Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 53(3); 2019 > Article
-
Review
Provisional Guideline Recommendation for EGFR Gene Mutation Testing in Liquid Samples of Lung Cancer Patients: A Proposal by the Korean Cardiopulmonary Pathology Study Group -
Dong Hoon Shin
 , Hyo Sup Shim1
, Hyo Sup Shim1 , Tae Jung Kim2
, Tae Jung Kim2 , Heae Surng Park3
, Heae Surng Park3 , Yun La Choi4
, Yun La Choi4 , Wan Seop Kim5
, Wan Seop Kim5 , Lucia Kim6
, Lucia Kim6 , Sun Hee Chang7
, Sun Hee Chang7 , Joon Seon Song8
, Joon Seon Song8 , Hyo jin Kim9
, Hyo jin Kim9 , Jung Ho Han4
, Jung Ho Han4 , Chang Hun Lee
, Chang Hun Lee , Geon Kook Lee10
, Geon Kook Lee10 , Se Jin Jang8
, Se Jin Jang8 , Korean Cardiopulmonary Pathology Study Group
, Korean Cardiopulmonary Pathology Study Group -
Journal of Pathology and Translational Medicine 2019;53(3):153-158.
DOI: https://doi.org/10.4132/jptm.2019.02.22
Published online: February 28, 2019
Department of Pathology, Pusan National University School of Medicine, Yangsan, Korea
1Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
2Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
3Department of Pathology, Ewha Womans University Mokdong Hospital, Seoul, Korea
4Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
5Department of Pathology, Konkuk University School of Medicine, Seoul, Korea
6Department of Pathology, Inha University School of Medicine, Incheon, Korea
7Department of Pathology, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
8Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
9Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea
10Department of Pathology, National Cancer Center, Goyang, Korea
- Corresponding Author Se Jin Jang, MD, PhD Department of Pathology, Asan Medical Centre, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea Tel: +82-2-3010-5966 Fax: +82-2-472-7898 E-mail: jangsejin@amc.seoul.kr
© 2019 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Liquid biopsy for detection of mutation from circulating tumor DNA is a new technology which is attractive in that it is non-invasive. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) is an effective first line drug for advanced non-small cell lung cancer patients who harbor activating EGFR mutation. During the course of treatment, resistance against TKI arises which can be contributed to EGFR T790M mutation in about 50–60% of patients. Third generation TKI may overcome the resistance. In patients who cannot undergo tissue biopsy due to variable reasons, liquid biopsy is an excellent alternative for the detection of EGFR T790M mutation. However, this relatively novel method requires standardization and vigorous quality insurance. Thus, a standard set of guideline recommendations for liquid biopsy for EGFR mutation testing suitable for the Korean medical community is necessary. In this article, we propose a set of provisional guideline recommendations that was discussed and approved by the Cardiopulmonary Pathology Study Group of the Korean Society of Pathologists.
- Liquid biopsy for the detection of EGFR mutation can play many roles in cancer diagnostics [12-14,16,17]. Patients diagnosed with lung adenocarcinoma harboring EGFR mutation will be the first candidates when they develop resistance against first-line TKIs. Especially, when the tumor is too small or located in a challenging region to be sampled, liquid biopsy can be a good alternative [14-18]. Patients with poor performance status can also benefit from this technique.
PATIENT ELIGIBILITY
- Sample collection and processing is a critical step in liquid biopsy. Since ctDNA is rapidly degraded by the nuclease in blood and contaminated by genomic DNA from blood cells, it is essential to separate plasma from the sample [13,14]. The routine venipuncture technique will be sufficient to collect blood from the patients. The sample collection tube should be chosen considering each institution’s setting. Conventional ethyldiaminetetraaceticacid (EDTA) tube can be used if the samples are processed without delay [19,20]. Recently, specialized tubes for delaying degradation of ctDNA are commercially available [19,20]. The tube from Streck (Omaha, NE, USA) has been the most widely used collection tube. Roche diagnostics and Qiagen have also marketed specialized tubes. According to a study [19], conventional EDTA tube and Streck tube do not show much difference in their performance when samples are processed within 6 hours. When incubated longer in EDTA tube, cell-free DNA may be released from the blood cells, and EDTA will hinder the polymerase chain reaction (PCR) [20]. Tubes from Roche and Qiagen showed similar performance, and they are slightly better than Streck tube [20]. Specialized tubes can sustain sample quality for several days at room temperature before processing further (Table 1).
