Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 53(4); 2019 > Article
-
Review
PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future -
Hyojin Kim1
 , Jin-Haeng Chung1,2
, Jin-Haeng Chung1,2
-
Journal of Pathology and Translational Medicine 2019;53(4):199-206.
DOI: https://doi.org/10.4132/jptm.2019.04.24
Published online: May 2, 2019
1Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea
2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
- Corresponding Author Jin-Haeng Chung, MD, PhD Department of Pathology, Seoul National University Bundang Hospital, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea Tel: +82-31-787-3379 Fax: +82-31-787-4412 E-mail: chungjh@snu.ac.kr
© 2019 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Abstract
- PROGRAMMED DEATH-LIGAND 1 PROTEIN EXPRESSION AS A BIOMARKER IN LUNG CANCER: RATIONALE AND PERFORMANCE
- VARIOUS PROGRAMMED DEATH-LIGAND 1 ASSAY AND HARMONIZATION
- INTERPRETATION AND PATHOLOGICAL REPORTING OF PROGRAMMED DEATH-LIGAND 1 IMMUNOHISTOCHEMISTRY
- ONGOING ISSUES
- BEYOND PROGRAMMED DEATH-LIGAND 1: IS THERE ANY PREDICTIVE BIOMARKER AS AN ALTERNATIVE TO PROGRAMMED DEATH-LIGAND 1?
- CONCLUSION
- NOTES
- Acknowledgments
- REFERENCES
Figure & Data
References
Citations

- Impact of patient and tumor characteristics on various aspects of the non-small cell lung cancer patient journey in Romania, Bulgaria, Serbia: A multicenter, non-interventional, retrospective cohort study
Alexandra C. Preda, Rossitza Krasteva, Mihailo Stjepanović, Krassimir Koynov, Assia Konsoulova, Georgeta P. Iorga, Anghel A. Udrea, Anca Zgura, Tudor-Eliade Ciuleanu, Raluca Patru, Jeliazko Arabadjiev, Ivan Tonev, Zoran Andrić, Goran Stojanović, Mihaela P
Cancer Treatment and Research Communications.2026; 46: 101056. CrossRef - Impact of First-Line Maintenance Immunotherapy With or Without Pemetrexed for Non-Squamous Advanced/Metastatic Non-Small Cell Lung Cancer Lacking Targetable Mutations: A Real-World Analysis in England
Fabio Gomes, Niall Gilding, Vivian Tan, Katerina Christoforou, Joanne Rule, Amine Aziez, Adam Januszewski, Anna Minchom
Clinical Lung Cancer.2026;[Epub] CrossRef - A patient similarity-embedded Bayesian approach to prognostic biomarker inference with application to thoracic cancer immunity
Duo Yu, Meilin Huang, Michael J Kane, Brian P Hobbs
Journal of the Royal Statistical Society Series C: Applied Statistics.2025; 74(3): 800. CrossRef - Programmed Cell Death Ligand 1 (PD-L1) and major Histocompatibility Complex Class I (MHC Class I) Expression Patterns and Their Pathologic Associations in triple-Negative Breast Cancer
Ponkrit Kaewkedsri, Piyapharom Intarawichian, Sirawich Jessadapattarakul, Waritta Kunprom, Supinda Koonmee, Malinee Thanee, Ongart Somintara, Anongporn Wongbuddha, Payia Chadbunchachai, Supajit Nawapun, Chaiwat Aphivatanasiri
Breast Cancer: Targets and Therapy.2025; Volume 17: 123. CrossRef - Exploring immune checkpoint inhibitors: Focus on PD-1/PD-L1 axis and beyond
Durre Aden, Samreen Zaheer, Niti Sureka, Monal Trisal, Jai Kumar Chaurasia, Sufian Zaheer
Pathology - Research and Practice.2025; 269: 155864. CrossRef - Prognostic value of HER2 expression in cervical adenocarcinoma: A retrospective cohort study
Qing Xu, Zhuomin Yin, Yueqi Li, Xiu Zhu, Hanmei Lou, Juan Ni
Oncology Letters.2025; 29(5): 1. CrossRef - PD-1/PD-L1 blockade therapy with atezolizumab: a new paradigm in the treatment of non-small cell lung cancer (NSCLC)
Samaneh Moradi, Pedram Sarikhani, Rafid Jihad Albadr, Waam Mohammed Taher, Mariem Alwan, Mahmood Jasem Jawad, Hiba Mushtaq, Niyousha Vakilzadehian
