Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 53(6); 2019 > Article
-
Original Article
A Multi-institutional Study of Prevalence and Clinicopathologic Features of Non-invasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features (NIFTP) in Korea -
Ja Yeong Seo
 , Ji Hyun Park
, Ji Hyun Park , Ju Yeon Pyo
, Ju Yeon Pyo , Yoon Jin Cha
, Yoon Jin Cha , Chan Kwon Jung1
, Chan Kwon Jung1 , Dong Eun Song2
, Dong Eun Song2 , Jeong Ja Kwak3
, Jeong Ja Kwak3 , So Yeon Park4
, So Yeon Park4 , Hee Young Na4
, Hee Young Na4 , Jang-Hee Kim5
, Jang-Hee Kim5 , Jae Yeon Seok6
, Jae Yeon Seok6 , Hee Sung Kim7
, Hee Sung Kim7 , Soon Won Hong
, Soon Won Hong
-
Journal of Pathology and Translational Medicine 2019;53(6):378-385.
DOI: https://doi.org/10.4132/jptm.2019.09.18
Published online: October 21, 2019
Department of Pathology, Thyroid Cancer Center, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
1Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
2Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
3Department of Pathology, Soonchunhyang University College of Medicine, Bucheon, Korea
4Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
5Department of Pathology, Ajou University School of Medicine, Suwon, Korea
6Department of Pathology, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
7Department of Pathology, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Korea
-
Corresponding Author: Chan Kwon Jung, MD, PhD, Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 222 Banpo-daero, Seocho-gu, Seoul 06591, Korea Tel: +82-2-2258-1622, Fax: +82-2-2258-1627, E-mail: ckjung@catholic.ac.kr
Corresponding Author: Soon Won Hong, MD, PhD, Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, 211 Eonju-ro, Gangnam-gu, Seoul 06273, Korea Tel: +82-2-2019-3540, Fax: +82-2-2019-3540, E-mail: SOONWONH@yuhs.ac
© 2019 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- In the present multi-institutional study, the prevalence and clinicopathologic characteristics of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) were evaluated among Korean patients who underwent thyroidectomy for papillary thyroid carcinoma (PTC).
-
Methods
- Data from 18,819 patients with PTC from eight university hospitals between January 2012 and February 2018 were retrospectively evaluated. Pathology reports of all PTCs and slides of potential NIFTP cases were reviewed. The strict criterion of no papillae was applied for the diagnosis of NIFTP. Due to assumptions regarding misclassification of NIFTP as non-PTC tumors, the lower boundary of NIFTP prevalence among PTCs was estimated. Mutational analysis for BRAF and three RAS isoforms was performed in 27 randomly selected NIFTP cases.
