Previous issues
- Page Path
- HOME > Articles and issues > Previous issues
Review
- Introduction to digital pathology and computer-aided pathology
- Soojeong Nam, Yosep Chong, Chan Kwon Jung, Tae-Yeong Kwak, Ji Youl Lee, Jihwan Park, Mi Jung Rho, Heounjeong Go
- J Pathol Transl Med. 2020;54(2):125-134. Published online February 13, 2020
- DOI: https://doi.org/10.4132/jptm.2019.12.31
- 17,864 View
- 614 Download
- 79 Web of Science
- 82 Crossref
-
 Abstract
Abstract
 PDF
PDF - Digital pathology (DP) is no longer an unfamiliar term for pathologists, but it is still difficult for many pathologists to understand the engineering and mathematics concepts involved in DP. Computer-aided pathology (CAP) aids pathologists in diagnosis. However, some consider CAP a threat to the existence of pathologists and are skeptical of its clinical utility. Implementation of DP is very burdensome for pathologists because technical factors, impact on workflow, and information technology infrastructure must be considered. In this paper, various terms related to DP and computer-aided pathologic diagnosis are defined, current applications of DP are discussed, and various issues related to implementation of DP are outlined. The development of computer-aided pathologic diagnostic tools and their limitations are also discussed.
-
Citations
Citations to this article as recorded by- Deep learning model to diagnose cardiac amyloidosis from haematoxylin/eosin-stained myocardial tissue
Takeshi Tohyama, Takeshi Iwasaki, Masataka Ikeda, Masato Katsuki, Tatsuya Watanabe, Kayo Misumi, Keisuke Shinohara, Takeo Fujino, Toru Hashimoto, Shouji Matsushima, Tomomi Ide, Junji Kishimoto, Koji Todaka, Yoshinao Oda, Kohtaro Abe
European Heart Journal - Imaging Methods and Practice.2025;[Epub] CrossRef - The current landscape of artificial intelligence in oral and maxillofacial surgery– a narrative review
Rushil Rajiv Dang, Balram Kadaikal, Sam El Abbadi, Branden R. Brar, Amit Sethi, Radhika Chigurupati
Oral and Maxillofacial Surgery.2025;[Epub] CrossRef - Assessing the quality of whole slide images in cytology from nuclei features
Paul Barthe, Romain Brixtel, Yann Caillot, Benoît Lemoine, Arnaud Renouf, Vianney Thurotte, Ouarda Beniken, Sébastien Bougleux, Olivier Lézoray
Journal of Pathology Informatics.2025; 17: 100420. CrossRef - An update on applications of digital pathology: primary diagnosis; telepathology, education and research
Shamail Zia, Isil Z. Yildiz-Aktas, Fazail Zia, Anil V. Parwani
Diagnostic Pathology.2025;[Epub] CrossRef - Artificial intelligence–driven digital pathology in urological cancers: current trends and future directions
Inyoung Paik, Geongyu Lee, Joonho Lee, Tae-Yeong Kwak, Hong Koo Ha
Prostate International.2025;[Epub] CrossRef - Label-free optical microscopy with artificial intelligence: a new paradigm in pathology
Chiho Yoon, Eunwoo Park, Donggyu Kim, Byullee Park, Chulhong Kim
Biophotonics Discovery.2025;[Epub] CrossRef - EPIIC: Edge-Preserving Method Increasing Nuclei Clarity for Compression Artifacts Removal in Whole-Slide Histopathological Images
Julia Merta, Michal Marczyk
Applied Sciences.2025; 15(8): 4450. CrossRef - Comparative analysis of a 5G campus network and existing networks for real-time consultation in remote pathology
Ilgar I. Guseinov, Arnab Bhowmik, Somaia AbuBaker, Anna E. Schmaus-Klughammer, Thomas Spittler
Journal of Pathology Informatics.2025; : 100444. CrossRef - The Evolution of Digital Pathology in Infrastructure, Artificial Intelligence and Clinical Impact
Chan Kwon Jung
International Journal of Thyroidology.2025; 18(1): 6. CrossRef - Role of Telepathology, Artificial Intelligence, and Emerging Technologies in Enhancing Diagnostic Accuracy
