Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 53(6); 2019 > Article
-
Original Article
Comparison of Squamous Cell Carcinoma of the Tongue between Young and Old Patients -
Gyuheon Choi
 , Joon Seon Song
, Joon Seon Song , Seung-Ho Choi1
, Seung-Ho Choi1 , Soon Yuhl Nam1
, Soon Yuhl Nam1 , Sang Yoon Kim1
, Sang Yoon Kim1 , Jong-Lyel Roh1
, Jong-Lyel Roh1 , Bu-Kyu Lee2
, Bu-Kyu Lee2 , Kyung-Ja Cho
, Kyung-Ja Cho
-
Journal of Pathology and Translational Medicine 2019;53(6):369-377.
DOI: https://doi.org/10.4132/jptm.2019.09.16
Published online: October 11, 2019
Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
1Department of Otolaryngology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
2Department of Oral and Maxillofacial Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- Corresponding Author: Kyung-Ja Cho, MD, PhD, Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea Tel: +82-2-3010-4545, Fax: +82-2-472-7898, E-mail: kjc@amc.seoul.kr
© 2019 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- The worldwide incidence of squamous cell carcinoma of the tongue (SCCOT) in young patients has been increasing. We investigated clinicopathologic features of this unique population and compared them with those of SCCOT in the elderly to delineate its pathogenesis.
-
Methods
- We compared clinicopathological parameters between patients under and over 45 years old. Immunohistochemical assays of estrogen receptor, progesterone receptor, androgen receptor, p53, p16, mdm2, cyclin D1, and glutathione S-transferase P1 were also compared between them.
-
Results
- Among 189 cases, 51 patients (27.0%) were under 45 years of age. A higher proportion of women was seen in the young group, but was not statistically significant. Smoking and drinking behaviors between age groups were similar. Histopathological and immunohistochemical analysis showed no significant difference by age and sex other than higher histologic grades observed in young patients.
-
Conclusions
- SCCOT in young adults has similar clinicopathological features to that in the elderly, suggesting that both progress via similar pathogenetic pathways.
- Study population
- After searching an anonymized research database at Asan Medical Center, 295 cases of histologically confirmed SCCOT were found from 2005 to 2012. Ninety-six cases were excluded due to the lack of an available tissue block or clinical data, and 189 cases were finally retrieved for this retrospective study.
- Clinical data collection
- Clinical parameters collected from electronic medical records included age at onset, sex, smoking status, alcohol consumption level, treatment history, recurrence, and survival. Patients under 45 years old were classified as young patients. Smoking status was classified as non- or ex-smoker (no smoking for at least 1 year), or current smoker. Smokers were further categorized into light (<1 pack of cigarettes/day) and heavy smokers (≥1 pack of cigarettes/day). Drinkers with a history of more than seven drinks per week were considered heavy drinkers. A drink was defined as roughly 14 g of pure alcohol regardless of beverage type, equivalent to approximately 12 ounces of regular beer. Cutoff values of heavy smoking and drinking were set by as described in previous work [24-26] wherein patients over these cutoff values showed a significantly increased risk of oral epithelial dysplasia or cancer. Disease-free survival (DFS) was calculated from the date of initial pathological diagnosis to the date of radiological or clinical recurrence, while overall survival (OS) was calculated to the date of patient death.
