Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 54(2); 2020 > Article
-
Case Study
Primary carcinoid tumor in the external auditory canal -
Dong Hae Chung1,*
 , Gyu Cheol Han2,*
, Gyu Cheol Han2,* , Na Rae Kim1
, Na Rae Kim1
-
Journal of Pathology and Translational Medicine 2020;54(2):184-187.
DOI: https://doi.org/10.4132/jptm.2019.11.07
Published online: November 13, 2019
1Department of Pathology, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
2Department of Otolaryngology, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- Corresponding Author: Na Rae Kim, MD, PhD, Department of Pathology, Gil Medical Center, Gachon University College of Medicine, 21 Namdong-daero 774 beon-gil, Namdong-gu, Incheon 21565, Korea Tel: +82-32-460-3073, Fax: +82-32-460-2394, E-mail: naraech@empal.com
- *Dong Hae Chung and Gyu Cheol Han contributed equally to this work.
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- First Report on a Rare Poorly Differentiated Neuroendocrine Tumour of the External Auditory Canal Involving Pinna
Akash Varshney, Amit Kumar Tyagi, Prashant Durgapal, Kajal Mahto, Akhilesh Chandra Yadav, Ankita Semwal
Indian Journal of Otolaryngology and Head & Neck Surgery.2025; 77(4): 1922. CrossRef - Incidental finding of a neuroendocrine neoplasm in a suspected ear canal exostosis
Alexander Wieck Fjaeldstad, Gerda Elisabeth Villadsen, Gitte Dam, Stephen Jacques Hamilton-Dutoit, Thomas Winther Frederiksen
Otolaryngology Case Reports.2022; 22: 100394. CrossRef - 68Ga-DOTATATE Uptake in Well-Differentiated Neuroendocrine Tumor of the External Auditory Canal
Özge Erol Fenercioğlu, Ediz Beyhan, Rahime Şahin, Mehmet Can Baloğlu, Tevfik Fikret Çermik
Clinical Nuclear Medicine.2022; 47(8): e552. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


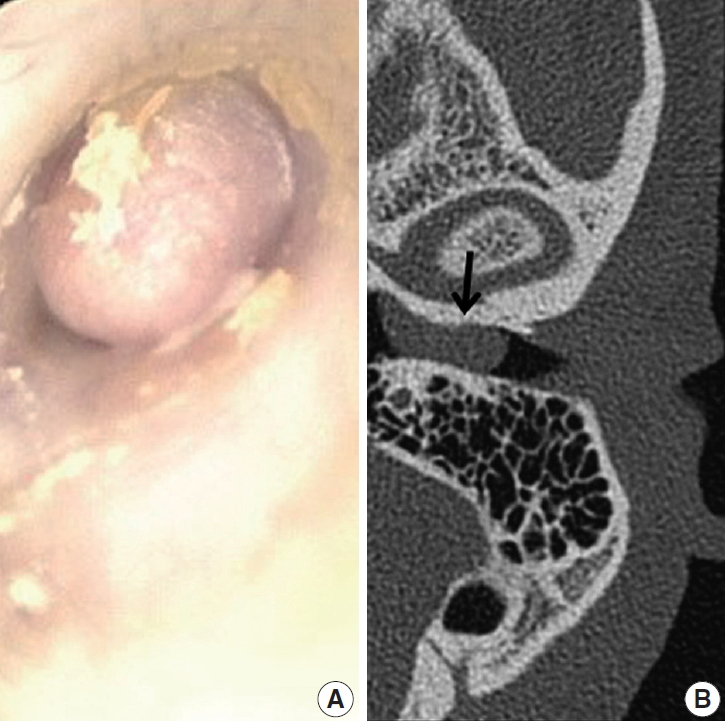
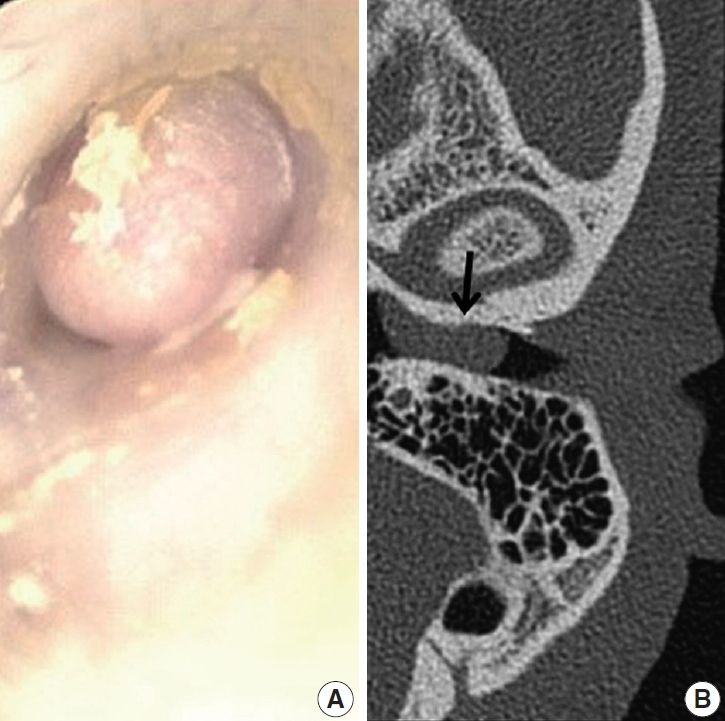
Fig. 1.
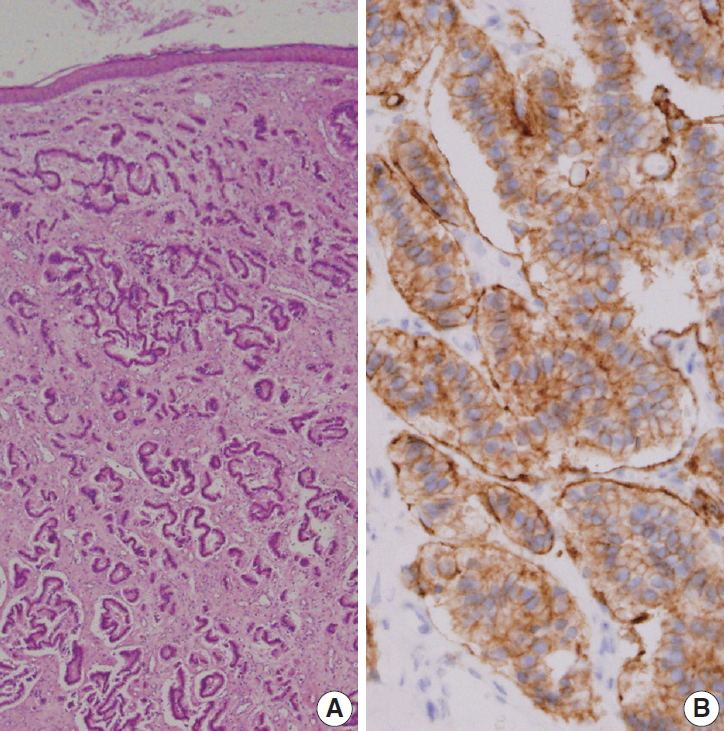
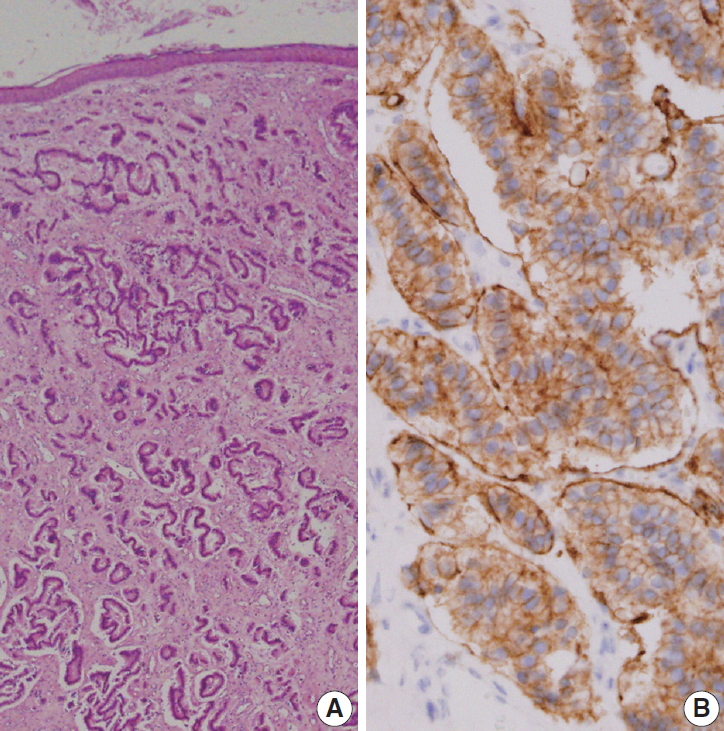
Fig. 2.