Yugeshwari R. Tiwade, Obaid Noman, Pratibha Dawande, Nandkishor J Bankar, Sweta Bahadure, Praful Patil
Journal of Nature and Science of Medicine.2025; 8(2): 115. CrossRef - Artificial intelligence for automatic detection of basal cell carcinoma from frozen tissue tangential biopsies
Dennis H Murphree, Yong-hun Kim, Kirk A Sidey, Nneka I Comfere, Nahid Y Vidal
Clinical and Experimental Dermatology.2024; 49(7): 719. CrossRef - Performance of externally validated machine learning models based on histopathology images for the diagnosis, classification, prognosis, or treatment outcome prediction in female breast cancer: A systematic review
Ricardo Gonzalez, Peyman Nejat, Ashirbani Saha, Clinton J.V. Campbell, Andrew P. Norgan, Cynthia Lokker
Journal of Pathology Informatics.2024; 15: 100348. CrossRef - Invisible for a few but essential for many: the role of Histotechnologists in the establishment of digital pathology
Gisela Magalhães, Rita Calisto, Catarina Freire, Regina Silva, Diana Montezuma, Sule Canberk, Fernando Schmitt
Journal of Histotechnology.2024; 47(1): 39. CrossRef - Using digital pathology to analyze the murine cerebrovasculature
Dana M Niedowicz, Jenna L Gollihue, Erica M Weekman, Panhavuth Phe, Donna M Wilcock, Christopher M Norris, Peter T Nelson
Journal of Cerebral Blood Flow & Metabolism.2024; 44(4): 595. CrossRef - PATrans: Pixel-Adaptive Transformer for edge segmentation of cervical nuclei on small-scale datasets
Hexuan Hu, Jianyu Zhang, Tianjin Yang, Qiang Hu, Yufeng Yu, Qian Huang
Computers in Biology and Medicine.2024; 168: 107823. CrossRef - CNAC-Seg: Effective segmentation for cervical nuclei in adherent cells and clusters via exploring gaps of receptive fields
Hexuan Hu, Jianyu Zhang, Tianjin Yang, Qiang Hu, Yufeng Yu, Qian Huang
Biomedical Signal Processing and Control.2024; 90: 105833. CrossRef - Artificial Intelligence-Enabled Prostate Cancer Diagnosis and Prognosis: Current State and Future Implications
Swati Satturwar, Anil V. Parwani
Advances in Anatomic Pathology.2024; 31(2): 136. CrossRef - Ensemble Deep Learning Model to Predict Lymphovascular Invasion in Gastric Cancer
Jonghyun Lee, Seunghyun Cha, Jiwon Kim, Jung Joo Kim, Namkug Kim, Seong Gyu Jae Gal, Ju Han Kim, Jeong Hoon Lee, Yoo-Duk Choi, Sae-Ryung Kang, Ga-Young Song, Deok-Hwan Yang, Jae-Hyuk Lee, Kyung-Hwa Lee, Sangjeong Ahn, Kyoung Min Moon, Myung-Giun Noh
Cancers.2024; 16(2): 430. CrossRef - Artificial intelligence’s impact on breast cancer pathology: a literature review
Amr Soliman, Zaibo Li, Anil V. Parwani
Diagnostic Pathology.2024;[Epub] CrossRef - Automated Analysis of Nuclear Parameters in Oral Exfoliative Cytology Using Machine Learning
Shubhangi Mhaske, Karthikeyan Ramalingam, Preeti Nair, Shubham Patel, Arathi Menon P, Nida Malik, Sumedh Mhaske
Cureus.2024;[Epub] CrossRef - Enhancing AI Research for Breast Cancer: A Comprehensive Review of Tumor-Infiltrating Lymphocyte Datasets
Alessio Fiorin, Carlos López Pablo, Marylène Lejeune, Ameer Hamza Siraj, Vincenzo Della Mea
Journal of Imaging Informatics in Medicine.2024; 37(6): 2996. CrossRef - Current Developments in Diagnosis of Salivary Gland Tumors: From Structure to Artificial Intelligence
Alexandra Corina Faur, Roxana Buzaș, Adrian Emil Lăzărescu, Laura Andreea Ghenciu
Life.2024; 14(6): 727. CrossRef - Comparative analysis of chronic progressive nephropathy (CPN) diagnosis in rat kidneys using an artificial intelligence deep learning model
Yeji Bae, Jongsu Byun, Hangyu Lee, Beomseok Han