- Pathological review and tissue microarray construction
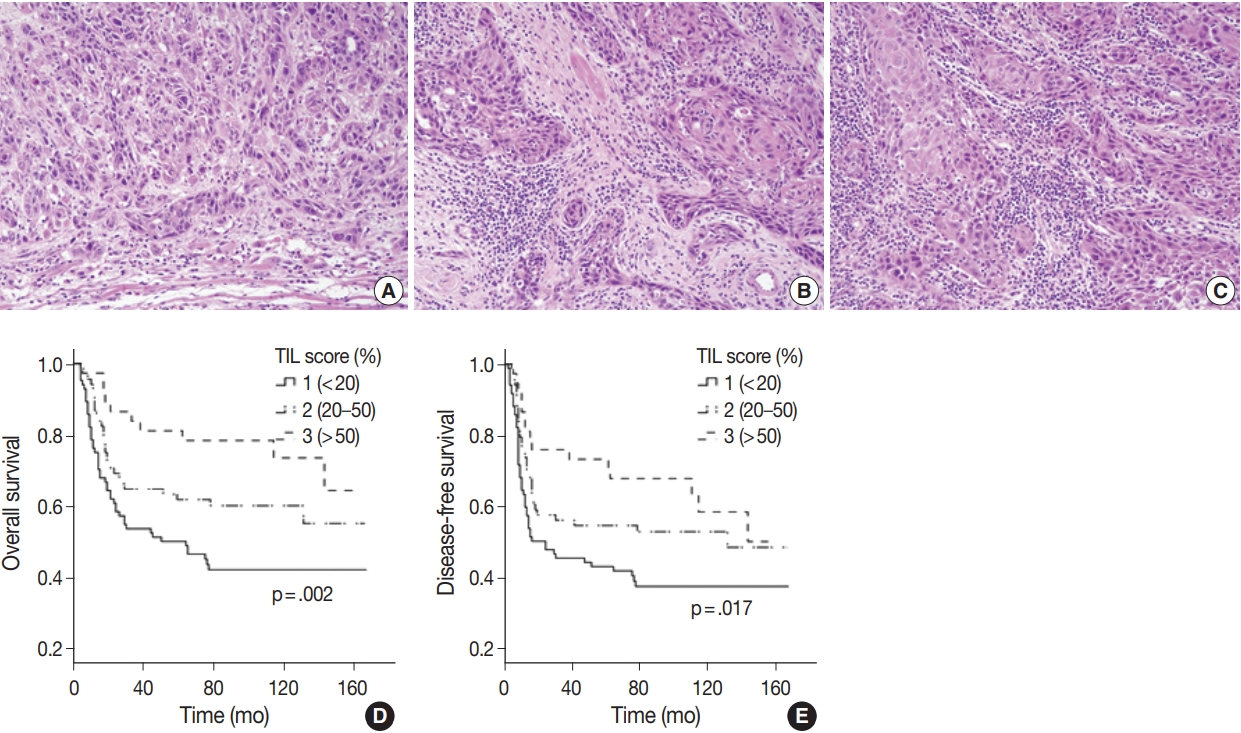
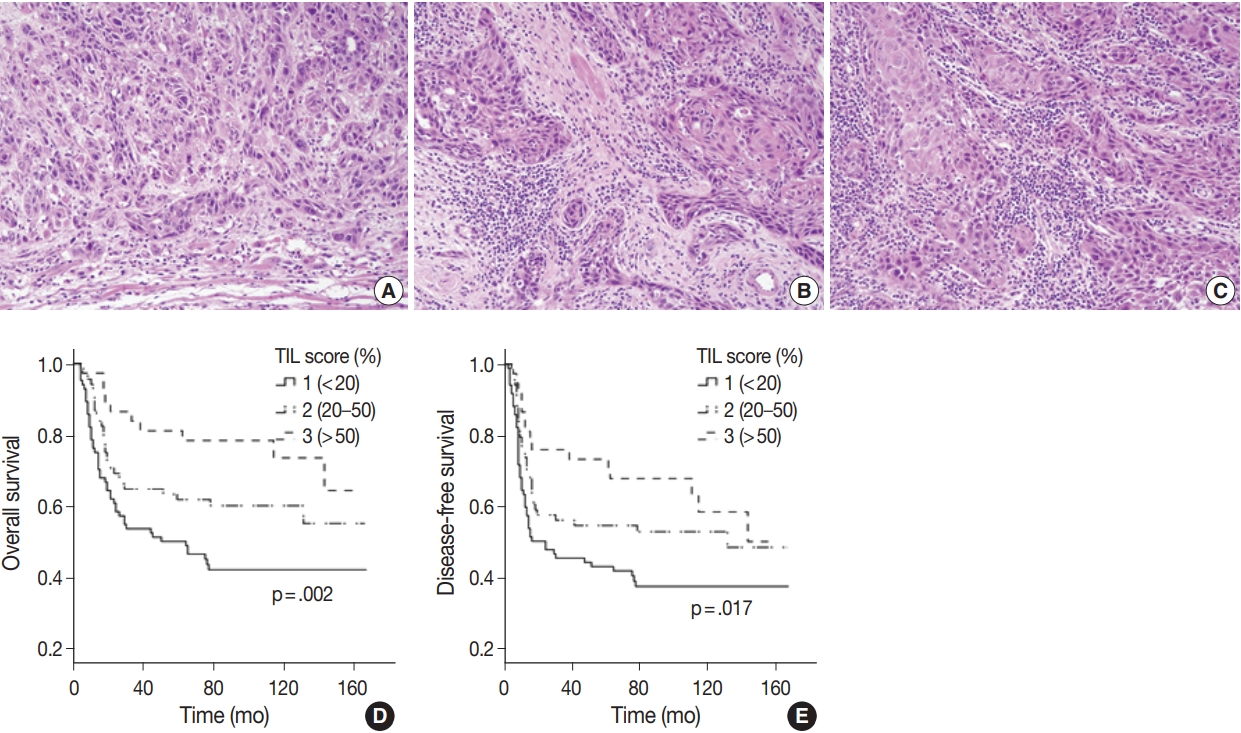
- All available hematoxylin and eosin slides were reviewed to obtain pathological parameters, such as tumor size, histologic grade, depth of invasion (DOI), stromal tumor-infiltrating lymphocytes (TIL) group, lymphovascular invasion (LVI), perineural invasion (PNI), and TNM stage. TNM stage for each case was revised based on the American Joint Committee on Cancer (AJCC), 8th edition [27]. TIL assessment criteria followed the International Immuno-Oncology Biomarker Working Group guidelines [28,29]. Each tumor was assigned to low, intermediate, or high TIL group according to the percentage of stromal area occupied by lymphocytic infiltrate (low, <20%; intermediate, 20%–50%; high, >50%). For the construction of tissue microarray (TMA) blocks, two tissue cores of approximately 2 mm were excised from the central area of the SCCOT and from tumor margins with normal mucosa.
- Immunohistochemistry
- IHC staining for p16 (1:6, clone E6H4, mouse mAb, Ventana Medical Systems, Tucson, AZ, USA), p53 (1:1,500, clone M7001, mouse mAb, Dako, Glostrup, Denmark), ER (1:200, clone 6F11, mouse mAb, Novocastra, Newcastle upon Tyne, UK), PR (1:200, clone 16, mouse mAb, Novocastra), AR (1:100, clone SP107, rabbit mAb, Cell Marque, Rocklin, CA, USA), mdm2 (1:50, clone SMP14, mouse mAb, Zeta, Arcadia, CA, USA), cyclin D1 (1:100, clone SP4, mouse mAb, Cell Marque), and GSTP1 (1:6,000, clone 3F2, mouse mAb, Cell Signaling, Danvers, MA, USA) was conducted in accordance with the manufacturer’s manual on a Ventana BenchMark XT Autostainer (Ventana Medical Systems). Serially cut 4-μm sections of the TMA block were deparaffinized, and antigen retrieval was carried out with EDTA buffer (cell conditioner #1) for 32 minutes (p16, p53, PR, AR, cyclin D1, and GSTP1) or 64 minutes (mdm2 and ER). After inactivation of endogenous peroxidase and rinsing with Tris buffer (reaction buffer), diluted primary antibodies were added and incubated for 16 minutes (p16, p53, PR, AR, cyclin D1, and GSTP1) or 32 minutes (mdm2 and ER) at 37°C.
- Expression levels of p53, p16, mdm2, and cyclin D1 were analyzed by a semiquantitative score based on the proportion of stained area (%) using criteria outlined in Table 1 and in reference to previous reports [19,30,31]. GSTP1 was interpreted as weak or strong by cytoplasmic staining intensity. IHC stains for ER, PR, and AR were considered as positive when nuclear expression was noted regardless of cellular proportion.
- Statistical analysis
- Statistical analysis was performed using SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA). Results for age, tumor size, and DOI were described with mean and 95% confidence interval. The Mann-Whitney U test was used to compare tumor size and DOI between age and sex groups. The Kruskal-Wallis test was used for comparisons among the four groups (young men, young women, old men, and old women). Other clinicopathological parameters and IHC results were compared using Pearson’s chi-square test and the Fisher’s exact test. OS and DFS were analyzed according to the Kaplan-Meier method with univariate analysis (log-rank test). All calculated p-values were 2-sided, and values less than 0.05 were considered statistically significant.