-
Results
- The prevalence of NIFTP was 1.3% (238/18,819) of all PTCs when the same histologic criteria were applied for NIFTP regardless of the tumor size but decreased to 0.8% (152/18,819) when tumors ≥1 cm in size were included. The mean follow-up was 37.7 months and no patient with NIFTP had evidence of lymph node metastasis, distant metastasis, or disease recurrence during the follow-up period. A difference in prevalence of NIFTP before and after NIFTP introduction was not observed. BRAFV600E mutation was not found in NIFTP. The mutation rate for the three RAS genes was 55.6% (15/27).
-
Conclusions
- The low prevalence and indolent clinical outcome of NIFTP in Korea was confirmed using the largest number of cases to date. The introduction of NIFTP may have a small overall impact in Korean practice.
- Study cohort
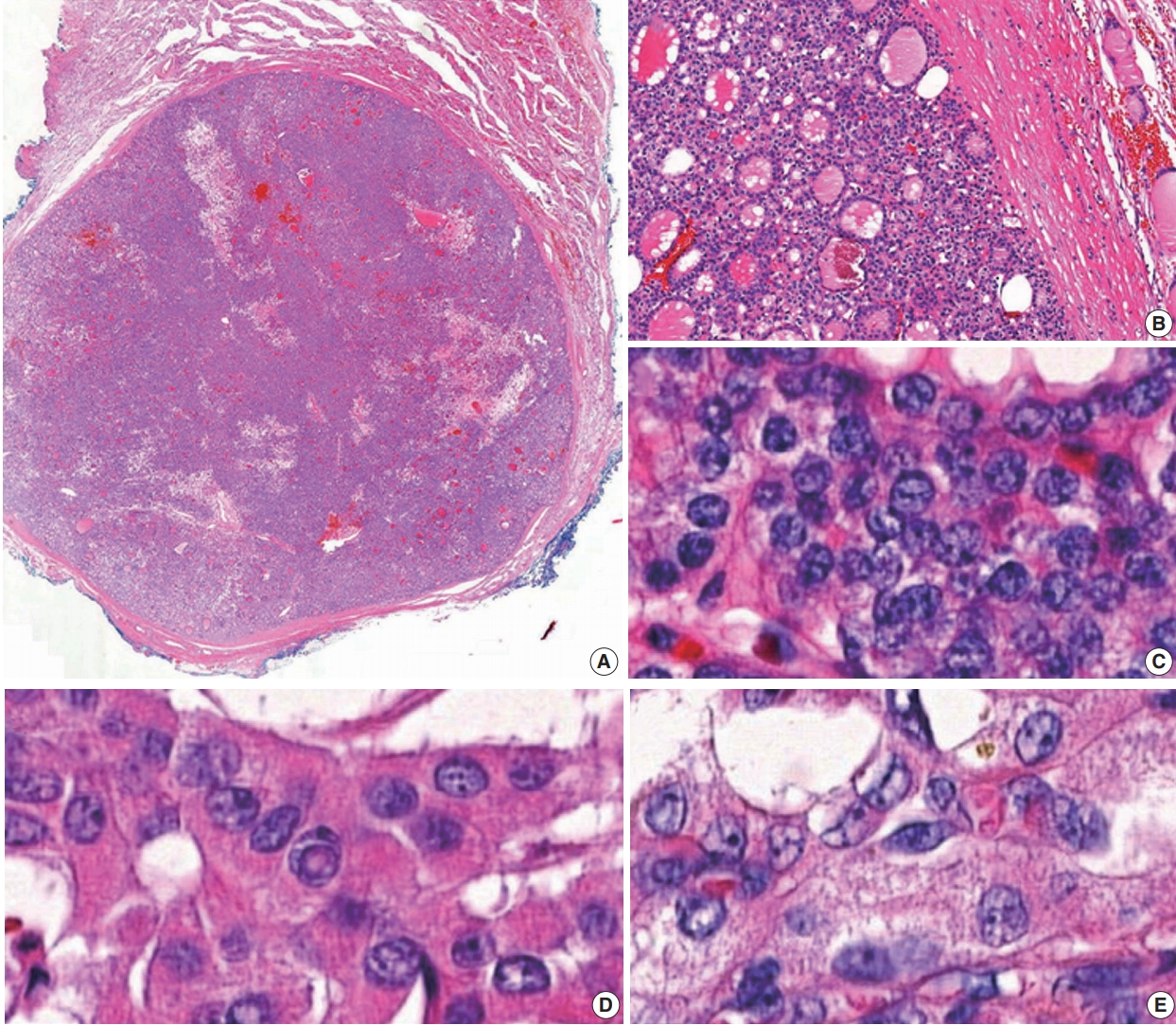
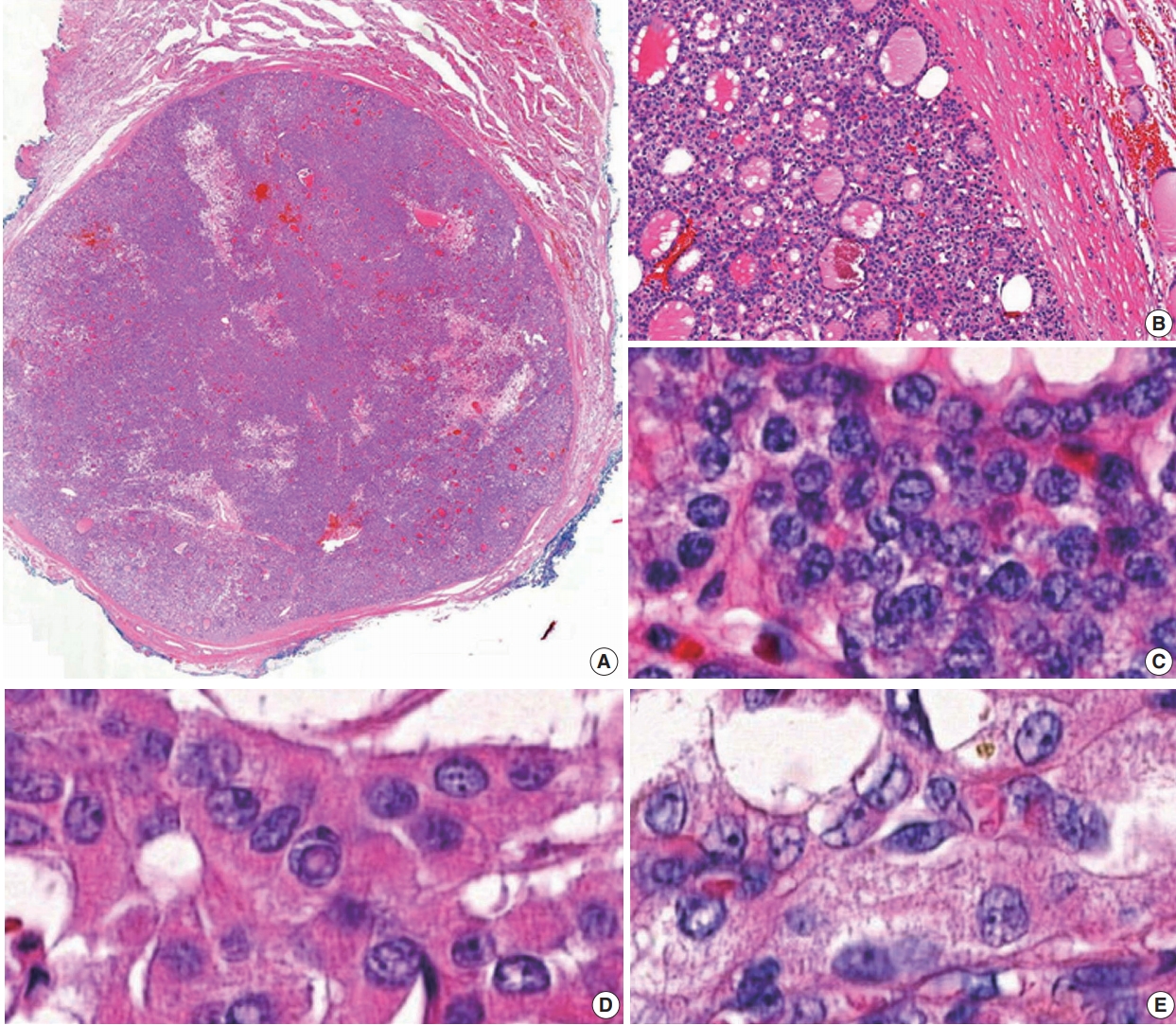
- Data from 18,819 patients with PTC from eight university hospitals were retrospectively analyzed. The tumors were diagnosed from surgically resected specimens between January 2012 and February 2018. The slides of all cases initially diagnosed as EFVPTC on pathology reports were reviewed and reclassified into NIFTP, invasive EFVPTC, and other PTC subtypes. The NIFTP was diagnosed according to the criteria of World Health Organization (WHO) Classification of Tumours of Endocrine [19] and recently revised diagnostic criteria [20]: (1) encapsulation or clear demarcation of the tumors (thick, thin, or partial capsule, or well circumscribed with a clear demarcation from adjacent thyroid tissues), (2) follicular growth pattern with no papillae (including microfollicular, normofollicular, or macrofollicular architecture with abundant colloid) with no psammoma bodies and <30% solid/trabecular/insular growth pattern, (3) nuclear score 2–3, (4) no capsular or vascular invasion (requires adequate microscopic examination of the tumor capsule interface), (5) no tumor necrosis, (6) no high mitotic activity (high mitotic activity defined as at least 3 mitoses per 10 high-power fields (400×) (Fig. 1). A nuclear score of 2–3 is diagnostic of NIFTP. However, if florid nuclear features (nuclear score 3) of PTC are present, a meticulous histopathologic examination of the entire tumor is required for the detection of any true papillae, psammoma bodies, aggressive histology, or invasion into tumor capsule or vessels.