Toxicological Research.2024; 40(4): 551. CrossRef - A Pan-Cancer Patient-Derived Xenograft Histology Image Repository with Genomic and Pathologic Annotations Enables Deep Learning Analysis
Brian S. White, Xing Yi Woo, Soner Koc, Todd Sheridan, Steven B. Neuhauser, Shidan Wang, Yvonne A. Evrard, Li Chen, Ali Foroughi pour, John D. Landua, R. Jay Mashl, Sherri R. Davies, Bingliang Fang, Maria Gabriela Raso, Kurt W. Evans, Matthew H. Bailey, Y
Cancer Research.2024; 84(13): 2060. CrossRef - Non-contrasted computed tomography (NCCT) based chronic thromboembolic pulmonary hypertension (CTEPH) automatic diagnosis using cascaded network with multiple instance learning
Mayang Zhao, Liming Song, Jiarui Zhu, Ta Zhou, Yuanpeng Zhang, Shu-Cheng Chen, Haojiang Li, Di Cao, Yi-Quan Jiang, Waiyin Ho, Jing Cai, Ge Ren
Physics in Medicine & Biology.2024; 69(18): 185011. CrossRef - MR_NET: A Method for Breast Cancer Detection and Localization from Histological Images Through Explainable Convolutional Neural Networks
Rachele Catalano, Myriam Giusy Tibaldi, Lucia Lombardi, Antonella Santone, Mario Cesarelli, Francesco Mercaldo
Sensors.2024; 24(21): 7022. CrossRef - Advances in AI-Enhanced Biomedical Imaging for Cancer Immunology
Willa Wen-You Yim, Felicia Wee, Zheng Yi Ho, Xinyun Feng, Marcia Zhang, Samuel Lee, Inti Zlobec, Joe Yeong, Mai Chan Lau
World Scientific Annual Review of Cancer Immunology.2024;[Epub] CrossRef - Blockchain: A safe digital technology to share cancer diagnostic results in pandemic times—Challenges and legacy for the future
Bruno Natan Santana Lima, Lucas Alves da Mota Santana, Rani Iani Costa Gonçalo, Carla Samily de Oliveira Costa, Daniel Pitanga de Sousa Nogueira, Cleverson Luciano Trento, Wilton Mitsunari Takeshita
Oral Surgery.2023; 16(3): 300. CrossRef - Pathologists’ acceptance of telepathology in the Ministry of National Guard Health Affairs Hospitals in Saudi Arabia: A survey study
Raneem Alawashiz, Sharifah Abdullah AlDossary
DIGITAL HEALTH.2023;[Epub] CrossRef - An Atrous Convolved Hybrid Seg-Net Model with residual and attention mechanism for gland detection and segmentation in histopathological images
Manju Dabass, Jyoti Dabass
Computers in Biology and Medicine.2023; 155: 106690. CrossRef - Validation of a Machine Learning Expert Supporting System, ImmunoGenius, Using Immunohistochemistry Results of 3000 Patients with Lymphoid Neoplasms
Jamshid Abdul-Ghafar, Kyung Jin Seo, Hye-Ra Jung, Gyeongsin Park, Seung-Sook Lee, Yosep Chong
Diagnostics.2023; 13(7): 1308. CrossRef - Diagnosing Infectious Diseases in Poultry Requires a Holistic Approach: A Review
Dieter Liebhart, Ivana Bilic, Beatrice Grafl, Claudia Hess, Michael Hess
Poultry.2023; 2(2): 252. CrossRef - Recent application of artificial intelligence on histopathologic image-based prediction of gene mutation in solid cancers
Mohammad Rizwan Alam, Kyung Jin Seo, Jamshid Abdul-Ghafar, Kwangil Yim, Sung Hak Lee, Hyun-Jong Jang, Chan Kwon Jung, Yosep Chong
Briefings in Bioinformatics.2023;[Epub] CrossRef - Canine Mammary Tumor Histopathological Image Classification via Computer-Aided Pathology: An Available Dataset for Imaging Analysis
Giovanni P. Burrai, Andrea Gabrieli, Marta Polinas, Claudio Murgia, Maria Paola Becchere, Pierfranco Demontis, Elisabetta Antuofermo
Animals.2023; 13(9): 1563. CrossRef - Rapid digital pathology of H&E-stained fresh human brain specimens as an alternative to frozen biopsy
Bhaskar Jyoti Borah, Yao-Chen Tseng, Kuo-Chuan Wang, Huan-Chih Wang, Hsin-Yi Huang, Koping Chang, Jhih Rong Lin, Yi-Hua Liao, Chi-Kuang Sun
Communications Medicine.2023;[Epub] CrossRef - Applied machine learning in hematopathology
Taher Dehkharghanian, Youqing Mu, Hamid R. Tizhoosh, Clinton J. V. Campbell