SAMPLE COLLECTION
- Before ctDNA extraction, blood should be processed into plasma through double centrifugation. Plasma samples are better than serum samples, which can be contaminated by DNA released from immune cells [13]. Since a small amount of ctDNA is present in plasma, isolation is a critical step in the process for saving tumor DNA. Several commercial kits for isolation are available in the market (Table 2) [21,22]. These are manual, semiautomatic, and fully automatic. Manual protocol uses column-based method while semi-automatic instrument works with magnetic beads. Previous studies showed variable results depending on the extraction kits, though they all had similar performances [21,22]. The technician’s skill and protocol optimization may be one of the critical factors for yielding better ctDNA. Table 1 summarizes commercial ctDNA extraction kits.
CIRCULATING TUMOR DNA ISOLATION
- High sensitivity detection methods are required to detect EGFR mutations from liquid samples. Kits for detecting mutations have been developed and are commercially available [23-25]. Each kit requires different quality and amount of DNA (Table 3). They depend on real time PCR technology with their own variations. Roche Cobas uses real time PCR with Taqman like probe and Qiagen has released ARMS based kits, Therascreen EGFR RGQ. Another PCR based technique uses peptide nucleic acid clamping and Panamutyper (Panagene, Daejeon, Korea). The Roche and Qiagen systems use their own PCR machine from Roche and Qiagen while Panamutyper can run on any qualified PCR machines. The number of mutations these kits can detect are different; however, together they include exon 19 deletion, T790M and L858R. Currently, only Roche kit has acquired FDA approval. The most important element of these kits is how sensitively and specifically they can detect mutations in liquid samples. There are certain studies to evaluate their performance and report sensitivities ranging from 62% to 67.5% and specificities ranging from 88% to 97% [26-29]. In the ASSESS study, these three kits showed high specificity, however, sensitivity was equal to or less than 75% [25]. For T790M, sensitivity was 41% and 29% for Cobas and Therascreen, respectively, and specificity was 100% for both kits from the patients enrolled in AURA trial [10]. Therefore, deciding the best kit will depend on the laboratory’s choice with consideration of their requirements. Features of these products are summarized in Table 3. Other platforms using digital PCR and next generation sequencing are still far from widespread use in clinical setting [24].
MUTATION DETECTION METHODS
- Once liquid biopsy for detecting T790M mutation is done, the reports should contain the following information: pathologic number, age, sex, hospital unit number, sample source, requesting physician, requesting department, adequacy for testing (amount of DNA extracted), receipt day, report day, storage tube, methodology used, exons tested and associated range of detectable mutations, mutation status, comments, testing technician, and corresponding pathologist. Since the patients already have sensitizing EGFR mutation, it is recommended to include the type of original EGFR alteration and previous histologic diagnosis.
REPORTING FORMAT
- Since liquid biopsy technique has not been validated yet, vigorous quality assurance is necessary. Although there is no recommended program for external quality assessment (EQA), one pilot trial for EGFR testing in blood is ongoing in Germany [30]. Another program for BRAF and KRAS is also being conducted [31]. Since patient derived standard sample is difficult to store and distribute, artificial sample mimicking the real one can be used instead [30,31]. We are in the process of developing Korean EQA program.
