of Invasion on Acral Melanoma by Increasing TGF-β1
Nastassa Gipsyianti, Afiati Aziz, Bethy S Hernowo, Hermin A Usman
Clinical, Cosmetic and Investigational Dermatology.2021; Volume 14: 209. CrossRef - More than just acral melanoma: the controversies of defining the disease
Sara S Bernardes, Ingrid Ferreira, David E Elder, Aretha B Nobre, Héctor Martínez‐Said, David J Adams, Carla Daniela Robles‐Espinoza, Patricia A Possik
The Journal of Pathology: Clinical Research.2021; 7(6): 531. CrossRef - CD103+ T Lymphocyte Count Linked to the Thickness of Invasion on Acral Melanoma without E-Cadherin Involvement
Fauzan Ali Zainal Abidin, Hermin Aminah Usman, Sri Suryanti, Bethy S Hernowo
Clinical, Cosmetic and Investigational Dermatology.2021; Volume 14: 1783. CrossRef - Translational pathology, genomics and the development of systemic therapies for acral melanoma
Yian Ann Chen, Jamie K. Teer, Zeynep Eroglu, Jheng-Yu Wu, John M. Koomen, Florian A. Karreth, Jane L. Messina, Keiran S.M. Smalley
Seminars in Cancer Biology.2020; 61: 149. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1.
Fig. 2.
Fig. 3.
| Variable | No. (%) (n = 96) |
|---|---|
| Age | |
| Mean ± SD | 61.730 ± 12.330 |
| Sex | |
| Male | 50 (52.1) |
| Female | 46 (47.9) |
| Depth of invasion (mm) | |
| Mean ± SD | 8.074 ± 6.902 |
| Clark Level | |
| I | 0 |
| II–III | 28 (29.2) |
| IV–V | 68 (70.8) |
| Ulceration | |
| No | 36 (37.5) |
| Yes | 60 (62.5) |
| TILs | |
| Grade 0–I | 49 (51.0) |
| Grade II–III | 47 (49.0) |
| NF-κB | |
| Negative | 44 (45.8) |
| Positive | 52 (54.2) |
| CD8+ T cells | |
| < 25 | 62 (64.6) |
| ≥ 25 | 34 (35.4) |
| PD-L1 | |
| < 5% | 68 (70.8) |
| ≥ 5% | 28 (29.2) |
| Variable | Depth of invasion (mm) | p-value |
|---|---|---|
| Age | 8.074 ± 6.902 | .109 |
| Sex | .086 | |
| Male | 8.770 ± 7.235 | |
| Female | 7.319 ± 6.515 | |
| Clark level | < .001 | |
| II–III | 3.969 ± 3.023 | |
| IV–V | 9.765 ± 7.343 | |
| Ulceration | < .001 | |
| No | 5.353 ± 4.896 | |
| Yes | 9.707 ± 7.433 | |
| TILs | .206 | |
| Grade 0–I | 8.465 ± 6.263 | |
| Grade II–III | 7.668 ± 7.558 | |
| NF-κB | <.001 | |
| Negative | 4.755 ± 3.075 | |
| Positive | 10.883 ± 7.951 | |
| CD8+ T cells | <.001 | |
| < 25 | 9.708 ± 6.970 | |
| ≥ 25 | 5.096 ± 5.760 | |
| PD-L1 | < .001 | |
| < 5% | 9.002 ± 7.034 | |
| ≥ 5% | 5.821 ± 6.115 |
| Variable | NF-κB |
p-value | |
|---|---|---|---|
| Negative (n = 44) | Positive (n = 52) | ||
| PD-L1 | .154 | ||
| < 5% | 28 (63.6) | 40 (76.9) | |
| ≥ 5% | 16 (36.4) | 12 (23.1) | |
| CD8+ T cells | .001 | ||
| < 25 | 21 (47.7) | 41 (78.8) | |
| ≥ 25 | 23 (52.3) | 11 (21.2) | |
| PD-L1 | CD8+ T cells |
p-value | |
|---|---|---|---|
| < 25 (n = 62) | ≥ 25 (n = 34) | ||
| < 5% | 60 (96.8) | 8 (23.5) | < .001 |
| ≥ 5% | 2 (3.2) | 26 (76.5) | |
| Variable | Unstandardized coefficients |
Standardized coefficients |
p-value | |
|---|---|---|---|---|
| B | Standard error | Beta | ||
| Clark level | 2,505.896 | 1,876.505 | 0.166 | .185 |
| Ulceration | 2,285.568 | 1,595.783 | 0.161 | .156 |
| TILs | -107.175 | 1,313.089 | -0.008 | .935 |
| NF-κB | 5,166.865 | 1,353.669 | 0.375 | < .001 |
| CD8+ T cells | 416.860 | 2,538.846 | 0.029 | .870 |
| PD-L1 | -1,541.337 | 2,248.71 | -0.102 | .495 |
SD, standard deviation; TIL, tumor-infiltrating lymphocyte; NF-κB, nuclear factor κB; PD-L1, programmed death-ligand 1.
Values are presented as mean ± standard deviation. TIL, tumor-infiltrating lymphocyte; NF-κB, nuclear factor κB; PD-L1, programmed death-ligand 1.
Values are presented as number (%). NF-κB, nuclear factor κB; PD-L1, programmed death-ligand 1.
Values are presented as number (%). PD-L1, programmed death-ligand 1.
Variables involved are significantly bivariable with p < .25. TIL, tumor-infiltrating lymphocyte; NF-κB, nuclear factor κB; PD-L1, programmed death-ligand 1.

 E-submission
E-submission