- Ethics statement
- All procedures performed for the current study were approved by the Institutional Review Board (IRB) of Asan Medical Center (approval No. 2018-0395) in accordance with the 1964 Helsinki declaration and its later amendments. Formal written informed consent was waived by the IRB.
MATERIALS AND METHODS
- Patients were divided into four groups according to age and sex; young men, young women, old men, and old women. Clinical characteristics are listed in Table 2, and histopathological data are listed in Table 3. We also compared patients between age and sex groups. Clinicopathological data therein are included in the Supplementary Materials (Supplementary Tables S1–4).
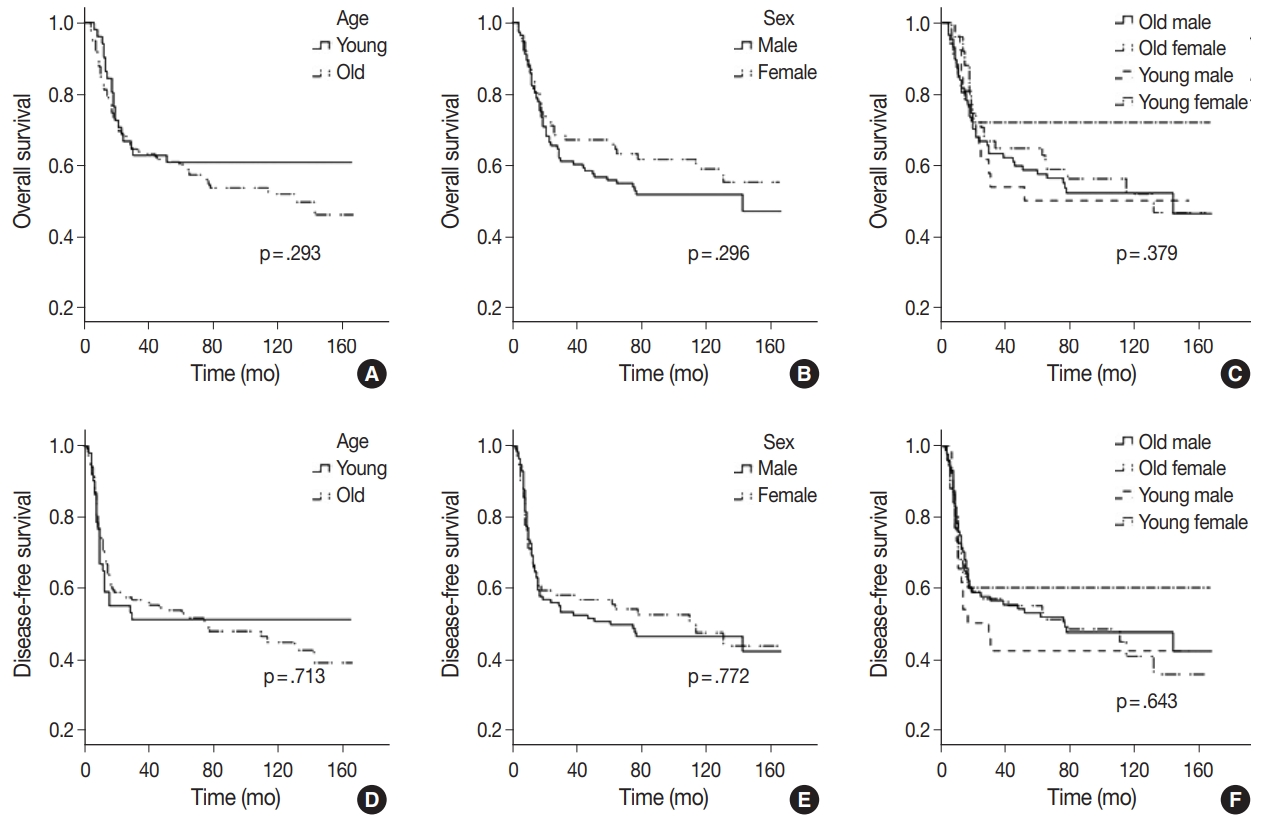
- Age at diagnosis ranged from 20.7 to 88.0 years (median, 56.1 years; 95% confidence interval, 52.88 to 57.26). Young patients accounted for 27.0% (51/189). Smoking and drinking status were markedly different between sexes, but not age groups (Tables 2, Supplementary Tables S1, 2). Most smokers (61/69, 88.4%) and regular drinkers (86/99, 86.9%) were men. Proportions of heavy smokers and drinkers were slightly lower in young patients, but these differences were not statistically significant. Women comprised 40.2% (72 of 189) of all patients. More women were in the young patient group (25 of 51, 49%), and more likely to be young non- or ex-smokers (21 of 30, 70%). OS and DFS were not significantly different by age (young or old) or sex (Fig. 1). Young women presented with relatively lower mortality rates (28.0%) than the other groups combined (48.2%), but this difference was not statistically significant (p=.082). Relatively poor survival among young men (50.0% vs 44.8% of others) was also observed but not statistically significant (p=.678).
- Histologic grade tended to be higher in young patients (p=.021) but did not vary between sexes (Supplementary Tables S3, 4). When we compared the four groups divided by sex and age, none of the histopathological parameters including tumor size, DOI, histologic grade, LVI, PNI, TIL group, T and N category were significantly different for any group (Table 3). Notably, higher TIL was correlated to better OS (p=.002) and DFS (p=.017) rates (Fig. 2).