Discover Oncology.2025;[Epub] CrossRef - PD‐L1 Scoring Models for Non‐Small Cell Lung Cancer in China: Current Status, AI‐Assisted Solutions and Future Perspectives
Ziling Huang, Shen Wang, Jiansong Zhou, Haiquan Chen, Yuan Li
Thoracic Cancer.2025;[Epub] CrossRef - Advancing Precision Medicine in PDAC: An Ethical Scoping Review and Call to Action for IHC Implementation
Lyanne A. Delgado-Coka, Lucia Roa-Peña, Andrew Flescher, Luisa F. Escobar-Hoyos, Kenneth R. Shroyer
Cancers.2025; 17(12): 1899. CrossRef - A Prospective Exploratory Study of Functional Immunity Assessed by Pretreatment Interferon Gamma Release Assay in Relation to Different Systemic Therapies in Patients With Advanced-Stage NSCLC
Hsu-Ching Huang, Han-Jhih Chang, Chi-Lu Chiang, Hsin-Yi Huang, Yung-Hung Luo, Yuh-Min Chen, Tsu-Hui Shiao, Chao-Hua Chiu
JTO Clinical and Research Reports.2025; 6(8): 100845. CrossRef - A panorama of lung cancer biomarkers
Ana Sofia Silva Mesquita, Maire Iumi Maeda, Juliana Cabral Duarte Brandão, Nicolle Cavalcante Gaglionone, Igor Campos da Silva, Milena Perez Mak, Ellen Caroline Toledo do Nascimento
Surgical and Experimental Pathology.2025;[Epub] CrossRef - Unraveling Resistance in Lung Cancer Immunotherapy: Clinical Milestones, Mechanistic Insights, and Future Strategies
Maria Vitale, Raffaella Pagliaro, Giuseppe Viscardi, Lucio Pastore, Giuseppe Castaldo, Fabio Perrotta, Susan F. Campbell, Andrea Bianco, Filippo Scialò
International Journal of Molecular Sciences.2025; 26(18): 9244. CrossRef - Precision Oncology in Lung Cancer Surgery
Patrick Bou-Samra, Sunil Singhal
Surgical Oncology Clinics of North America.2024; 33(2): 311. CrossRef - Expression of PD-L1 clones (22C3 and 28-8) in hepatocellular carcinoma: a tertiary cancer care hospital experience
Kashif Asghar, Shaarif Bashir, Muhammad Hassan, Asim Farooq, Muhammad Abu Bakar, Sundus Bilal, Maryam Hameed, Shafqat Mehmood, Asif Loya
Egyptian Liver Journal.2024;[Epub] CrossRef - Engineering Proteus mirabilis improves antitumor efficacy via enhancing cytotoxic T cell responses
Hong Zhang, Yinlin Luo, Xincheng Zhao, Xiande Liu
Molecular Therapy: Oncology.2024; 32(1): 200770. CrossRef - Unlocking the potential of oncology biomarkers: advancements in clinical theranostics
Ankit Kumar Dubey, Ishnoor Kaur, Reecha Madaan, Shikha Raheja, Rajni Bala, Manoj Garg, Suresh Kumar, Viney Lather, Vineet Mittal, Deepti Pandita, Rohit Gundamaraju, Rajeev K. Singla, Rohit Sharma
Drug Metabolism and Personalized Therapy.2024; 39(1): 5. CrossRef - Exploring histological predictive biomarkers for immune checkpoint inhibitor therapy response in non–small cell lung cancer
Uiju Cho, Soyoung Im, Hyung Soon Park
Journal of Pathology and Translational Medicine.2024; 58(2): 49. CrossRef - Predicting efficacy assessment of combined treatment of radiotherapy and nivolumab for NSCLC patients through virtual clinical trials using QSP modeling
Miriam Schirru, Hamza Charef, Khalil-Elmehdi Ismaili, Frédérique Fenneteau, Didier Zugaj, Pierre-Olivier Tremblay, Fahima Nekka
Journal of Pharmacokinetics and Pharmacodynamics.2024; 51(4): 319. CrossRef - Predictions of PD-L1 Expression Based on CT Imaging Features in Lung Squamous Cell Carcinoma
Seong Hee Yeo, Hyun Jung Yoon, Injoong Kim, Yeo Jin Kim, Young Lee, Yoon Ki Cha, So Hyeon Bak
Journal of the Korean Society of Radiology.2024; 85(2): 394. CrossRef - Assessment of Programmed Cell Death Ligand-1 Expression in Non-small Cell Lung Carcinomas by Immunohistochemistry