International Journal of Laboratory Hematology.2023; 45(S2): 87. CrossRef - Automated diagnosis of 7 canine skin tumors using machine learning on H&E-stained whole slide images
Marco Fragoso-Garcia, Frauke Wilm, Christof A. Bertram, Sophie Merz, Anja Schmidt, Taryn Donovan, Andrea Fuchs-Baumgartinger, Alexander Bartel, Christian Marzahl, Laura Diehl, Chloe Puget, Andreas Maier, Marc Aubreville, Katharina Breininger, Robert Klopf
Veterinary Pathology.2023; 60(6): 865. CrossRef - Artificial Intelligence in the Pathology of Gastric Cancer
Sangjoon Choi, Seokhwi Kim
Journal of Gastric Cancer.2023; 23(3): 410. CrossRef - Efficient Convolution Network to Assist Breast Cancer Diagnosis and Target Therapy
Ching-Wei Wang, Kai-Lin Chu, Hikam Muzakky, Yi-Jia Lin, Tai-Kuang Chao
Cancers.2023; 15(15): 3991. CrossRef - Multi-Configuration Analysis of DenseNet Architecture for Whole Slide Image Scoring of ER-IHC
Wan Siti Halimatul Munirah Wan Ahmad, Mohammad Faizal Ahmad Fauzi, Md Jahid Hasan, Zaka Ur Rehman, Jenny Tung Hiong Lee, See Yee Khor, Lai-Meng Looi, Fazly Salleh Abas, Afzan Adam, Elaine Wan Ling Chan, Sei-Ichiro Kamata
IEEE Access.2023; 11: 79911. CrossRef - Digitization of Pathology Labs: A Review of Lessons Learned
Lars Ole Schwen, Tim-Rasmus Kiehl, Rita Carvalho, Norman Zerbe, André Homeyer
Laboratory Investigation.2023; 103(11): 100244. CrossRef - Artificial Intelligence in Endoscopic Ultrasonography-Guided Fine-Needle Aspiration/Biopsy (EUS-FNA/B) for Solid Pancreatic Lesions: Opportunities and Challenges
Xianzheng Qin, Taojing Ran, Yifei Chen, Yao Zhang, Dong Wang, Chunhua Zhou, Duowu Zou
Diagnostics.2023; 13(19): 3054. CrossRef - Deep Learning for the Pathologic Diagnosis of Hepatocellular Carcinoma, Cholangiocarcinoma, and Metastatic Colorectal Cancer
Hyun-Jong Jang, Jai-Hyang Go, Younghoon Kim, Sung Hak Lee
Cancers.2023; 15(22): 5389. CrossRef - AIR-UNet++: a deep learning framework for histopathology image segmentation and detection
Anusree Kanadath, J. Angel Arul Jothi, Siddhaling Urolagin
Multimedia Tools and Applications.2023; 83(19): 57449. CrossRef - Deep Learning-Based Dermatological Condition Detection: A Systematic Review With Recent Methods, Datasets, Challenges, and Future Directions
Stephanie S. Noronha, Mayuri A. Mehta, Dweepna Garg, Ketan Kotecha, Ajith Abraham
IEEE Access.2023; 11: 140348. CrossRef - Digital pathology and artificial intelligence in translational medicine and clinical practice
Vipul Baxi, Robin Edwards, Michael Montalto, Saurabh Saha
Modern Pathology.2022; 35(1): 23. CrossRef - Artificial Intelligence in Toxicological Pathology: Quantitative Evaluation of Compound-Induced Follicular Cell Hypertrophy in Rat Thyroid Gland Using Deep Learning Models
Valeria Bertani, Olivier Blanck, Davy Guignard, Frederic Schorsch, Hannah Pischon
Toxicologic Pathology.2022; 50(1): 23. CrossRef - Investigating the genealogy of the literature on digital pathology: a two-dimensional bibliometric approach
Dayu Hu, Chengyuan Wang, Song Zheng, Xiaoyu Cui
Scientometrics.2022; 127(2): 785. CrossRef - Digital Dermatopathology and Its Application to Mohs Micrographic Surgery
Yeongjoo Oh, Hye Min Kim, Soon Won Hong, Eunah Shin, Jihee Kim, Yoon Jung Choi
Yonsei Medical Journal.2022; 63(Suppl): S112. CrossRef - Assessment of parathyroid gland cellularity by digital slide analysis
Rotem Sagiv, Bertha Delgado, Oleg Lavon, Vladislav Osipov, Re'em Sade, Sagi Shashar, Ksenia M. Yegodayev, Moshe Elkabets, Ben-Zion Joshua
Annals of Diagnostic Pathology.2022; 58: 151907. CrossRef - PancreaSys: An Automated Cloud-Based Pancreatic Cancer Grading System