| Case No. | Study | Age (yr)/Sex | Symptom | Pathologic finding | WHO classification of NEN according to 4th edition [8] (NEN-2018 WHO) [9] | Treatment | Prognosis (duration of follow-up) |
|---|---|---|---|---|---|---|---|
| 1 | Manipoud et al. (1994) [2] | NA | NA | NA | Merkel cell carcinoma (NEN-2018 WHO) | NA | NA |
| 2 | Litofsky et al. (1998) [3] | 86/F | Otalgia and hearing loss | SYN+ Vimentin+ NF+ CK+ Chromogranin-focal+ S100– HMB45– GFAP– Serotonin– ER– PR– | Merkel cell carcinoma (NEN-2018 WHO) | Gross total resection, RT | No evidence of recurrence (8 mo) |
| 3 | Mahalingam et al. (2006) [4] | 32/F | Progressive hearing loss | NSE+ SYN+ PanCKweak+ Chromogranin– CK20– S100– HMB45– Melan A– | NEC G1 (NET G1-2018 WHO) | Second-look tympanomastoidectomy | No evidence of recurrence (8 mo) |
| 4 | Palma et al. (2007) [5] | 72/M | Painless swelling of retroauricular region and sudden onset of bleeding | CK perinuclear dot+ Chromogranin+ NSE+ SYN+ | Merkel cell carcinoma (NEC-2018 WHO) | CTx | Died due to underlying multiple malignancies |
| 5 | Li et al. (2012) [6] | 62/M | Painless mass at EAC | CK+ NSE+ Vimentin+ | Merkel cell carcinoma (NEC-2018 WHO) | Sleeve mastoidectomy and tympanotomy, RT, CTx | No evidence of recurrence (2 yr) |
| 6 | McCrary et al. (2017) [7] | 38/F | Otalgia, aural fullness, and decreased hearing | PanCK+ CD56+ SYN+ Chromogranin+ CK7– Mucin– | NEC G1 (NET G1-2018 WHO) | Gross total resection | Recurrence (8 yr) |
| 7 | Present case | 39/M | Progressive hearing loss | SYN+ CD56+ Chromogranin– CK7– CK20– | NEC G1 (NET G1-2018 WHO) | Gross total resection | No evidence of recurrence (20 mo) |
EAC, external auditory canal; NET, neuroendocrine tumor; WHO, World Health Organization; NEN, neuroendocrine neoplasm; NA, not available; NEC, neuroendocrine carcinoma; SYN, synaptophysin; NF, neurofilament; CK, cytokeratin; HMB45, human melanoma black 45; GFAP, glial fibrillary acidic protein; ER, estrogen receptor; PR, progesteron receptor; RT, radiotherapy; G1, grade 1; NSE, neuron specific enolase; CTx, chemotherapy.

 E-submission
E-submission