Sandeep Mani, Archana Lakshmanan, Annapurneswari Subramanyan
Apollo Medicine.2024; 21(1): 57. CrossRef - The efficacy of immune checkpoint inhibitors on low PD‐L1 cervical cancer: A meta‐analysis
Wutao Chen, Nan Zhang, Zhihong He, Qing Li, You Wang, Weihua Lou, Wen Di
Health Science Reports.2024;[Epub] CrossRef - Molecular Characteristics and Pretreatment Neutrophil-to-Lymphocyte Ratio as Predictors of Durable Clinical Benefit from Immune Checkpoint Inhibition in Non-Small Cell Lung Cancer
Arpeet T. Shah, Isabelle Blanchard, Sukhmani K. Padda, Heather A. Wakelee, Joel W. Neal
Clinical Lung Cancer.2024; 25(6): 550. CrossRef - Optimizing immunofluorescence with high-dynamic-range imaging to enhance PD-L1 expression evaluation for 3D pathology assessment from NSCLC tumor tissue
Hsien-Neng Huang, Chun-Wei Kuo, Yu-Ling Hung, Chia-Hung Yang, Yu-Han Hsieh, Yu-Chieh Lin, Margaret Dah-Tsyr Chang, Yen-Yin Lin, Jen-Chung Ko
Scientific Reports.2024;[Epub] CrossRef - The Role of Programmed Cell Death Ligand 1 Expression in Pituitary Tumours: Lessons from the Current Literature
Mariana Lopes-Pinto, Ema Lacerda-Nobre, Ana Luísa Silva, Francisco Tortosa, Pedro Marques
Neuroendocrinology.2024; 114(8): 709. CrossRef - Real‑world evidence of advanced non‑small cell lung carcinoma treated with an immune checkpoint inhibitor plus chemotherapy
Zihan Xu, Huien Zhang, Guikai Ma, Wenjuan Meng, Junliang Du, Xin Wu, Baohong Yang, Ningning Wang, Yanhong Ding, Qingyun Zhang, Na Li, Xuede Zhang, Guohua Yu, Shuzhen Liu, Zhenhua Li
Oncology Letters.2024;[Epub] CrossRef - Clinical impact of concomitant BIO-three use in advanced or recurrent non-small cell lung cancer treated with immune-checkpoint inhibitor
Hitomi Nakatsukasa, Masaya Takahashi, Masahito Shibano, Yusuke Ishigami, Tomoya Kawaguchi, Yasutaka Nakamura, Hiroyasu Kaneda
International Journal of Clinical Oncology.2024; 29(12): 1840. CrossRef - Special issue “The advance of solid tumor research in China”: FGFR4 alterations predict efficacy of immune checkpoint inhibitors in nonsmall cell lung cancer
Guanghui Gao, Longgang Cui, Fei Zhou, Tao Jiang, Wanying Wang, Shiqi Mao, Fengying Wu, Fangli Jiang, Bei Zhang, Ting Bei, Wenchuan Xie, Cheng Zhang, Hougang Zhang, Chan Gao, Xiaochen Zhao, Yuezong Bai, Caicun Zhou, Shengxiang Ren
International Journal of Cancer.2023; 152(1): 79. CrossRef - Molecular Classification of Extrapulmonary Neuroendocrine Carcinomas With Emphasis on POU2F3-positive Tuft Cell Carcinoma
Jiwon Koh, Haeryoung Kim, Kyung Chul Moon, Cheol Lee, Kyoungbun Lee, Han Suk Ryu, Kyeong Cheon Jung, Yoon Kyung Jeon
American Journal of Surgical Pathology.2023; 47(2): 183. CrossRef - Biomarkers for Predicting Response to Personalized Immunotherapy in Gastric Cancer
Moonsik Kim, Ji Yun Jeong, An Na Seo
Diagnostics.2023; 13(17): 2782. CrossRef - PD-L1 Expression in Pituitary Neuroendocrine Tumors/Pituitary Adenomas
Giulia Cossu, Stefano La Rosa, Jean Philippe Brouland, Nelly Pitteloud, Ethan Harel, Federico Santoni, Maxime Brunner, Roy Thomas Daniel, Mahmoud Messerer
Cancers.2023; 15(18): 4471. CrossRef - Prognostic Significance of Programmed Cell Death Ligand 1 Expression in High-Grade Serous Ovarian Carcinoma: A Systematic Review and Meta-Analysis
Jeongwan Kang, Kang Min Han, Hera Jung, Hyunchul Kim
Diagnostics.2023; 13(20): 3258. CrossRef - A targeted expression panel for classification, gene fusion detection and PD-L1 measurements – Can molecular profiling replace immunohistochemistry in non-small cell lung cancer?