- Molecular analysis of NRAS and HRAS genes
- Molecular analysis of 27 NIFTP cases obtained from a single institution was performed. Genomic DNA was extracted from paraffin-embedded thyroid specimen blocks. The representative slides were selected and the tumor tissues were manually dissected under a stereomicroscope and stored in a 1.5 mL tube. Genomic DNAs were extracted from 5–10-μm-thick tissue sections using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Exon 3 of NRAS and HRAS genes was amplified using polymerase chain reaction (PCR) with the following primers: (1) NRAS-exon 3–197 bp, forward (5'-CCCCTTACCCTCCACACC-3') and reverse (5'-GAGGTTAATATCCGCAAATGACTT-3'); (2) HRAS-exon 3–201 bp, forward (5'-GTCCTCCTGCAGGATTCCTA-3') and reverse (5'-CGGGGTTCACCTGTACT-3'). The PCR cycling conditions for NRAS and HRAS mutations were as follows: initial activation at 94°C for 15 minutes; 35 cycles at 94°C for 30 seconds, 51°C–57°C for 30 seconds, 72°C for 30 seconds, final extension at 72°C for 10 minutes. The amplicons were analyzed using 2% agarose gel electrophoresis and purified using QIAquick PCR purification kit (Qiagen). The amplified PCR products were sequenced using Sanger sequencing. The amplicons were evaluated on 2% agarose gel electrophoresis and purified using the QIAquick PCR purification kit (Qiagen). The amplified PCR products were analyzed using the automated sequencing machine ABI 3730 (Applied Biosystems, Foster City, CA, USA), which performed Sanger sequencing using the same PCR primers [17].
- Molecular analysis of KRAS gene
- For detection of the KRAS mutation, a PNAClamp KRAS mutation detection kit (Panagene, Daejeon, Korea) was used. The following reagents were used in all PCR reactions (total volume, 20 µL): 10 ng template DNA, primer, peptide nucleic acid (PNA) probe set, and SYBR Green PCR Master Mix. A CFX 96 (Bio-Rad, Hercules, CA, USA) was used for the real-time PCR of PNA-mediated clamping. PCR cycling conditions consisted of the following sequential steps: 5-minute hold at 94°C followed by 40 cycles at 94°C for 30 seconds, 70°C for 20 seconds, 63°C for 30 seconds, and 72°C for 30 seconds. All seven mutations in the KRAS gene were detected using one-step PNA-mediated real-time PCR clamping. PNA probes and DNA primers were used for the corresponding clamping reaction. SYBR Green fluorescent dye was applied for the detection of positive reaction signals. The amplification of the wild-type target was suppressed by the PNA probe sequence complementary to wild-type DNA. Due to this suppression, the amplification of mutant sequences was specifically preferred by competitive inhibition of DNA primers binding to wild-type DNA. The threshold cycle (Ct) value was used to evaluate PCR efficiency. The SYBR Green amplification plots were generated to analyze Ct values for the control and mutation assays. Mutation status was determined by Ct value differences ≥2, which were obtained between the control and samples.
- Molecular analysis of BRAF gene
- Mutational analysis of BRAF was performed using two different methods. The PNAClamp BRAF mutation detection kit (Panagene) was used to detect the BRAFV600E mutation. Each reaction tube had a total volume of 20 µL and included a mixture of template DNA, primers, PNA probe, and SYBR Green PCR Master Mix. Real-time PCR of PNA-clamping PCR was performed using a CFX96 real-time PCR system (Bio-Rad, Pleasanton, CA, USA). The PNA probe was complementary to wild-type (V600). PCR was performed under the following conditions: 5-minute hold at 94°C, 40 cycles of 30 seconds at 94°C, 20 seconds at 70°C, 30 seconds at 63°C, and 30 seconds at 72°C. The PNA probe and primers incorporated in the assay were separate oligonucleotides, and the PNA probe location was placed between forward and reverse primers within the template. Intercalation of SYBR Green fluorescent dye was used to detect positive signals.
- Pyrosequencing for the BRAF mutation analysis was performed as described in detail elsewhere [21]. The primers used for PCR were the following: forward primer (5'-GAAGACCTCACAGTAAAAATAG-3') and reverse primer (5'-biotin-ATAGCCTCAATTCTTACCATCC-3'). The pyrosequencing reaction was performed with a sequencing primer (5'-biotin-ATAGCCTCAATTCTTACCATCC-3') on a Pyromark Q24 instrument (Qiagen). The PyroMark Q24 software (Qiagen) was used for analysis of the pyrogram results.
- Statistical analysis
- Clinicopathological parameters of NIFTP before and after NIFTP introduction were analyzed using the chi-square test or Fisher’s exact test for categorical variables and the t-test for continuous variables. All statistical analyses were performed using SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
- Ethics statement
- The current study was approved by the Institutional Review Boards (IRBs) of eight institutions. Ethics approval for all procedures performed in the current study was obtained from the IRB (approval No. 3-2018-0271). Formal written informed consent was waived by the IRB.
MATERIALS AND METHODS
- The prevalence of NIFTP in PTC cases
- To evaluate the incidence of NIFTP in the Korean population, data were retrospectively collected from the eight university hospitals in Korea. The incidence of NIFTP in each institution is shown in Table 1. After reviewing pathology reports of 18,819 patients with PTC, 378 patients (2.0%) were initially diagnosed with non-invasive EFVPTC as potential NIFTP cases; 140 cases were excluded from NIFTP diagnosis after review of pathology slides acquired from 378 cases. The most common reason for exclusion was the presence of small, but true papillae that were rediagnosed as conventional PTC. Other reasons for exclusion were reclassification as infiltrative FVPTC or unavailability for slide review. Finally, 238 (1.3%) of all PTCs were eligible for the diagnosis of NIFTP after slide review.