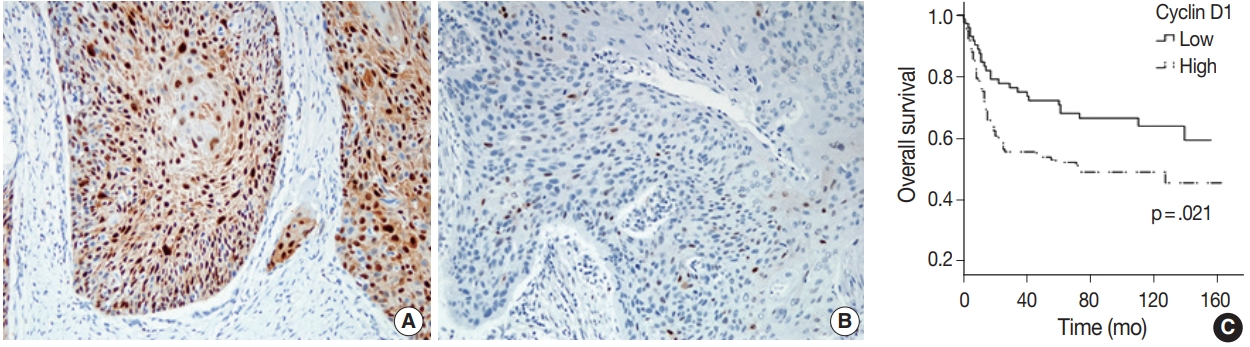
- IHC stains for sex hormone receptors were positive in a small number of patients. Only a single case presented with nuclear expression of ER (51.1-year-old male, non-smoker) and two cases presented with expression of AR (case 1, 40.2-year-old female, non-smoker; case 2, 56.7-year-old, male, non-smoker). PR was not expressed in any of the study participant samples. Expression levels as determined by IHC stainings of p16, p53, mdm2, GSTP1, and cyclin D1 were compared between patients grouped by age, sex, and smoking and drinking status, and no significant differences were found for any cohort (Table 4). Among immunomarkers, cyclin D1 expression was correlated to OS (p=.009) and DFS (p=.011) (Fig. 3). Other markers (p53, mdm2, p16, and GSTP1) were not correlated to any clinicopathological parameters.
RESULTS
- SCCOT in young and non-smoking patients has been reported since the 1980s but became a substantial issue after 2000 when epidemiologic evidence demonstrated an increasing incidence in this group. Previously, physicians thought that young SCCOT patients had poorer prognoses, and more aggressive treatments were used in this population. However, this notion has yet to be empirically substantiated [7]. Many studies attempted to find biological factors unique to young patients with SCCOT, but distinctive features were not found [11,32,33]. Indeed, SCCOT in young and old patients has not been found to exhibit relevant difference at the molecular level [34]. Pickering et al. [10] found certain genomic similarities between SCCOT in young patients and older smokers by whole-exome sequencing. However, these studies were mainly conducted by Western countries so data from Asian populations are insufficient. Recently, Sun et al. [35] reported that prognoses for young Chinese patients with oral SCC were similar to those for older patients. In this study, we also found equivalent results for old and young patients from Korea.
- Briefly, the only clinicopathological parameter that differed between young and old patients was histological grade (Table 3), which was found to be worse in the young group. Male-to-female ratios were also not statistically different by age, although a relatively higher proportion of women was observed in young patients. This tendency became more significant in the young, non-smoking group where women were predominant (21 of 27, 77.8%). This finding recapitulated those from previous studies reporting that young women with no history of tobacco or alcohol use were more vulnerable to SCCOT than their male counterparts [4]. This epidemiologic peculiarity aroused our interest in a potential relationship between SCCOT and sex hormone receptor expression.
- Previous studies reported ER expression in 11% to 50% of SCCOT cases [13,14,36]. In this study, however, we only observed focal ER expression in a single patient, despite the use of automated IHC staining with a well-established primary antibody and staining protocol. The only ER-expressing tumor found was that of an older male with no history of smoking. Interestingly, in other reports, most patients presenting with ER-positive head and neck cancer were also older males [13,14]. These results suggest that ER involvement might be primarily related to the original patient group of older men. PR was negative in all cases; this is also consistent with a previous report [14]. Immunoreactivity with nuclear expression for AR was seen in two cases, in contrast to previous reports reporting AR positivity in up to 67% of patients, most of them expressing AR in the cytoplasm [37]. Since true positivity for AR requires nuclear expression, the importance of AR expression in young SCCOT patients seems to be limited.
- Inter-individual variation in metabolic capacity for toxins could influence the carcinogenesis of SCCOT in young patients. GSTP1 is an important detoxifying enzyme, but a relationship between GSTP1 and oral carcinogenesis remains unclear. Genetic polymorphisms in the GSTP1 gene has been reported to be associated with impaired metabolism of carcinogens, thereby elevating the risk of several tumors, including head and neck cancer [21,38]. Soares et al. [17] observed increased GSTP1 expression in non-tumor margins in both smoking and drinking patients and suggested that this result could be a reaction to carcinogen exposure. In the current study, all tumors presented with diffuse GSTP1 expression and heterogeneous staining intensities. Overall intensity scores did not vary by age, sex or smoking or drinking status. We also found that strong GSTP1 expression was not related to prognosis. Epithelial tissues in non-tumor margins showed similar or slightly weaker intensities than those of matched tumor cells. These results suggest that expression of GSTP1 is not a suitable marker for individual cancer susceptibility or toxin exposure related to SCCOT.
- Other IHC markers, p53, cyclin D1, mdm2, and p16, were variably positive in a significant proportion of cases but their expression levels were not statistically different by age, sex or smoking or drinking status. Strong expression of cyclin D1 was associated with poor prognosis, a finding that is consistent with previous reports [15,19,39]. However, the lack of standardized interpretation criteria of cyclin D1 expression parameters compromises the reliability and integrity of these results. The relationship between cyclin D1 and SCCOT is worthy of further investigation, particularly with respect to standardizing the interpretation criteria. No standardized TIL assessment guideline for oral SCC exists, either. Several reports suggest a prognostic impact of TIL on oral SCC [40,41] but they used different assessment methods and demonstrated conflicting results. We applied the TIL assessment method used in breast cancer [29] and found a significant correlation between TIL and OS, implying that this method might be a candidate for standardized assessment.
- This study evaluated the clinicopathological parameters and expression profiles of several tumorigenic candidate proteins of SCCOT and found no significant difference between young and old patients nor between male and female patients. Despite epidemiologic idiosyncrasy, SCCOT in young women appears to be similar to that in older men. Previous studies reported similar data and came to similar conclusions [6-8,10,11,42]. These results, combined with epidemiological data, suggest the presence of unknown carcinogenic factors contributing to an emerging incidence of SCCOT in young women via a similar pathogenetic sequence to that associated with known risk factors. We investigated two candidates for these factors, hormone receptors and GSTP1, but significant findings were not observed. Considering that known risk factors are primarily associated with toxin exposure (tobacco, betel quid, or alcohol), extrinsic factors appear to be more important than individual factors of age, sex, or intrinsic metabolic activity in the pathogenesis of SCCOT. Possible exposure to toxic materials associated with altered lifestyles or new environmental pollutants should thereby be investigated in the young female SCCOT population.
DISCUSSION
Electronic Supplementary Material
Author contributions
Conceptualization: KJC.