PROPOSAL FOR AN EXTERNAL QUALITY ASSESSMENT PROGRAM
- Performance and interpretation of liquid biopsy require broad knowledge in lung cancer pathology. Pathologists have an important role in the diagnosis and management of cancer and thus can interpret liquid biopsy results in conjunction with the histologic diagnosis, previous status of EGFR-activating mutation, and clinical situation. The liquid biopsy in lung cancer is usually performed in patients whose previous EGFR mutation status has been known. The sole purpose of this technique is to detect a T790M mutation responsible for TKI resistance. Unlike tissue specimens, in which the pathologists can determine the percentage of tumor cells, it is extremely difficult to estimate whether the blood sample contains a sufficient amount of tumor DNA. If the sample is adequate, the test generally finds the original EGFR-activating mutation, which may act as an internal control for the presence of ctDNA [13]. When it has not detected any EGFR-activating mutation including previously existing one or reported mutations other than the preexisting ones or T790M, pathologists should be able to interpret the result. In the former, test should be repeated because the samples might have been degraded and contain insufficient ctDNA. In the latter, the newly emerged mutation, in the presence of newly developed lesion, may indicate a metachronous primary tumor. The communication between pathologists, clinicians, and radiologists is important for further diagnosis and management of cancer. Moreover, lung adenocarcinoma undergoes frequent transformation into small cell carcinoma when it is treated with TKI, while maintaining the original EGFR mutation [7,8,32]. Recommended interpretation is suggested in Table 4.
ROLE OF PATHOLOGISTS
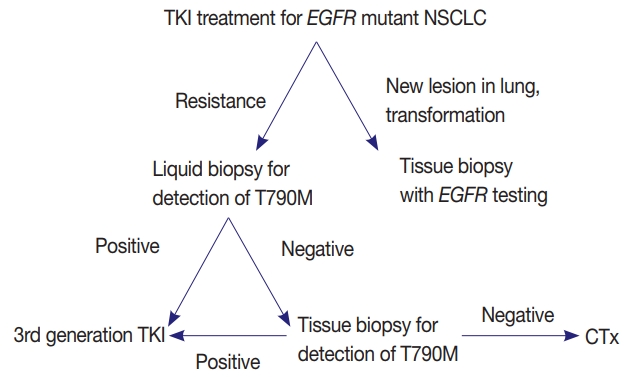
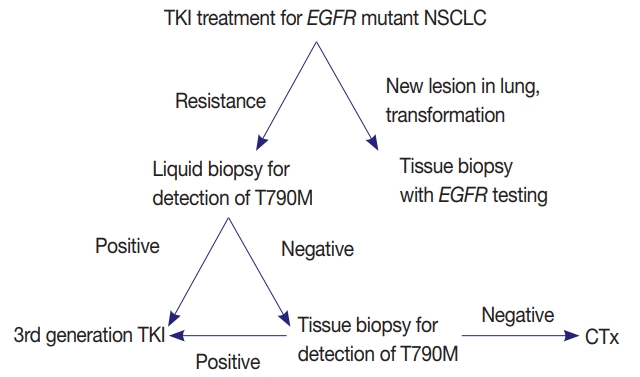
- EGFR mutation testing performed with blood or other liquid sample is a non-invasive method, which can be more widely adopted. Laboratories must get familiar with liquid samples and develop their own protocols to handle these specimens. They can choose appropriate sample tubes, extraction kits, detection methods, and other instruments. They should select the most suitable combination in accordance with their requirements, unless the detection kits indicate specific methods and instruments [31]. Although sensitivity of tissue biopsy is higher than liquid biopsy, both are far from perfection and T790M mutation can be detected only in one of the two methods. Reportedly, allele fraction of T790M mutation tends to correlate with treatment efficacy of osimertinib [33]. Therefore, absence of T790M in tumor tissue while it is detected in plasma might reflect low allele frequency and lead to poor response. Therefore, the two methods are complementary to each other and should be selected according to each patient’s condition (Fig. 1).