- Among 238 cases of NIFTP, 174 cases (73.3%) had only NIFTP; in the remaining 64 cases (26.7%), NIFTP coexisted with other malignancies, such as conventional PTC, infiltrative FVPTC, tall cell variant PTC, oncocytic variant PTC, follicular carcinoma, or poorly differentiated carcinoma (Table 2).
- Because not all cases were available for slide review and NIFTP can be misclassified as non-PTC tumors, the lower boundary for the prevalence of NIFTP among PTCs was estimated. When the same histologic criteria was applied for the diagnosis of NIFTP regardless of the tumor size, the prevalence of NIFTP was 238 (1.3%) among all PTCs. Among the reclassified 238 NIFTP cases, 86 had a tumor <1.0 cm and 152 had a tumor ≥1.0 cm (Table 2). Therefore, the expected lower boundary for the prevalence of NIFTP was 0.8% (152/18,819) when tumors ≥1.0 cm were included for the diagnosis of NIFTP.
- Clinicopathologic characteristics of patients with NIFTP
- Among 152 patients with NIFTP ≥1.0 cm in size, 125 (82.2%) patients had only NIFTP and the remaining 27 patients (17.8%) had NIFTP coexisting with thyroid cancer. The clinicopathologic characteristics were evaluated in patients with only NIFTP. The demographics of 125 NIFTP patients are shown in Table 3. The mean age was 46.7 years (range, 23 to 73 years). The NIFTP patients included 93 females (74.4%) and 32 males (25.6%). The median primary tumor size was 26.2 mm (range, 10 to 80 mm). Among 125 patients, 101 (80.8%) underwent cervical lymph node dissection and lymph node metastasis was not found. In addition, no patient had lymphatic/vascular invasion or distant metastases. Regarding surgical methods, a total of 44 patients (35.2%) underwent total thyroidectomy and 81 patients (64.8%) received lobectomy or isthmectomy. Furthermore, 29 patients (23.2%) underwent radioactive iodine (RAI) remnant ablation therapy based on the initial tumor size. Disease recurrence was not observed in any NIFTP patient during a median follow-up period of 25.1 months.
- Impact of NIFTP on pathologic examination and clinical practice
- A difference in the prevalence of NIFTP before and after NIFTP introduction was not observed (Table 3). The number of paraffin blocks for diagnosis of NIFTP did not increase after the introduction of NIFTP (average 5.2 per tumor before April 2016 and average 5.5 per tumor after April 2016).
- The rate of lymph node dissection increased from 72.7% before April 2016 to 89.8% after April 2016. The rate of total thyroidectomy decreased from 43.9% before April 2016 to 25.4% after April 2016. The number of patients undergoing postoperative RAI therapy was significantly reduced from 25.8% before April 2016 to 20.3% after April 2016 (Table 3). RAI treatment was performed due to coexisting thyroid cancers in most cases.
- BRAF and RAS mutations in NIFTPs
- Twenty-seven patients diagnosed with NIFTP at the Gangnam Severance Hospital after April 2016 were randomly selected for gene analysis; BRAFV600E mutation was not observed. As shown in Table 4, the overall frequency of three RAS gene mutations was 55.6% (15/27). The mutation rates of NRAS, HRAS, and KRAS were 22.2%, 22.2%, and 11.1%, respectively.
RESULTS
- The overall prevalence of NIFTP was 1.3% (range, 0.4% to 2.6%) of all PTCs in the present study, which included the largest cohort size researched to date among the Korean population (Table 1). According to the recent study by Nikiforov et al. [8], any masses <1.0 cm were not included in the diagnosis of NIFTP. Moreover, in another recent NIFTP study, data on the sub-centimeter NIFTP were limited [22]. Thus, the expected lower boundary for the prevalence of NIFTP was 0.8% when tumors ≥1 cm in size were included in the diagnosis of NIFTP.
- These results are consistent with the findings from a previous study in which the mean prevalence of NIFTP was 1.5% (range, 0% to 4.7%) in nine institutions from six Asian countries, including Korea [23]. The prevalence of NIFTP is constantly lower in Asian studies than in Western population-based studies [22,24-27].
- NIFTPs frequently have RAS mutations but no BRAFV600E mutation [11-14]. However, the BRAFV600E mutation was found in some NIFTP cases in several studies immediately performed after the initial publication of NIFTP [17,18,28,29]. Table 5 summarizes the results of BRAF mutation in NIFTP reported in previous studies from six Korean institutions [17,18,28-31]. When the strict criterion of “0% papillae” was applied in the present study, no BRAF mutation was found in NIFTPs. In the present case series, a case of BRAFV600E-positive tumor originally diagnosed as non-invasive EFVPTC was found. The pathology slides were re-examined after cutting deeper sections and a true papillary structure was found in a focal area. Therefore, the case was reclassified as encapsulated classic PTC with predominant follicular growth pattern. This finding reconfirms the strict diagnostic criteria for NIFTP are helpful for excluding true PTC. Because the diagnostic criteria for NIFTP have been updated, NIFTP should no longer include any follicular patterned tumors with well-formed papillae or high-risk gene mutations such as BRAFV600E, TERT promoter, or TP53 [20].
- The present study had several technical limitations. First, due to the retrospective multicenter-based nature of this study, interobserver variability in the diagnosis of NIFTP based on histology was not considered. Second, since only the cases diagnosed with PTC were included, the possibility that NIFTP was diagnosed as benign, such as nodular hyperplasia or follicular adenoma, may result in differences in actual prevalence. However, the prevalence of NIFTP did not change significantly even after the introduction of NIFTP. Third, although the overall study cohort size was relatively large, genetic analysis of BRAF and RAS mutations were performed in only a limited number of NIFTP cases. Nevertheless, the results were consistent with previous study results showing NIFTP has no BRAFV600E mutation and frequent RAS mutations [8,15,20].
- The results from this study has shown the low prevalence of NIFTP among PTCs in Korea using the largest number of cases to date. Adverse outcomes were not experienced by any patient with NIFTP during the follow-up period. The introduction of NIFTP may have a small overall impact in Korean practice.
DISCUSSION
Author contributions
Conceptualization: SWH, CKJ.
Data curation: JYS, JHP, JYP, YJC, CKJ.