Data curation: KJC, GC.
Formal analysis: GC.
Funding acquisition: KJC.
Investigation: GC.
Methodology: KJC.
Project administration: GC.
Resources: SHC, SYN, SYK, JLR, BKL.
Supervision: KJC.
Validation: KJC, JSS.
Visualization: GC.
Writing – original draft preparation: GC.
Writing – review & editing: KJC, JSS.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding
No funding to declare.

| Marker | Score | Cells stained (%) |
|---|---|---|
| p53 | 0 | 0 |
| 1+ | < 10 | |
| 2+ | 10 to < 50 | |
| 3+ | ≥ 50 | |
| mdm2 | 0 | 0 |
| 1+ | < 10 | |
| 2+ | 10 to < 50 | |
| 3+ | ≥ 50 | |
| Cyclin D1 | Low | < 50 |
| High | ≥ 50 | |
| p16 | 0 | 0 |
| 1+ | < 5 | |
| 2+ | 5 to < 25 | |
| 3+ | ≥ 25 |
- 1. Scully C, Bagan J. Oral squamous cell carcinoma: overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis 2009; 15: 388-99. ArticlePubMed
- 2. Ng JH, Iyer NG, Tan MH, Edgren G. Changing epidemiology of oral squamous cell carcinoma of the tongue: a global study. Head Neck 2017; 39: 297-304. ArticlePubMedPDF
- 3. Patel SC, Carpenter WR, Tyree S, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol 2011; 29: 1488-94. ArticlePubMed
- 4. Harris SL, Kimple RJ, Hayes DN, Couch ME, Rosenman JG. Never-smokers, never-drinkers: unique clinical subgroup of young patients with head and neck squamous cell cancers. Head Neck 2010; 32: 499-503. ArticlePubMed
- 5. Choi SW, Moon EK, Park JY, et al. Trends in the incidence of and survival rates for oral cavity cancer in the Korean population. Oral Dis 2014; 20: 773-9. PubMed
- 6. Blanchard P, Belkhir F, Temam S, et al. Outcomes and prognostic factors for squamous cell carcinoma of the oral tongue in young adults: a single-institution case-matched analysis. Eur Arch Otorhinolaryngol 2017; 274: 1683-90. ArticlePubMedPDF
- 7. Goepfert RP, Kezirian EJ, Wang SJ. Oral tongue squamous cell carcinoma in young women: a matched comparison-do outcomes justify treatment intensity? ISRN Otolaryngol 2014; 2014: 529395.ArticlePubMedPMCPDF
- 8. Majchrzak E, Szybiak B, Wegner A, et al. Oral cavity and oropharyngeal squamous cell carcinoma in young adults: a review of the literature. Radiol Oncol 2014; 48: 1-10. ArticlePubMedPMCPDF
- 9. van Monsjou HS, Wreesmann VB, van den Brekel MW, Balm AJ. Head and neck squamous cell carcinoma in young patients. Oral Oncol 2013; 49: 1097-102. ArticlePubMed
- 10. Pickering CR, Zhang J, Neskey DM, et al. Squamous cell carcinoma of the oral tongue in young non-smokers is genomically similar to tumors in older smokers. Clin Cancer Res 2014; 20: 3842-8. ArticlePubMedPMCPDF
- 11. Santos HB, dos Santos TK, Paz AR, et al. Clinical findings and risk factors to oral squamous cell carcinoma in young patients: a 12-year retrospective analysis. Med Oral Patol Oral Cir Bucal 2016; 21: e151-6. ArticlePubMedPMC
- 12. Lingen MW, Chang KW, McMurray SJ, et al. Overexpression of p53 in squamous cell carcinoma of the tongue in young patients with no known risk factors is not associated with mutations in exons 5-9. Head Neck 2000; 22: 328-35. ArticlePubMed
- 13. Grimm M, Biegner T, Teriete P, et al. Estrogen and progesterone hormone receptor expression in oral cavity cancer. Med Oral Patol Oral Cir Bucal 2016; 21: e554-8. ArticlePubMedPMC
- 14. Colella G, Izzo G, Carinci F, et al. Expression of sexual hormones receptors in oral squamous cell carcinoma. Int J Immunopathol Pharmacol 2011; 24(2 Suppl): 129-32. ArticlePDF
- 15. Kaminagakura E, Werneck da Cunha I, Soares FA, Nishimoto IN, Kowalski LP. CCND1 amplification and protein overexpression in oral squamous cell carcinoma of young patients. Head Neck 2011; 33: 1413-9. ArticlePubMed
- 16. Lang J, Song X, Cheng J, Zhao S, Fan J. Association of GSTP1 Il-e105Val polymorphism and risk of head and neck cancers: a metaanalysis of 28 case-control studies. PLoS One 2012; 7: e48132. ArticlePubMedPMC
- 17. Soares PO, Maluf Cury P, Mendoza Lopez RV, et al. GTSP1 expression in non-smoker and non-drinker patients with squamous cell carcinoma of the head and neck. PLoS One 2017; 12: e0182600. ArticlePubMedPMC
- 18. Yanamoto S, Kawasaki G, Yoshitomi I, Mizuno A. p53, mdm2, and p21 expression in oral squamous cell carcinomas: relationship with clinicopathologic factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002; 94: 593-600. ArticlePubMed
- 19. Mineta H, Miura K, Takebayashi S, et al. Cyclin D1 overexpression correlates with poor prognosis in patients with tongue squamous cell carcinoma. Oral Oncol 2000; 36: 194-8. ArticlePubMed
- 20. Harris SL, Thorne LB, Seaman WT, Hayes DN, Couch ME, Kimple RJ. Association of p16(INK4a) overexpression with improved outcomes in young patients with squamous cell cancers of the oral tongue. Head Neck 2011; 33: 1622-7. ArticlePubMed
- 21. Karen-Ng LP, Marhazlinda J, Rahman ZA, et al. Combined effects of isothiocyanate intake, glutathione S-transferase polymorphisms and risk habits for age of oral squamous cell carcinoma development. Asian Pac J Cancer Prev 2011; 12: 1161-6. PubMed
- 22. Koenigs MB, Lefranc-Torres A, Bonilla-Velez J, et al. Association of estrogen receptor alpha expression with survival in oropharyngeal cancer following chemoradiation therapy. J Natl Cancer Inst 2019; 111: 933-42. ArticlePubMedPMCPDF
- 23. Mohamed H, Aro K, Jouhi L, et al. Expression of hormone receptors in oropharyngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol 2018; 275: 1289-300. ArticlePubMedPDF
- 24. Morse DE, Katz RV, Pendrys DG, et al. Smoking and drinking in relation to oral epithelial dysplasia. Cancer Epidemiol Biomarkers Prev 1996; 5: 769-77. PubMed
- 25. Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988; 48: 3282-7. PubMed
- 26. Morse DE, Psoter WJ, Cleveland D, et al. Smoking and drinking in relation to oral cancer and oral epithelial dysplasia. Cancer Causes Control 2007; 18: 919-29. ArticlePubMedPMCPDF
- 27. Amin MB, Edge S, Greene F, et al. AJCC cancer staging manual. 8th. New York: Springer, 2017.