Anita Tranberg Simonsen, Amalie Utke, Johanne Lade-Keller, Lasse Westphal Thomsen, Torben Steiniche, Magnus Stougaard
Experimental and Molecular Pathology.2022; 125: 104749. CrossRef - Program death ligand‐1 immunocytochemistry in lung cancer cytological samples: A systematic review
Swati Satturwar, Ilaria Girolami, Enrico Munari, Francesco Ciompi, Albino Eccher, Liron Pantanowitz
Diagnostic Cytopathology.2022; 50(6): 313. CrossRef - Computer-assisted three-dimensional quantitation of programmed death-ligand 1 in non-small cell lung cancer using tissue clearing technology
Yen-Yu Lin, Lei-Chi Wang, Yu-Han Hsieh, Yu-Ling Hung, Yung-An Chen, Yu-Chieh Lin, Yen-Yin Lin, Teh-Ying Chou
Journal of Translational Medicine.2022;[Epub] CrossRef - Correlation Between Pretreatment Neutrophil-to-Lymphocyte Ratio and Programmed Death-Ligand 1 Expression as Prognostic Markers in Non-Small Cell Lung Cancer
Cristina-Florina Pirlog, Horia Teodor Cotan, Andreea Parosanu, Cristina Orlov Slavu, Ana Maria Popa, Cristian Iaciu, Mihaela Olaru, Alexandru Vlad Oprita, Irina Nita, Cornelia Nitipir
Cureus.2022;[Epub] CrossRef - Expert opinion on NSCLC small specimen biomarker testing — Part 2: Analysis, reporting, and quality assessment
Frédérique Penault-Llorca, Keith M. Kerr, Pilar Garrido, Erik Thunnissen, Elisabeth Dequeker, Nicola Normanno, Simon J. Patton, Jenni Fairley, Joshua Kapp, Daniëlle de Ridder, Aleš Ryška, Holger Moch
Virchows Archiv.2022; 481(3): 351. CrossRef - Novel Inflammasome-Based Risk Score for Predicting Survival and Efficacy to Immunotherapy in Early-Stage Non-Small Cell Lung Cancer
Chih-Cheng Tsao, Hsin-Hung Wu, Ying-Fu Wang, Po-Chien Shen, Wen-Ting Wu, Huang-Yun Chen, Yang-Hong Dai
Biomedicines.2022; 10(7): 1539. CrossRef - Comparative Analysis of Mutation Status and Immune Landscape for Squamous Cell Carcinomas at Different Anatomical sites
Wenqi Ti, Tianhui Wei, Jianbo Wang, Yufeng Cheng
Frontiers in Immunology.2022;[Epub] CrossRef - Pan-cancer analysis of the angiotensin II receptor-associated protein as a prognostic and immunological gene predicting immunotherapy responses in pan-cancer
Kai Hong, Yingjue Zhang, Lingli Yao, Jiabo Zhang, Xianneng Sheng, Lihua Song, Yu Guo, Yangyang Guo
Frontiers in Cell and Developmental Biology.2022;[Epub] CrossRef - Molecular imaging of immune checkpoints in oncology: Current and future applications
Shushan Ge, Tongtong Jia, Jihui Li, Bin Zhang, Shengming Deng, Shibiao Sang
Cancer Letters.2022; 548: 215896. CrossRef - PD-L1 expression and association with genetic background in pheochromocytoma and paraganglioma
Katerina Hadrava Vanova, Ondrej Uher, Leah Meuter, Suman Ghosal, Sara Talvacchio, Mayank Patel, Jiri Neuzil, Karel Pacak
Frontiers in Oncology.2022;[Epub] CrossRef - Case report: Variable response to immunotherapy in ovarian cancer: Our experience within the current state of the art
Nicoletta Provinciali, Marco Greppi, Silvia Pesce, Mariangela Rutigliani, Irene Maria Briata, Tania Buttiron Webber, Marianna Fava, Andrea DeCensi, Emanuela Marcenaro