Formal analysis: JYS, JHP, JYP.
Funding acquisition: SWH.
Investigation: JYS, JHP, JYP, YJC, CKJ, DES, JJK, SYP, HYN, JHK, JYS, HSK, SWH.
Methodology: JYS, JYP, YJC, CKJ, SWH.
Project administration: CKJ, SWH.
Resources: JYS, JYP, YJC, CKJ, DES, JJK, SYP, HYN, JHK, JYS, HSK, SWH.
Supervision: CKJ, SWH.
Validation: JYS, JYP, YJC, CKJ, SWH.
Visualization: JYS.
Writing—original draft: JYS, CKJ, SWH.
Writing—review & editing: JYS, CKJ, SWH.
Conflicts of Interest
CKJ and SYP, editors-in-chief of the Journal of Pathology and Translational Medicine and SWH, an editorial board member of the Journal of Pathology and Translational Medicine, were not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding
This research was supported and funded by the Korean Society of Pathologists (grant number: 2017-05-001).
| Total | ≥ 1.0 cm | < 1.0 cm | |
|---|---|---|---|
| All cases of NIFTP | 238 (1.3) | 152 (0.8) | 86 (0.5) |
| NIFTP alone | 174 (73.3) | 125 (82.2) | 49 (57.0) |
| NIFTP coexisting with malignancy | 64 (26.7) | 27 (17.8) | 37 (43.0) |
| Overall | Before NIFTP introductiona | After NIFTP introduction | p-value | |
|---|---|---|---|---|
| Prevalence of NIFTP | 125/18,819 (0.7) | 66/9,656 (0.6) | 59/9,163 (0.7) | .739 |
| Sex | .004 | |||
| Male | 32 (25.6) | 24 (36.4) | 8 (13.6) | |
| Female | 93 (74.4) | 42 (63.6) | 51 (86.4) | |
| Age, mean ± SD (range, yr) | 46.7 ± 12.5 (23–73) | 47.9 ± 13.1 (23–73) | 45.3 ± 11.9 (25–73) | .238 |
| Tumor size, median (range, mm) | 26.2 (10–80) | 24.5 (10–61) | 28.0 (10–80) | .195 |
| No. of paraffin blocks, median (range) | 5.3 (1–26) | 5.2 (2–26) | 5.5 (1–18) | .398 |
| Lymph node dissection | .385 | |||
| Performed | 101 (80.8) | 48 (72.7) | 53 (89.8) | |
| Not performed | 24 (19.2) | 18 (27.3) | 6 (10.2) | |
| Lymph node metastases | > .99 | |||
| Positive | 0 | 0 | 0 | |
| Negativeb | 125 (100) | 66 (100) | 59 (100) | |
| Surgical procedure | .021 | |||
| Lobectomy or isthmectomy | 81 (64.8) | 37 (56.1) | 44 (74.6) | |
| Total thyroidectomy | 44 (35.2) | 29 (43.9) | 15 (25.4) | |
| Lymphatic invasion | 1.000 | |||
| Positive | 0 | 0 | 0 | |
| Negative | 125 (100) | 66 (100) | 59 (100) | |
| Vascular invasion | 1.000 | |||
| Positive | 0 | 0 | 0 | |
| Negative | 125 (100) | 66 (100) | 59 (100) | |
| Distant metastasis | 1.000 | |||
| Positive | 0 | 0 | 0 | |
| Negative | 125 (100) | 66 (100) | 59 (100) | |
| Postoperative radioactive iodine therapy | .365 | |||
| Performed | 29 (23.2) | 17 (25.8) | 12 (20.3) | |
| Not performed | 96 (76.8) | 49 (74.2) | 47 (79.7) | |
| Follow-up, median ± SD (range, mo) | 25.1 ± 19.1 (0–60) | 36.2 ± 14.5 (0–60) | 10.7 ± 6.6 (1–24) | |
| Recurrence of disease | 1.000 | |||
| Positive | 0 | 0 | 0 | |
| Negative | 125 (100) | 66 (100) | 59 (100) |
| Mutation | No. (%) |
|---|---|
| BRAFV600E | |
| Present | 0 |
| Absent | 27 (100) |
| All RAS mutation | |
| Present | 15 (55.6) |
| NRAS | 6 (22.2) |
| c.181C > A (p.Gln61Lys) | 3 (50.0) |
| c.182A > G (p.Gln61Arg) | 3 (50.0) |
| HRAS | 6 (22.2) |
| c.182A > G (p.Gln61Arg) | 6 (100) |
| KRAS codon 61 mutationa | 3 (11.1) |
| Study | Period | Diagnostic criteria | PTC | NIFTP |
No. (%) |
|||
|---|---|---|---|---|---|---|---|---|
| BRAFV600E mutation | RAS mutation | Lymph node metastasis | Distant metastasis | |||||
| Cho et al. (2017) [17] | 2008–2014 | < 1% papillae | 6,269 | 105 | 10 (10.0) | - | 3 (2.9) | 1 (1.0) |
| 0% papillae | 6,269 | 95 | 0 | 48/89 (53.9) | 2 (2.1) | 0 | ||
| Kim et al. (2017) [28] | 2009–2014 | < 1% papillae | 6,548 | 43 | 3 (7.0) | - | 1 (2.3) | 0 |
| Lee et al. (2017) [18] | 2010–2014 | < 1% papillae | 769 | 21 | 5 (23.8) | 12 (57.1) | 1 (4.7) | 0 |
| Kim et al. (2018) [30] | 2011–2012 | < 1% papillae | 1,411 | 2 | 0 | - | 0 | 0 |
| Kim et al. (2018) [31] | 2013–2016 | 0% papillae | - | 32 | 0 | 15 (46.9) | 0 | 0 |
| Kim et al. (2018) [29] | 2014–2016 | < 1% papillae | 2,853 | 73 | 9 (12.3) | 36 (49.3) | 9 (12.3) | 0 |
- 1. Lang BH, Lo CY, Chan WF, Lam AK, Wan KY. Classical and follicular variant of papillary thyroid carcinoma: a comparative study on clinicopathologic features and long-term outcome. World J Surg 2006; 30: 752-8. ArticlePubMedPDF
- 2. Baloch ZW, Shafique K, Flannagan M, Livolsi VA. Encapsulated classic and follicular variants of papillary thyroid carcinoma: comparative clinicopathologic study. Endocr Pract 2010; 16: 952-9. ArticlePubMedPDF
- 3. Zidan J, Karen D, Stein M, Rosenblatt E, Basher W, Kuten A. Pure versus follicular variant of papillary thyroid carcinoma: clinical features, prognostic factors, treatment, and survival. Cancer 2003; 97: 1181-5. ArticlePubMed