- 28. Hendry S, Salgado R, Gevaert T, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immunooncology Biomarkers Working Group: Part 1: assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol 2017; 24: 235-51. ArticlePubMedPMC
- 29. Hendry S, Salgado R, Gevaert T, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol 2017; 24: 311-35. ArticlePubMedPMC
- 30. Ofner D, Maier H, Riedmann B, et al. Immunohistochemically detectable p53 and mdm-2 oncoprotein expression in colorectal carcinoma: prognostic significance. Clin Mol Pathol 1995; 48: M12-6. ArticlePubMedPMC
- 31. Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer 2001; 92: 276-84. ArticlePubMed
- 32. Zhang YY, Wang DC, Su JZ, Jia LF, Peng X, Yu GY. Clinicopathological characteristics and outcomes of squamous cell carcinoma of the tongue in different age groups. Head Neck 2017; 39: 2276-82. ArticlePubMedPDF
- 33. Dediol E, Sabol I, Virag M, Grce M, Muller D, Manojlovic S. HPV prevalence and p16INKa overexpression in non-smoking non-drinking oral cavity cancer patients. Oral Dis 2016; 22: 517-22. PubMed
- 34. Dos Santos Costa SF, Brennan PA, Gomez RS, et al. Molecular basis of oral squamous cell carcinoma in young patients: is it any different from older patients? J Oral Pathol Med 2018; 47: 541-6. ArticlePubMedPDF
- 35. Sun Q, Fang Q, Guo S. A comparison of oral squamous cell carcinoma between young and old patients in a single medical center in China. Int J Clin Exp Med 2015; 8: 12418-23. PubMedPMC
- 36. Chang YL, Hsu YK, Wu TF, et al. Regulation of estrogen receptor alpha function in oral squamous cell carcinoma cells by FAK signaling. Endocr Relat Cancer 2014; 21: 555-65. PubMed
- 37. Wu TF, Luo FJ, Chang YL, et al. The oncogenic role of androgen receptors in promoting the growth of oral squamous cell carcinoma cells. Oral Dis 2015; 21: 320-7. ArticlePubMed
- 38. Mutallip M, Nohata N, Hanazawa T, et al. Glutathione S-transferase P1 (GSTP1) suppresses cell apoptosis and its regulation by miR133alpha in head and neck squamous cell carcinoma (HNSCC). Int J Mol Med 2011; 27: 345-52. PubMed
- 39. Zhao Y, Yu D, Li H, et al. Cyclin D1 overexpression is associated with poor clinicopathological outcome and survival in oral squamous cell carcinoma in Asian populations: insights from a metaanalysis. PLoS One 2014; 9: e93210. ArticlePubMedPMC
- 40. Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol 2005; 29: 167-78. PubMed
- 41. Cho YA, Yoon HJ, Lee JI, Hong SP, Hong SD. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol 2011; 47: 1148-53. ArticlePubMed
- 42. Chang TS, Chang CM, Ho HC, et al. Impact of young age on the prognosis for oral cancer: a population-based study in Taiwan. PLoS One 2013; 8: e75855. ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Epidemiological trends and characteristics of oral tongue cancer in females: systematic review and meta-analysis
J. Hu, N. Kaunein, A. DeAngelis
International Journal of Oral and Maxillofacial Surgery.2026; 55(3): 267. CrossRef - Oral Tongue Squamous Cell Carcinoma in Young Adults in Brazil: Temporal Trends From 2013 to 2023
Natália Santos Barcelos, Yohana Cordeiro de Miranda Magno, Juliana Maria Braga Sclauser, Renata de Castro Martins, Rodnei Alves Marques, Maria Cássia Ferreira de Aguiar, Patrícia Carlos Caldeira
Oral Diseases.2026;[Epub] CrossRef - The Tongue Tells a Tale: Clinicopathological Case Study of Squamous Cell Carcinoma
Pankaj Kshirsagar, Manu S. Babu
Medical Journal of Dr. D.Y. Patil Vidyapeeth.2026; 19(2): 158. CrossRef - Oral Cavity Squamous Cell Carcinoma in Young Patients: A Multi-Institutional Study of the Canadian Head & Neck Collaborative Research Initiative
Xinyuan Hong, Alexandra E. Quimby, Dorsa Mavedatnia, A. Travis Pickett, Martin Corsten, Tinghua Zhang, Angelina Tohme, Stephanie Johnson-Obaseki, Carlos Khalil, Mark Khoury, Antoine Eskander, Hesameddin Noroozi, David Goldstein, John De Almeida, James Fow
Journal of Otolaryngology - Head & Neck Surgery.2025;[Epub] CrossRef - High Failure Rates in Young Nonsmoker Nondrinkers With Squamous Cell Carcinoma of the Oral Tongue