Frontiers in Immunology.2022;[Epub] CrossRef - PD-L1 Is Preferentially Expressed in PIT-1 Positive Pituitary Neuroendocrine Tumours
John Turchini, Loretta Sioson, Adele Clarkson, Amy Sheen, Anthony J. Gill
Endocrine Pathology.2021; 32(3): 408. CrossRef - Comprehensive tumor molecular profile analysis in clinical practice
Mustafa Özdoğan, Eirini Papadopoulou, Nikolaos Tsoulos, Aikaterini Tsantikidi, Vasiliki-Metaxa Mariatou, Georgios Tsaousis, Evgenia Kapeni, Evgenia Bourkoula, Dimitrios Fotiou, Georgios Kapetsis, Ioannis Boukovinas, Nikolaos Touroutoglou, Athanasios Fassa
BMC Medical Genomics.2021;[Epub] CrossRef - CT-Based Hand-crafted Radiomic Signatures Can Predict PD-L1 Expression Levels in Non-small Cell Lung Cancer: a Two-Center Study
Zekun Jiang, Yinjun Dong, Linke Yang, Yunhong Lv, Shuai Dong, Shuanghu Yuan, Dengwang Li, Liheng Liu
Journal of Digital Imaging.2021; 34(5): 1073. CrossRef - Deep Learning of Histopathological Features for the Prediction of Tumour Molecular Genetics
Pierre Murchan, Cathal Ó’Brien, Shane O’Connell, Ciara S. McNevin, Anne-Marie Baird, Orla Sheils, Pilib Ó Broin, Stephen P. Finn
Diagnostics.2021; 11(8): 1406. CrossRef - Molecular Imaging and the PD-L1 Pathway: From Bench to Clinic
David Leung, Samuel Bonacorsi, Ralph Adam Smith, Wolfgang Weber, Wendy Hayes
Frontiers in Oncology.2021;[Epub] CrossRef - A Comparative Study of Immunotherapy as Second-Line Treatment and beyond in Patients with Advanced Non-Small-Cell Lung Carcinoma
Jerónimo Rafael Rodríguez-Cid, Sonia Carrasco-Cara Chards, Iván Romarico González-Espinoza, Vanessa García-Montes, Julio César Garibay-Díaz, Osvaldo Hernández-Flores, Rodrigo Riera-Sala, Anna Gozalishvili-Boncheva, Jorge Arturo Alatorre-Alexander, Luis Ma
Lung Cancer Management.2021;[Epub] CrossRef - Clinical utility of the C‐reactive protein:albumin ratio in non‐small cell lung cancer patients treated with nivolumab
Taisuke Araki, Kazunari Tateishi, Kei Sonehara, Shuko Hirota, Masamichi Komatsu, Manabu Yamamoto, Shintaro Kanda, Hiroshi Kuraishi, Masayuki Hanaoka, Tomonobu Koizumi
Thoracic Cancer.2021; 12(5): 603. CrossRef - Programmed Cell Death Ligand 1‐Transfected Mouse Bone Marrow Mesenchymal Stem Cells as Targeted Therapy for Rheumatoid Arthritis
Qiong-ying Hu, Yun Yuan, Yu-chen Li, Lu-yao Yang, Xiang-yu Zhou, Da-qian Xiong, Zi-yi Zhao, Hiroshi Tanaka
BioMed Research International.2021;[Epub] CrossRef - PD-L1 testing and clinical management of newly diagnosed metastatic non-small cell lung cancer in Spain: MOREL study
Belen Rubio-Viqueira, Margarita Majem Tarruella, Martín Lázaro, Sergio Vázquez Estévez, Juan Felipe Córdoba-Ortega, Inmaculada Maestu Maiques, Jorge García González, Ana Blasco Cordellat, Javier Valdivia-Bautista, Carmen González Arenas, Jose Miguel Sánch
Lung Cancer Management.2021;[Epub] CrossRef - Can Systems Biology Advance Clinical Precision Oncology?