- 4. Yu XM, Schneider DF, Leverson G, Chen H, Sippel RS. Follicular variant of papillary thyroid carcinoma is a unique clinical entity: a population-based study of 10,740 cases. Thyroid 2013; 23: 1263-8. ArticlePubMedPMC
- 5. Liu J, Singh B, Tallini G, et al. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer 2006; 107: 1255-64. ArticlePubMed
- 6. Jung CK, Little MP, Lubin JH, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab 2014; 99: E276-85. ArticlePubMedPDF
- 7. Hirokawa M, Carney JA, Goellner JR, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol 2002; 26: 1508-14. ArticlePubMed
- 8. Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2016; 2: 1023-9. ArticlePubMedPMC
- 9. Baloch ZW, Seethala RR, Faquin WC, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): a changing paradigm in thyroid surgical pathology and implications for thyroid cytopathology. Cancer Cytopathol 2016; 124: 616-20. ArticlePubMed
- 10. Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol 2011; 7: 569-80. ArticlePubMedPDF
- 11. Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma: an unusually high prevalence of RAS mutations. Am J Clin Pathol 2003; 120: 71-7. ArticlePubMedPDF
- 12. Adeniran AJ, Zhu Z, Gandhi M, et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol 2006; 30: 216-22. ArticlePubMed
- 13. Zhao L, Dias-Santagata D, Sadow PM, Faquin WC. Cytological, molecular, and clinical features of noninvasive follicular thyroid neoplasm with papillary-like nuclear features versus invasive forms of follicular variant of papillary thyroid carcinoma. Cancer Cytopathol 2017; 125: 323-31. ArticlePubMedPDF
- 14. McFadden DG, Dias-Santagata D, Sadow PM, et al. Identification of oncogenic mutations and gene fusions in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab 2014; 99: E2457-62. ArticlePubMedPMC
- 15. Howitt BE, Paulson VA, Barletta JA. Absence of BRAF V600E in non-infiltrative, non-invasive follicular variant of papillary thyroid carcinoma. Histopathology 2015; 67: 579-82. ArticlePubMed
- 16. Song RY, Kang KH, Kim HS, Park SJ. Significance of follicular variant of papillary thyroid carcinoma: study from a thyroid cancer center. Int J Thyroidol 2017; 10: S162.
- 17. Cho U, Mete O, Kim MH, Bae JS, Jung CK. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol 2017; 30: 810-25. ArticlePubMedPDF
- 18. Lee SE, Hwang TS, Choi YL, et al. Molecular profiling of papillary thyroid carcinoma in Korea with a high prevalence of BRAFV600E mutation. Thyroid 2017; 27: 802-10. ArticlePubMed
- 19. Nikiforov YE, Ghossein RA, Kakudo K. Non-invasive follicular thyroid neoplasm with papillary-like nuclear features.In: Lloyd RV, Osamura RY, Klöppel G, Rosai J, eds. WHO classification of tumours of endocrine organs. 4th. Lyon: IARC Press, 2017; 78-80.
- 20. Nikiforov YE, Baloch ZW, Hodak SP, et al. Change in diagnostic criteria for noninvasive follicular thyroid neoplasm with papillary-like nuclear features. JAMA Oncol 2018; 4: 1125-6. ArticlePubMedPMC
- 21. Kang SH, Pyo JY, Yang SW, Hong SW. Detection of BRAF V600E mutation with thyroid tissue using pyrosequencing: comparison with PNA-clamping and real-time PCR. Am J Clin Pathol 2013; 139: 759-64. ArticlePubMedPDF
- 22. Bychkov A, Jung CK, Liu Z, Kakudo K. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice: perspectives for surgical pathology and cytopathology. Endocr Pathol 2018; 29: 276-88. ArticlePubMedPMCPDF
- 23. Bychkov A, Hirokawa M, Jung CK, et al. Low rate of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Thyroid 2017; 27: 983-4. ArticlePubMed
- 24. Satoh S, Yamashita H, Kakudo K. Thyroid cytology: the Japanese system and experience at Yamashita Thyroid Hospital. J Pathol Transl Med 2017; 51: 548-54. ArticlePubMedPMCPDF
- 25. Liu Z, Song Y, Han B, Zhang X, Su P, Cui X. Non-invasive follicular thyroid neoplasm with papillary-like nuclear features and the practice in Qilu Hospital of Shandong University, China. J Basic Clin Med 2017; 6: 22-5.
- 26. Paulson VA, Shivdasani P, Angell TE, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features accounts for more than half of “carcinomas” harboring RAS mutations. Thyroid 2017; 27: 506-11. ArticlePubMed
- 27. Jung CK, Kim C. Effect of lowering the diagnostic threshold for encapsulated follicular variant of papillary thyroid carcinoma on the prevalence of non-invasive follicular thyroid neoplasm with papillary-like nuclear features: a single-institution experience in Korea. J Basic Clin Med 2017; 6: 26-8.