Brianna M. Jones, Dillan F. Villavisanis, Eric J. Lehrer, Daniel R. Dickstein, Kunal K. Sindhu, Krzysztof J. Misiukiewicz, Marshall Posner, Jerry T. Liu, Vishal Gupta, Sonam Sharma, Scott A. Roof, Marita Teng, Eric M. Genden, Richard L. Bakst
The Laryngoscope.2023; 133(5): 1110. CrossRef - Characteristics of oral squamous cell carcinoma focusing on cases unaffected by smoking and drinking: A multicenter retrospective study
Hiroyuki Harada, Masahiro Kikuchi, Ryo Asato, Kiyomi Hamaguchi, Hisanobu Tamaki, Masanobu Mizuta, Ryusuke Hori, Tsuyoshi Kojima, Keigo Honda, Takashi Tsujimura, Yohei Kumabe, Kazuyuki Ichimaru, Yoshiharu Kitani, Koji Ushiro, Morimasa Kitamura, Shogo Shino
Head & Neck.2023; 45(7): 1812. CrossRef - Genetic characteristics of advanced oral tongue squamous cell carcinoma in young patients
Sehui Kim, Chung Lee, Hyangmi Kim, Sun Och Yoon
Oral Oncology.2023; 144: 106466. CrossRef - Oral Squamous Cell Carcinoma Frequency in Young Patients from Referral Centers Around the World
Rafael Ferreira e Costa, Marina Luiza Baião Leão, Maria Sissa Pereira Sant’Ana, Ricardo Alves Mesquita, Ricardo Santiago Gomez, Alan Roger Santos-Silva, Syed Ali Khurram, Artysha Tailor, Ciska-Mari Schouwstra, Liam Robinson, Willie F. P. van Heerden, Rami
Head and Neck Pathology.2022; 16(3): 755. CrossRef - Early-onset oral cancer as a clinical entity: aetiology and pathogenesis
E.S. Kolegova, M.R. Patysheva, I.V. Larionova, I.K. Fedorova, D.E. Kulbakin, E.L. Choinzonov, E.V. Denisov
International Journal of Oral and Maxillofacial Surgery.2022; 51(12): 1497. CrossRef - The effect of age on the clinicopathological features of oral squamous cell carcinoma
Alaa S Saeed, Bashar H Abdullah
Journal of Baghdad College of Dentistry.2022; 34(1): 25. CrossRef - Survival Outcomes in Oral Tongue Cancer: A Mono-Institutional Experience Focusing on Age
Mohssen Ansarin, Rita De Berardinis, Federica Corso, Gioacchino Giugliano, Roberto Bruschini, Luigi De Benedetto, Stefano Zorzi, Fausto Maffini, Fabio Sovardi, Carolina Pigni, Donatella Scaglione, Daniela Alterio, Maria Cossu Rocca, Susanna Chiocca, Sara
Frontiers in Oncology.2021;[Epub] CrossRef - A Meta-analysis of Oral Squamous Cell Carcinoma in Young Adults with a Comparison to the Older Group Patients (2014–2019)
Khadijah Mohideen, C. Krithika, Nadeem Jeddy, Thayumanavan Balakrishnan, R. Bharathi, S. Leena Sankari
Contemporary Clinical Dentistry.2021; 12(3): 213. CrossRef - Modern perspectives of oral squamous cell carcinoma
A.A. Ivina
Arkhiv patologii.2020; 82(3): 55. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1.
Fig. 2.
Fig. 3.
| Marker | Score | Cells stained (%) |
|---|---|---|
| p53 | 0 | 0 |
| 1+ | < 10 | |
| 2+ | 10 to < 50 | |
| 3+ | ≥ 50 | |
| mdm2 | 0 | 0 |
| 1+ | < 10 | |
| 2+ | 10 to < 50 | |
| 3+ | ≥ 50 | |
| Cyclin D1 | Low | < 50 |
| High | ≥ 50 | |
| p16 | 0 | 0 |
| 1+ | < 5 | |
| 2+ | 5 to < 25 | |
| 3+ | ≥ 25 |
| Characteristic | Young (< 45 yr, n = 51) |
Old (≥ 45 yr, n = 138) |
p-value | ||
|---|---|---|---|---|---|
| Men (n = 26) | Women (n = 25) | Men (n = 87) | Women (n = 51) | ||
| Age (yr) | < .001 | ||||
| Mean (95% CI) | 35.5 (32.8–38.2) | 34.8 (32.0–37.6) | 61.5 (59.6–63.5) | 64.0 (60.8–67.2) | |
| Smoking status | < .001 | ||||
| Non- or ex-smoker | 9 (34.6) | 21 (84.0) | 43 (49.4) | 47 (92.2) | |
| Light smoker | 7 (26.9) | 3 (12.0) | 10 (11.5) | 1 (2.0) | |
| Heavy smoker | 10 (38.5) | 1 (4.0) | 34 (39.1) | 3 (5.9) | |
| Alcohol use | < .001 | ||||
| Abstain | 6 (23.1) | 20 (80.0) | 24 (27.6) | 45 (88.2) | |
| Light drinker | 15 (57.7) | 3 (12.0) | 35 (40.2) | 6 (11.8) | |
| Heavy drinker | 5 (19.2) | 2 (8.0) | 28 (32.2) | 0 | |
| Adjuvant treatment | .550 | ||||
| None | 9 (34.6) | 11 (44.0) | 42 (48.3) | 25 (49.0) | |
| Radiotherapy | 12 (46.2) | 8 (32.0) | 26 (29.9) | 20 (39.2) | |
| Chemotherapy | 0 | 0 | 5 (5.7) | 1 (2.0) | |
| Chemo + radiotherapy | 5 (19.2) | 6 (24.0) | 14 (16.1) | 5 (9.8) | |
| Recurrence (%) | 11 (42.3) | 9 (36.0) | 25 (28.7) | 18 (35.3) | .597 |
| Deceased (%) | 13 (50.0) | 7 (28.0) | 42 (48.3) | 24 (47.1) | .309 |
| Characteristic | Young (< 45, n = 51) |
Old (≥ 45, n = 138) |
p-value | ||
|---|---|---|---|---|---|
| Men (n = 26) | Women (n = 25) | Men (n = 87) | Women (n = 51) | ||
| Tumor size (cm) | .868 | ||||