PERSPECTIVES AND ADDITIONAL RECOMMENDATIONS
- Liquid biopsy is a promising method, which is safe and convenient. Before more experiences and data are accumulated, liquid biopsy should be performed with great caution. There are a few steps in liquid biopsy, which can produce false negative or false positive results. Interpretation requires profound knowledge of lung cancer including diagnosis, treatment, and prognosis. However, in debatable cases, discussion between pathologists, physicians, and radiologists is critical. This method will soon play a major role in early diagnosis, monitoring of treatment, and detection of minimal residual disease. Currently, it cannot replace the conventional pretreatment tissue diagnosis [14]. It is important to validate and improve the performance of this technique before it is widely used in clinical practice. Liquid biopsy performed in EGFR has provided a platform for determining gene mutations in KRAS, ALK, PI3CA, and BRAF as well.
CONCLUSIONS
Author contributions
Conceptualization: DHS, HSS, TJK, HSP, YLC, WSK, LK, SHC, JSS, HJK, JHH, CHL, GKL, SJJ.
Data curation: DHS.
Formal analysis: DHS, HSS, TJK.
Investigation: DHS, HSP, YLC, WSK, LK.
Methodology: DHS, SHC, JSS, HJK, JHH.
Project administration: JHH, CHL, GKL, SJJ.
Writing—original draft: DHS.
Writing—review & editing: DHS, HSS, TJK, HSP, YLC, WSK, LK, SHC, JSS, HJK, JHH, CHL, GKL, SJJ.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
| Company | Trade name | Volume (mL) | Temperature (°C) | Storage duration (day) |
|---|---|---|---|---|
| Streck | cfDNA BCT | 10 | 6–37 | 14 |
| Roche | Cell-Free DNA Collection Tube | 8.5 | 18–25 | 7 |
| Qiagen | PAXgene Blood ccfDNA Tube | 10 | 18–25 | 7 |
| Company | Trade name | Method | Automation |
|---|---|---|---|
| ThermoFisher | MagMAX | Magnetic beads | Semiauto |
| Promega | Maxwell RSC | Magnetic beads | Semiauto |
| Roche | Cobas | Silica membrane | Manual |
| Qiagen | QIAamp | Silica membrane | Semiauto |
- 1. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129-39. PubMed
- 2. Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497-500. ArticlePubMed
- 3. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947-57. ArticlePubMed
- 4. Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res 2006; 12: 6494-501. ArticlePubMedPDF
- 5. Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015; 21: 560-2. ArticlePubMedPMCPDF
- 6. Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011; 3: 75ra26.PubMedPMC
- 7. Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011; 3: 75ra26.PubMedPMC
- 8. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013; 19: 2240-7. ArticlePubMedPMCPDF
- 9. Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016; 17: 1643-52. ArticlePubMed
- 10. Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in pretreated T790Mpositive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol 2017; 35: 1288-96. ArticlePubMed
- 11. Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016; 22: 5130-40. ArticlePubMedPDF
- 12. Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med 2008; 14: 985-90. ArticlePubMedPDF
- 13. Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 2017; 17: 223-38. ArticlePubMedPDF
- 14. Sholl LM, Aisner DL, Allen TC, et al. Liquid biopsy in lung cancer: a perspective from members of the Pulmonary Pathology Society. Arch Pathol Lab Med 2016; 140: 825-9. ArticlePubMedPDF
- 15. Cobas EGFR Mutation Test v2, PMA 150047. FDA summary of safety and effectiveness data [Internet]. Silverspring: US Food and Drug Administration, 2016 [cited 2018 Jan 2]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150047B.pdf.
- 16. Mino-Kenudson M. Cons: Can liquid biopsy replace tissue biopsy?: the US experience. Transl Lung Cancer Res 2016; 5: 424-7. ArticlePubMedPMC
- 17. Ilie M, Hofman P. Pros: can tissue biopsy be replaced by liquid biopsy? Transl Lung Cancer Res 2016; 5: 420-3. ArticlePubMedPMC
- 18. Mock TS, Carbone DP, Hirsch FR. IASLC atlas of EGFR testing in lung cancer. Aurora: International Association for the Study of Lung Cancer, 2017.