Andrea Rocca, Boris N. Kholodenko
Cancers.2021; 13(24): 6312. CrossRef - Do we need PD‐L1 as a biomarker for thyroid cytologic and histologic specimens?
Marc P. Pusztaszeri, Massimo Bongiovanni, Fadi Brimo
Cancer Cytopathology.2020; 128(3): 160. CrossRef - Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment
Andrea Botticelli, Pamela Vernocchi, Federico Marini, Andrea Quagliariello, Bruna Cerbelli, Sofia Reddel, Federica Del Chierico, Francesca Di Pietro, Raffaele Giusti, Alberta Tomassini, Ottavia Giampaoli, Alfredo Miccheli, Ilaria Grazia Zizzari, Marianna
Journal of Translational Medicine.2020;[Epub] CrossRef - Immune checkpoint inhibitors in advanced non–small cell lung cancer: A metacentric experience from India
Santosh Kumar, Srujana Joga, Bivas Biswas, Deepak Dabkara, Kuruswamy Thurai Prasad, Navneet Singh, Prabhat Singh Malik, Sachin Khurana, Sandip Ganguly, Valliappan Muthu, Ullas Batra
Current Problems in Cancer.2020; 44(3): 100549. CrossRef - Utility of CT radiomics for prediction of PD‐L1 expression in advanced lung adenocarcinomas
Jiyoung Yoon, Young Joo Suh, Kyunghwa Han, Hyoun Cho, Hye‐Jeong Lee, Jin Hur, Byoung Wook Choi
Thoracic Cancer.2020; 11(4): 993. CrossRef - Immune checkpoint inhibitors of the PD-1/PD-L1-axis in non-small cell lung cancer: promise, controversies and ambiguities in the novel treatment paradigm
Lars H. Breimer, Petros Nousios, Louise Olsson, Hans Brunnström
Scandinavian Journal of Clinical and Laboratory Investigation.2020; 80(5): 360. CrossRef - Tumour mutational burden as a biomarker for immunotherapy: Current data and emerging concepts
Jean-David Fumet, Caroline Truntzer, Mark Yarchoan, Francois Ghiringhelli
European Journal of Cancer.2020; 131: 40. CrossRef - Precision Medicine for NSCLC in the Era of Immunotherapy: New Biomarkers to Select the Most Suitable Treatment or the Most Suitable Patient
Giovanni Rossi, Alessandro Russo, Marco Tagliamento, Alessandro Tuzi, Olga Nigro, Giacomo Vallome, Claudio Sini, Massimiliano Grassi, Maria Giovanna Dal Bello, Simona Coco, Luca Longo, Lodovica Zullo, Enrica Teresa Tanda, Chiara Dellepiane, Paolo Pronzato
Cancers.2020; 12(5): 1125. CrossRef - Current status and future perspectives of liquid biopsy in non-small cell lung cancer