| Mean (95% CI) | 2.7 (2.1–3.3) | 2.6 (2.0–3.1) | 2.5 (2.2–2.8) | 3.0 (2.1–3.8) | |
| Depth of invasion (mm) | .479 | ||||
| Mean (95% CI) | 11.9 (9.0–14.8) | 11.9 (8.4–15.3) | 10.1 (8.5–11.6) | 10.7 (9.0–12.4) | |
| Histological grade | .102 | ||||
| Well differentiated | 11 (42.3) | 7 (28.0) | 45 (51.7) | 26 (51.0) | |
| Moderately differentiated | 8 (30.8) | 13 (52.0) | 35 (40.2) | 19 (37.3) | |
| Poorly differentiated | 7 (26.9) | 5 (20.0) | 7 (8.0) | 6 (11.8) | |
| Lymphovascular invasion | .625 | ||||
| Present | 6 (23.1) | 6 (24.0) | 63 (72.4) | 42 (82.4) | |
| Absent | 20 (76.9) | 19 (76.0) | 24 (27.6) | 9 (17.6) | |
| Perineural invasion | .661 | ||||
| Present | 11 (42.3) | 10 (40.0) | 29 (33.3) | 22 (43.1) | |
| Absent | 15 (57.7) | 15 (60.0) | 58 (66.7) | 29 (56.9) | |
| Tumor-infiltrating lymphocyte | .335 | ||||
| Low | 13 (50.0) | 14 (56.0) | 53 (60.9) | 28 (54.9) | |
| Intermediate | 10 (38.5) | 7 (28.0) | 24 (27.6) | 10 (19.6) | |
| High | 3 (11.5) | 4 (16.0) | 10 (11.5) | 13 (25.5) | |
| T category | .569 | ||||
| T1 | 4 (15.4) | 2 (8.0) | 23 (26.4) | 26.4 (10.0) | |
| T2 | 9 (34.6) | 13 (52.0) | 30 (34.5) | 34.5 (17.0) | |
| T3 | 13 (50.0) | 10 (40.0) | 33 (37.9) | 37.9 (24.0) | |
| T4 | 0 | 0 | 1 (1.1) | 0 | |
| N category | .849 | ||||
| N0 | 11 (42.3) | 13 (52.0) | 47 (54.0) | 29 (56.9) | |
| N1 | 5 (19.2) | 4 (16.0) | 12 (13.8) | 9 (17.6) | |
| N2 | 6 (23.1) | 3 (12.0) | 18 (20.7) | 9 (17.6) | |
| N3 | 4 (15.4) | 5 (20.0) | 10 (11.5) | 4 (7.8) | |
| Age |
p-value | Sex |
p-value | Smoking |
p-value | Alcohol use |
p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Young (< 45 yr) | Old (≥ 45 yr) | Male | Female | Non- or ex-smoker | Light smoker | Heavy smoker | Abstain | Light drinker | Heavy drinker | |||||
| p16 | .072 | .893 | .465 | .307 | ||||||||||
| 0 | 27 (52.9) | 77 (55.8) | 60 (53.1) | 44 (57.9) | 61 (50.8) | 12 (57.1) | 31 (64.6) | 52 (54.7) | 29 (49.2) | 23 (65.7) | ||||
| 1+ | 8 (15.7) | 39 (28.3) | 29 (25.7) | 18 (23.7) | 34 (28.3) | 4 (19.0) | 9 (18.8) | 21 (22.1) | 21 (35.6) | 5 (14.3) | ||||
| 2+ | 7 (13.7) | 11 (8.0) | 12 (10.6) | 6 (7.9) | 12 (10.0) | 1 (4.8) | 5 (10.4) | 10 (10.5) | 4 (6.8) | 4 (11.4) | ||||
| 3+ | 9 (17.6) | 11 (8.0) | 12 (10.6) | 8 (10.5) | 13 (10.8) | 4 (19.0) | 3 (6.3) | 12 (12.6) | 5 (8.5) | 3 (8.6) | ||||
| p53 | .443 | .596 | .739 | .676 | ||||||||||
| 0 | 9 (17.6) | 20 (14.5) | 18 (15.9) | 11 (14.5) | 19 (15.8) | 5 (23.8) | 5 (10.4) | 13 (13.7) | 11 (18.6) | 5 (14.3) | ||||
| 1+ | 15 (29.4) | 28 (20.3) | 25 (22.1) | 18 (23.7) | 26 (21.7) | 5 (23.8) | 12 (25.0) | 18 (18.9) | 14 (23.7) | 11 (31.4) | ||||
| 2+ | 3 (5.9) | 13 (9.4) | 12 (10.6) | 4 (5/3) | 9 (7.5) | 1 (4.8) | 6 (12.5) | 8 (8.4) | 6 (10.2) | 2 (5.7) | ||||
| 3+ | 24 (47.1) | 77 (55.8) | 58 (51.3) | 43 (56.6) | 66 (55.0) | 10 (47.6) | 25 (52.1) | 56 (58.9) | 28 (47.5) | 17 (48.6) | ||||
| mdm2 | .442 | .403 | .464 | .877 | ||||||||||
| 0 | 31 (60.8) | 72 (52.2) | 57 (50.4) | 46 (60.5) | 69 (57.5) | 9 (42.9) | 25 (52.1) | 55 (57.9) | 30 (50.8) | 18 (51.4) | ||||
| 1+ | 19 (37.3) | 59 (42.8) | 51 (45.1) | 27 (35.5) | 45 (37.5) | 12 (57.1) | 21 (43.8) | 36 (37.9) | 26 (44.1) | 16 (45.7) | ||||
| 2+ | 1 (2.0) | 7 (5.1) | 5 (4.4) | 3 (3.9) | 6 (5.0) | 0 | 2 (4.2) | 4 (4.2) | 3 (5.1) | 1 (2.9) | ||||
| 3+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Cyclin D1 | .247 | .771 | .883 | .662 | ||||||||||
| Low | 16 (31.4) | 56 (40.6) | 44 (38.9) | 28 (36.8) | 46 (38.3) | 7 (33.3) | 19 (39.6) | 38 (40.0) | 23 (39.0) | 11 (31.4) | ||||
| High | 35 (68.6) | 82 (59.4) | 69 (61.1) | 48 (63.2) | 74 (61.7) | 14 (66.7) | 29 (60.4) | 57 (60.0) | 36 (61.0) | 24 (68.6) | ||||
| GSTP1 | .082 | .952 | .599 | .266 | ||||||||||
| Weak | 11 (21.6) | 16 (11.6) | 16 (14.2) | 11 (14.5) | 18 (15.0) | 4 (19.0) | 5 (10.4) | 16 (16.8) | 9 (15.3) | 2 (5.7) | ||||
| Strong | 40 (78.4) | 122 (88.0) | 97 (85.8) | 65 (85.5) | 102 (85.0) | 17 (81.0) | 43 (89.6) | 79 (83.2) | 50 (84.7) | 33 (94.3) | ||||
Values are presented as number (%) unless otherwise indicated. CI, confidence interval.
Values are presented as number (%) unless otherwise indicated. CI, confidence interval.

 E-submission
E-submission