- 28. Kim MJ, Won JK, Jung KC, et al. Clinical characteristics of subtypes of follicular variant papillary thyroid carcinoma. Thyroid 2018; 28: 311-8. ArticlePubMed
- 29. Kim TH, Lee M, Kwon AY, et al. Molecular genotyping of the noninvasive encapsulated follicular variant of papillary thyroid carcinoma. Histopathology 2018; 72: 648-61. ArticlePubMedPDF
- 30. Kim H, Kim BH, Kim YK, et al. Prevalence of BRAFV600E mutation in follicular variant of papillary thyroid carcinoma and non-invasive follicular tumor with papillary-like nuclear features (NIFTP) in a BRAFV600E prevalent area. J Korean Med Sci 2018; 33: e75.ArticlePubMedPMCPDF
- 31. Kim M, Jeon MJ, Oh HS, et al. BRAF and RAS mutational status in noninvasive follicular thyroid neoplasm with papillary-like nuclear features and invasive subtype of encapsulated follicular variant of papillary thyroid carcinoma in Korea. Thyroid 2018; 28: 504-10. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Case report & review: Bilateral NIFTP harboring concomitant HRAS and KRAS mutation: Report of an unusual case and literature review
Marianna Rita Brogna, Francesca Collina, Maria Grazia Chiofalo, Debora De Bartolo, Angela Montone, Maria Rosaria Schiano, Michele Del Sesto, Nubia Pizza, Gerardo Ferrara
Molecular Carcinogenesis.2024; 63(12): 2273. CrossRef - Non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): what do we need to know?
Andrés Coca-Pelaz, Juan P. Rodrigo, Abbas Agaimy, Dana M. Hartl, Göran Stenman, Vincent Vander Poorten, Antti A. Mäkitie, Mark Zafereo, Karthik N. Rao, Gregory W. Randolph, Alessandra Rinaldo, Alfio Ferlito
Virchows Archiv.2024; 485(6): 977. CrossRef - Study of non-invasive follicular thyroid neoplasm: A borderline entity
Rupali Bavikar, Ruchi S. Randive, Anubhaw Verma, Madhuri Singh, Vidya Viswanathan, Arpana Dharwadkar
Journal of Cancer Research and Therapeutics.2024; 20(5): 1365. CrossRef - Analysis of a pre-2017 follicular variant papillary thyroid carcinoma cohort reclassified as noninvasive follicular thyroid neoplasm with papillary-like features (NIFTP): an 11-year retrospective single institution experience
Shaham Beg, Sana Irfan Khan, Isabella Cui, Theresa Scognamiglio, Rema Rao
Journal of the American Society of Cytopathology.2023; 12(2): 112. CrossRef - Noninvasive Follicular Thyroid Neoplasm With Papillary-Like Nuclear Features: What a Surgeon Should Know
Jabir Alharbi, Thamer Alraddadi, Haneen Sebeih, Mohammad A Alessa, Haddad H Alkaf, Ahmed Bahaj, Sherif K Abdelmonim
Cureus.2023;[Epub] CrossRef - NTRK Fusion in a Cohort of BRAF p. V600E Wild-Type Papillary Thyroid Carcinomas
Seung Eun Lee, Mi-Sook Lee, Heejin Bang, Mi Young Kim, Yoon-La Choi, Young Lyun Oh
Modern Pathology.2023; 36(8): 100180. CrossRef - A Comprehensive Study on the Diagnosis and Management of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features
Bayan A. Alzumaili, Lauren N. Krumeich, Reagan Collins, Timothy Kravchenko, Emad I. Ababneh, Adam S. Fisch, William C. Faquin, Vania Nosé, Maria Martinez-Lage, Gregory W. Randolph, Rajshri M. Gartland, Carrie C. Lubitz, Peter M. Sadow
Thyroid.2023; 33(5): 566. CrossRef - Clinical-Pathological and Molecular Evaluation of 451 NIFTP Patients from a Single Referral Center
Paola Vignali, Agnese Proietti, Elisabetta Macerola, Anello Marcello Poma, Liborio Torregrossa, Clara Ugolini, Alessio Basolo, Antonio Matrone, Teresa Rago, Ferruccio Santini, Rossella Elisei, Gabriele Materazzi, Fulvio Basolo
Cancers.2022; 14(2): 420. CrossRef - Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: its updated diagnostic criteria, preoperative cytologic diagnoses and impact on the risk of malignancy
Hee Young Na, So Yeon Park
Journal of Pathology and Translational Medicine.2022; 56(6): 319. CrossRef - SFE-AFCE-SFMN 2022 Consensus on the management of thyroid nodules : Follow-up: How and how long?
Sophie Leboulleux, Livia Lamartina, Emmanuelle Lecornet Sokol, Fabrice Menegaux, Laurence Leenhardt, Gilles Russ
Annales d'Endocrinologie.2022; 83(6): 407. CrossRef - Different Threshold of Malignancy for RAS-like Thyroid Tumors Causes Significant Differences in Thyroid Nodule Practice
Kennichi Kakudo
Cancers.2022; 14(3): 812. CrossRef - Clinicopathological parameters for predicting non-invasive follicular thyroid neoplasm with papillary features (NIFTP)
Eunju Jang, Kwangsoon Kim, Chan Kwon Jung, Ja Seong Bae, Jeong Soo Kim
Therapeutic Advances in Endocrinology and Metabolism.2021;[Epub] CrossRef - The Incidence of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features: A Meta-Analysis Assessing Worldwide Impact of the Reclassification
Chanchal Rana, Huy Gia Vuong, Thu Quynh Nguyen, Hoang Cong Nguyen, Chan Kwon Jung, Kennichi Kakudo, Andrey Bychkov
Thyroid.2021;[Epub] CrossRef - The Genomic Landscape of Thyroid Cancer Tumourigenesis and Implications for Immunotherapy
Amandeep Singh, Jeehoon Ham, Joseph William Po, Navin Niles, Tara Roberts, Cheok Soon Lee
Cells.2021; 10(5): 1082. CrossRef - Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) is rare, benign lesion using modified stringent diagnostic criteria: Reclassification and outcome study
David Cubero Rego, Hwajeong Lee, Anne Boguniewicz, Timothy A. Jennings
Annals of Diagnostic Pathology.2020; 44: 151439. CrossRef - Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features: From Echography to Genetic Profile
Francesca Maletta, Enrico Costantino Falco, Alessandro Gambella, Jasna Metovic, Mauro Papotti
The Tohoku Journal of Experimental Medicine.2020; 252(3): 209. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

Fig. 1.