Sunhee Chang, Jae Young Hur, Yoon-La Choi, Chang Hun Lee, Wan Seop Kim
Journal of Pathology and Translational Medicine.2020; 54(3): 204. CrossRef - Digital Pathology and PD-L1 Testing in Non Small Cell Lung Cancer: A Workshop Record
Fabio Pagni, Umberto Malapelle, Claudio Doglioni, Gabriella Fontanini, Filippo Fraggetta, Paolo Graziano, Antonio Marchetti, Elena Guerini Rocco, Pasquale Pisapia, Elena V. Vigliar, Fiamma Buttitta, Marta Jaconi, Nicola Fusco, Massimo Barberis, Giancarlo
Cancers.2020; 12(7): 1800. CrossRef - PD-L1 in Systemic Immunity: Unraveling Its Contribution to PD-1/PD-L1 Blockade Immunotherapy
Ana Bocanegra, Ester Blanco, Gonzalo Fernandez-Hinojal, Hugo Arasanz, Luisa Chocarro, Miren Zuazo, Pilar Morente, Ruth Vera, David Escors, Grazyna Kochan
International Journal of Molecular Sciences.2020; 21(16): 5918. CrossRef - PD-1 blockade in recurrent or metastatic cervical cancer: Data from cemiplimab phase I expansion cohorts and characterization of PD-L1 expression in cervical cancer
Danny Rischin, Marta Gil-Martin, Antonio González-Martin, Irene Braña, June Y. Hou, Daniel Cho, Gerald S. Falchook, Silvia Formenti, Salma Jabbour, Kathleen Moore, Aung Naing, Kyriakos P. Papadopoulos, Joaquina Baranda, Wen Fury, Minjie Feng, Elizabeth St
Gynecologic Oncology.2020; 159(2): 322. CrossRef - Atezolizumab: A Review in Extensive-Stage SCLC
James E. Frampton
Drugs.2020; 80(15): 1587. CrossRef - Prognostic and clinicopathological roles of programmed death‐ligand 1 (PD‐L1) expression in thymic epithelial tumors: A meta‐analysis
Hyun Min Koh, Bo Gun Jang, Hyun Ju Lee, Chang Lim Hyun
Thoracic Cancer.2020; 11(11): 3086. CrossRef - Präzisionsmedizin bei NSCLC im Zeitalter der Immuntherapie: Neue Biomarker zur Selektion der am besten geeigneten Therapie oder des am besten geeigneten Patienten
Giovanni Rossi, Alessandro Russo, Marco Tagliamento, Alessandro Tuzi, Olga Nigro, Giacomo Vollome, Claudio Sini, Massimiliano Grassi, Maria Giovanna Dal Bello, Simona Coco, Luca Longo, Lodovica Zullo, Enrica Teresa Tanda, Chiara Dellepiane, Paolo Pronzato
Kompass Pneumologie.2020; 8(6): 300. CrossRef - Association with PD-L1 Expression and Clinicopathological Features in 1000 Lung Cancers: A Large Single-Institution Study of Surgically Resected Lung Cancers with a High Prevalence of EGFR Mutation