- 19. Alidousty C, Brandes D, Heydt C, et al. Comparison of blood collection tubes from three different manufacturers for the collection of cell-free DNA for liquid biopsy mutation testing. J Mol Diagn 2017; 19: 801-4. ArticlePubMed
- 20. Kang Q, Henry NL, Paoletti C, et al. Comparative analysis of circulating tumor DNA stability In K3EDTA, Streck, and CellSave blood collection tubes. Clin Biochem 2016; 49: 1354-60. ArticlePubMed
- 21. Fleischhacker M, Schmidt B, Weickmann S, et al. Methods for isolation of cell-free plasma DNA strongly affect DNA yield. Clin Chim Acta 2011; 412: 2085-8. ArticlePubMed
- 22. Sorber L, Zwaenepoel K, Deschoolmeester V, et al. A comparison of cell-free DNA isolation kits: isolation and quantification of cellfree DNA in plasma. J Mol Diagn 2017; 19: 162-8. PubMed
- 23. Vendrell JA, Mau-Them FT, Beganton B, Godreuil S, Coopman P, Solassol J. Circulating cell free tumor DNA detection as a routine tool for lung cancer patient management. Int J Mol Sci 2017; 18: E264.Article
- 24. Thress KS, Brant R, Carr TH, et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: a cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer 2015; 90: 509-15. ArticlePubMed
- 25. Reck M, Hagiwara K, Han B, et al. ctDNA determination of EGFR mutation status in European and Japanese patients with advanced NSCLC: the ASSESS study. J Thorac Oncol 2016; 11: 1682-9. ArticlePubMed
- 26. Bernabe R, Hickson N, Wallace A, Blackhall FH. What do we need to make circulating tumour DNA (ctDNA) a routine diagnostic test in lung cancer? Eur J Cancer 2017; 81: 66-73. ArticlePubMed
- 27. Qiu M, Wang J, Xu Y, et al. Circulating tumor DNA is effective for the detection of EGFR mutation in non-small cell lung cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2015; 24: 206-12. ArticlePubMedPDF
- 28. Luo J, Shen L, Zheng D. Diagnostic value of circulating free DNA for the detection of EGFR mutation status in NSCLC: a systematic review and meta-analysis. Sci Rep 2014; 4: 6269.ArticlePubMedPMCPDF
- 29. Wu Y, Liu H, Shi X, Song Y. Can EGFR mutations in plasma or serum be predictive markers of non-small-cell lung cancer? A meta-analysis. Lung Cancer 2015; 88: 246-53. ArticlePubMed
- 30. Fassunke J, Ihle MA, Lenze D, et al. EGFR T790M mutation testing of non-small cell lung cancer tissue and blood samples artificially spiked with circulating cell-free tumor DNA: results of a round robin trial. Virchows Arch 2017; 471: 509-20. ArticlePubMedPDF
- 31. Haselmann V, Ahmad-Nejad P, Geilenkeuser WJ, et al. Results of the first external quality assessment scheme (EQA) for isolation and analysis of circulating tumour DNA (ctDNA). Clin Chem Lab Med 2018; 56: 220-8. ArticlePubMedPDF
- 32. Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol 2015; 16: e165-72. ArticlePubMedPMC
- 33. Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol 2016; 34: 3375-82. ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Comparison of tissue-based and plasma-based testing for EGFR mutation in non–small cell lung cancer patients
Yoon Kyung Kang, Dong Hoon Shin, Joon Young Park, Chung Su Hwang, Hyun Jung Lee, Jung Hee Lee, Jee Yeon Kim, JooYoung Na
Journal of Pathology and Translational Medicine.2025; 59(1): 60. CrossRef - Improving non-small-cell lung cancer survival through molecular characterization: Perspective of a multidisciplinary expert panel