| Institution | Period | PTC | Invasive EFVPTC | NIFTP |
|---|---|---|---|---|
| A | 2012–2017 | 1,427 | 44 (3.1) | 26 (1.8) |
| B | 2013–2016 | 1,342 | 35 (2.6) | 35 (2.6) |
| C | 2013–2017 | 3,927 | 192 (4.9) | 100 (2.5) |
| D | 2013–2017 | 6,200 | 134 (2.2) | 24 (0.4) |
| E | 2013–2017 | 3,083 | 37 (1.2) | 23 (0.7) |
| F | 2014–2017 | 734 | 9 (1.2) | 5 (0.7) |
| G | 2015–2017 | 1,077 | 2 (0.2) | 20 (1.9) |
| H | 2015–2018 | 1,029 | 14 (1.4) | 5 (0.5) |
| Total | 18,819 | 467 (2.5) | 238 (1.3) |
| Total | ≥ 1.0 cm | < 1.0 cm | |
|---|---|---|---|
| All cases of NIFTP | 238 (1.3) | 152 (0.8) | 86 (0.5) |
| NIFTP alone | 174 (73.3) | 125 (82.2) | 49 (57.0) |
| NIFTP coexisting with malignancy | 64 (26.7) | 27 (17.8) | 37 (43.0) |
| Overall | Before NIFTP introduction |
After NIFTP introduction | p-value | |
|---|---|---|---|---|
| Prevalence of NIFTP | 125/18,819 (0.7) | 66/9,656 (0.6) | 59/9,163 (0.7) | .739 |
| Sex | .004 | |||
| Male | 32 (25.6) | 24 (36.4) | 8 (13.6) | |
| Female | 93 (74.4) | 42 (63.6) | 51 (86.4) | |
| Age, mean ± SD (range, yr) | 46.7 ± 12.5 (23–73) | 47.9 ± 13.1 (23–73) | 45.3 ± 11.9 (25–73) | .238 |
| Tumor size, median (range, mm) | 26.2 (10–80) | 24.5 (10–61) | 28.0 (10–80) | .195 |
| No. of paraffin blocks, median (range) | 5.3 (1–26) | 5.2 (2–26) | 5.5 (1–18) | .398 |
| Lymph node dissection | .385 | |||
| Performed | 101 (80.8) | 48 (72.7) | 53 (89.8) | |
| Not performed | 24 (19.2) | 18 (27.3) | 6 (10.2) | |
| Lymph node metastases | > .99 | |||
| Positive | 0 | 0 | 0 | |
| Negative |
125 (100) | 66 (100) | 59 (100) | |
| Surgical procedure | .021 | |||
| Lobectomy or isthmectomy | 81 (64.8) | 37 (56.1) | 44 (74.6) | |
| Total thyroidectomy | 44 (35.2) | 29 (43.9) | 15 (25.4) | |
| Lymphatic invasion | 1.000 | |||
| Positive | 0 | 0 | 0 | |
| Negative | 125 (100) | 66 (100) | 59 (100) | |
| Vascular invasion | 1.000 | |||
| Positive | 0 | 0 | 0 | |
| Negative | 125 (100) | 66 (100) | 59 (100) | |
| Distant metastasis | 1.000 | |||
| Positive | 0 | 0 | 0 | |
| Negative | 125 (100) | 66 (100) | 59 (100) | |
| Postoperative radioactive iodine therapy | .365 | |||
| Performed | 29 (23.2) | 17 (25.8) | 12 (20.3) | |
| Not performed | 96 (76.8) | 49 (74.2) | 47 (79.7) | |
| Follow-up, median ± SD (range, mo) | 25.1 ± 19.1 (0–60) | 36.2 ± 14.5 (0–60) | 10.7 ± 6.6 (1–24) | |
| Recurrence of disease | 1.000 | |||
| Positive | 0 | 0 | 0 | |
| Negative | 125 (100) | 66 (100) | 59 (100) |
| Mutation | No. (%) |
|---|---|
| BRAFV600E | |
| Present | 0 |
| Absent | 27 (100) |
| All RAS mutation | |
| Present | 15 (55.6) |
| NRAS | 6 (22.2) |
| c.181C > A (p.Gln61Lys) | 3 (50.0) |
| c.182A > G (p.Gln61Arg) | 3 (50.0) |
| HRAS | 6 (22.2) |
| c.182A > G (p.Gln61Arg) | 6 (100) |
| KRAS codon 61 mutation |
3 (11.1) |
| Study | Period | Diagnostic criteria | PTC | NIFTP | No. (%) |
|||
|---|---|---|---|---|---|---|---|---|
| BRAFV600E mutation | RAS mutation | Lymph node metastasis | Distant metastasis | |||||
| Cho et al. (2017) [17] | 2008–2014 | < 1% papillae | 6,269 | 105 | 10 (10.0) | - | 3 (2.9) | 1 (1.0) |
| 0% papillae | 6,269 | 95 | 0 | 48/89 (53.9) | 2 (2.1) | 0 | ||
| Kim et al. (2017) [28] | 2009–2014 | < 1% papillae | 6,548 | 43 | 3 (7.0) | - | 1 (2.3) | 0 |
| Lee et al. (2017) [18] | 2010–2014 | < 1% papillae | 769 | 21 | 5 (23.8) | 12 (57.1) | 1 (4.7) | 0 |
| Kim et al. (2018) [30] | 2011–2012 | < 1% papillae | 1,411 | 2 | 0 | - | 0 | 0 |
| Kim et al. (2018) [31] | 2013–2016 | 0% papillae | - | 32 | 0 | 15 (46.9) | 0 | 0 |
| Kim et al. (2018) [29] | 2014–2016 | < 1% papillae | 2,853 | 73 | 9 (12.3) | 36 (49.3) | 9 (12.3) | 0 |
Values are presented as number (%). NIFTP, non-invasive follicular thyroid neoplasm with papillary-like nuclear features; PTC, papillary thyroid carcinoma; EFVPTC, encapsulated follicular variant of PTC.
Values are presented as number (%). NIFTP, non-invasive follicular thyroid neoplasm with papillary-like nuclear features.
Values are presented as number (%) unless otherwise indicated. NIFTP, non-invasive follicular thyroid neoplasm with papillary-like nuclear features; SD, standard deviation. Before April, 2016; Includes pN0 and pNx stages.
The assay cannot identify specific mutation types.
NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; PTC, papillary thyroid carcinoma.

 E-submission
E-submission