Seung Eun Lee, Yu Jin Kim, Minjung Sung, Mi-Sook Lee, Joungho Han, Hong Kwan Kim, Yoon-La Choi
International Journal of Molecular Sciences.2019; 20(19): 4794. CrossRef - Detailed Characterization of Immune Cell Infiltrate and Expression of Immune Checkpoint Molecules PD-L1/CTLA-4 and MMR Proteins in Testicular Germ Cell Tumors Disclose Novel Disease Biomarkers
João Lobo, Ângelo Rodrigues, Rita Guimarães, Mariana Cantante, Paula Lopes, Joaquina Maurício, Jorge Oliveira, Carmen Jerónimo, Rui Henrique
Cancers.2019; 11(10): 1535. CrossRef - Basis of PD1/PD-L1 Therapies
Barbara Seliger
Journal of Clinical Medicine.2019; 8(12): 2168. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
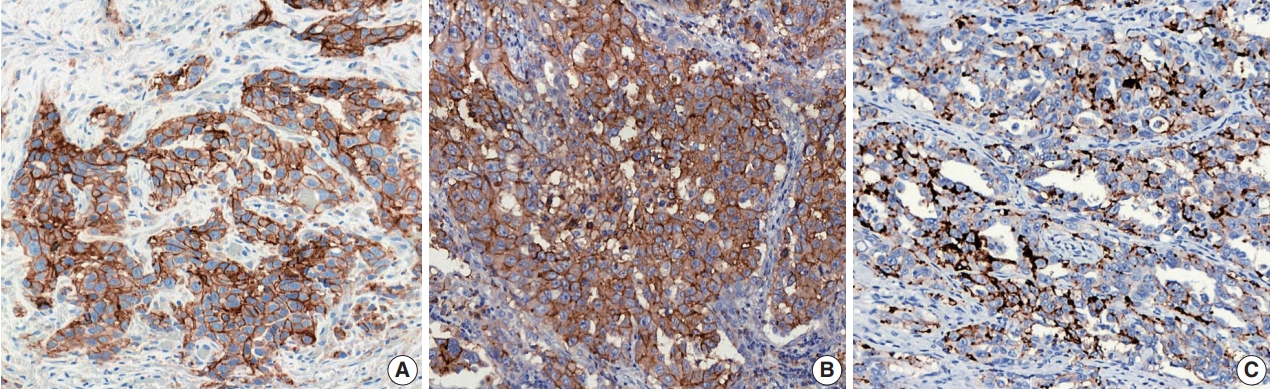
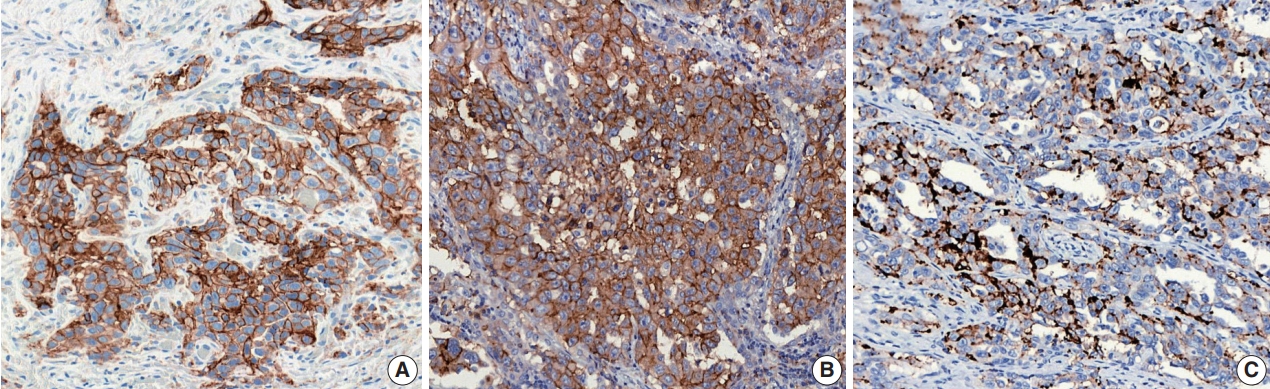
- Figure

Fig. 1.
| PD-L1 assay | |||||
|---|---|---|---|---|---|
| Performance | Clone | 22C3 | 28-8 | SP263 | SP142 |
| Developer | Dako | Dako | Ventana | Ventana | |
| Host species | Mouse monoclonal | Rabbit monoclonal | Rabbit monoclonal | Rabbit monoclonal | |
| Epitope location | Extracellular domain | Extracellular domain | Cytoplasmic domain | Cytoplasmic domain | |
| Platform | Link 48 autostainer | Link 48 autostainer | Benchmark ultra | Benchmark ultra | |
| Detection kit | Envision FLEX | Envision FLEX | Optiview | Optiview | |
| Amplification | No | No | No | Yes | |
| Interpretation | Scoring | TC | TC | TC | TC and IC |
| Staining pattern for positivity | Membranous | Membranous | Membranous ± cytoplasmic | Membranous±cytoplasmic | |
| Minimum TC number | 100 | 100 | 100 | 50 with associated stroma | |
| Cut-off (mandatory) | ≥ 50% (≥ 2nd line), ≥ 1% (1st line) | All comer | All comer | All comer | |
| Cut-off (proven survival benefit) | ≥ 1%, ≥ 5%, ≥ 10% | ≥ 25% (for durvalumab), ≥ 10% (for nivolumab) |
TC ≥ 5% or IC ≥ 5% | ||
| Pharma | Immune checkpoint inhibitor | Pembrolizumab | Nivolumab | Durvalumab Nivolumab |
Atezolizumab |
| FDA approval | Companion | Complementary | Complementary | Complementary | |
PD-L1, programmed death-ligand 1; TC, tumor cell; IC, immune cell; FDA, Food and Drug Administration. Applied only in Korea; Approved by Conformite Europeenne (CE) and Korea Food and Drug Administration.

 E-submission
E-submission