M.G.O. Fernandes, A.S. Vilariça, B. Fernandes, C. Camacho, C. Saraiva, F. Estevinho, H. Novais e Bastos, J.M. Lopes, P. Fidalgo, P. Garrido, S. Alves, S. Silva, T. Sequeira, F. Barata
Pulmonology.2024; 30(1): 4. CrossRef - Unlocking the future of cancer diagnosis – promises and challenges of ctDNA-based liquid biopsies in non-small cell lung cancer
Chiara Reina, Berina Šabanović, Chiara Lazzari, Vanesa Gregorc, Christopher Heeschen
Translational Research.2024; 272: 41. CrossRef - Exosomes in Lung Cancer: Actors and Heralds of Tumor Development
Amaia Sandúa, Estibaliz Alegre, Álvaro González
Cancers.2021; 13(17): 4330. CrossRef - Molecular biomarker testing for non–small cell lung cancer: consensus statement of the Korean Cardiopulmonary Pathology Study Group
Sunhee Chang, Hyo Sup Shim, Tae Jung Kim, Yoon-La Choi, Wan Seop Kim, Dong Hoon Shin, Lucia Kim, Heae Surng Park, Geon Kook Lee, Chang Hun Lee
Journal of Pathology and Translational Medicine.2021; 55(3): 181. CrossRef - Current status and future perspectives of liquid biopsy in non-small cell lung cancer
Sunhee Chang, Jae Young Hur, Yoon-La Choi, Chang Hun Lee, Wan Seop Kim
Journal of Pathology and Translational Medicine.2020; 54(3): 204. CrossRef - Prevalence of T790M mutation among TKI-therapy resistant Lebanese lung cancer patients based on liquid biopsy analysis: a first report from a major tertiary care center
Hazem Assi, Arafat Tfayli, Nada Assaf, Sarah Abou Daya, Aram H. Bidikian, Dima Kawsarani, Puzant Fermanian, Ghazi Zaatari, Rami Mahfouz
Molecular Biology Reports.2019; 46(4): 3671. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

Fig. 1.
| Company | Trade name | Volume (mL) | Temperature (°C) | Storage duration (day) |
|---|---|---|---|---|
| Streck | cfDNA BCT | 10 | 6–37 | 14 |
| Roche | Cell-Free DNA Collection Tube | 8.5 | 18–25 | 7 |
| Qiagen | PAXgene Blood ccfDNA Tube | 10 | 18–25 | 7 |
| Company | Trade name | Method | Automation |
|---|---|---|---|
| ThermoFisher | MagMAX | Magnetic beads | Semiauto |
| Promega | Maxwell RSC | Magnetic beads | Semiauto |
| Roche | Cobas | Silica membrane | Manual |
| Qiagen | QIAamp | Silica membrane | Semiauto |
| Company | Trade Name | Method | Detectable mutations |
|---|---|---|---|
| Roche | Cobas | Real time PCR | EX19Del, S768I, T790M, L858R, L861Q, G719X, EX20Ins |
| Qiagen | Therascreen RGQ | ARMS | Ex19Del, T790M, L858R |
| Panagene | Mutyper | PNA clamp | EX19Del, S768I, T790M, L858R, L861Q, G719X, EX20Ins |
| Bio-Rad | rimePCR ddPCR Mutation Assay | Digital PCR | Ex19Del, T790M, L858R, L861Q |
| Sysmex-Inostics | OncoBEAM | Digital PCR | Ex19Del, T790M, L858R, C797S |
| Sensitizing mutation | T790M | Interpretation |
|---|---|---|
| Detected | Detected | T790M positive: start treatment with third generation TKI |
| Detected | Not detected | T790M negative: tissue biopsy recommended |
| Not detected | Detected | T790M positive: confirmation is necessary |
| Not detected | Not detected | Non-informative: tissue biopsy strongly recommended |
ctDNA, circulating tumor DNA.
ctDNA, circulating tumor DNA.
EGFR, epidermal growth factor receptor; PCR, polymerase chain reaction; PNA, peptide nucleic acid.
EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.

 E-submission
E-submission