Muhammad Nurmahir Mohamad Sehmi, Mohammad Faizal Ahmad Fauzi, Wan Siti Halimatul Munirah Wan Ahmad, Elaine Wan Ling Chan
Frontiers in Signal Processing.2022;[Epub] CrossRef - Classification of Mouse Lung Metastatic Tumor with Deep Learning
Ha Neul Lee, Hong-Deok Seo, Eui-Myoung Kim, Beom Seok Han, Jin Seok Kang
Biomolecules & Therapeutics.2022; 30(2): 179. CrossRef - Techniques for digital histological morphometry of the pineal gland
Bogdan-Alexandru Gheban, Horaţiu Alexandru Colosi, Ioana-Andreea Gheban-Roșca, Carmen Georgiu, Dan Gheban, Doiniţa Crişan, Maria Crişan
Acta Histochemica.2022; 124(4): 151897. CrossRef - Current Trend of Artificial Intelligence Patents in Digital Pathology: A Systematic Evaluation of the Patent Landscape
Muhammad Joan Ailia, Nishant Thakur, Jamshid Abdul-Ghafar, Chan Kwon Jung, Kwangil Yim, Yosep Chong
Cancers.2022; 14(10): 2400. CrossRef - Recent Applications of Artificial Intelligence from Histopathologic Image-Based Prediction of Microsatellite Instability in Solid Cancers: A Systematic Review
Mohammad Rizwan Alam, Jamshid Abdul-Ghafar, Kwangil Yim, Nishant Thakur, Sung Hak Lee, Hyun-Jong Jang, Chan Kwon Jung, Yosep Chong
Cancers.2022; 14(11): 2590. CrossRef - Development of a prognostic prediction support system for cervical intraepithelial neoplasia using artificial intelligence-based diagnosis
Takayuki Takahashi, Hikaru Matsuoka, Rieko Sakurai, Jun Akatsuka, Yusuke Kobayashi, Masaru Nakamura, Takashi Iwata, Kouji Banno, Motomichi Matsuzaki, Jun Takayama, Daisuke Aoki, Yoichiro Yamamoto, Gen Tamiya
Journal of Gynecologic Oncology.2022;[Epub] CrossRef - Digital Pathology and Artificial Intelligence Applications in Pathology
Heounjeong Go
Brain Tumor Research and Treatment.2022; 10(2): 76. CrossRef - Mass spectrometry imaging to explore molecular heterogeneity in cell culture
Tanja Bien, Krischan Koerfer, Jan Schwenzfeier, Klaus Dreisewerd, Jens Soltwisch
Proceedings of the National Academy of Sciences.2022;[Epub] CrossRef - Integrating artificial intelligence in pathology: a qualitative interview study of users' experiences and expectations
Jojanneke Drogt, Megan Milota, Shoko Vos, Annelien Bredenoord, Karin Jongsma
Modern Pathology.2022; 35(11): 1540. CrossRef - Deep Learning on Basal Cell Carcinoma In Vivo Reflectance Confocal Microscopy Data
Veronika Shavlokhova, Michael Vollmer, Patrick Gholam, Babak Saravi, Andreas Vollmer, Jürgen Hoffmann, Michael Engel, Christian Freudlsperger
Journal of Personalized Medicine.2022; 12(9): 1471. CrossRef - Deep Learning-Based Classification of Uterine Cervical and Endometrial Cancer Subtypes from Whole-Slide Histopathology Images
JaeYen Song, Soyoung Im, Sung Hak Lee, Hyun-Jong Jang
Diagnostics.2022; 12(11): 2623. CrossRef - A self-supervised contrastive learning approach for whole slide image representation in digital pathology
Parsa Ashrafi Fashi, Sobhan Hemati, Morteza Babaie, Ricardo Gonzalez, H.R. Tizhoosh
Journal of Pathology Informatics.2022; 13: 100133. CrossRef - A Matched-Pair Analysis of Nuclear Morphologic Features Between Core Needle Biopsy and Surgical Specimen in Thyroid Tumors Using a Deep Learning Model
Faridul Haq, Andrey Bychkov, Chan Kwon Jung
Endocrine Pathology.2022; 33(4): 472. CrossRef - Development of quality assurance program for digital pathology by the Korean Society of Pathologists
Yosep Chong, Jeong Mo Bae, Dong Wook Kang, Gwangil Kim, Hye Seung Han
Journal of Pathology and Translational Medicine.2022; 56(6): 370. CrossRef - Machine learning in renal pathology
Matthew Nicholas Basso, Moumita Barua, Julien Meyer, Rohan John, April Khademi
Frontiers in Nephrology.2022;[Epub] CrossRef - Whole Slide Image Quality in Digital Pathology: Review and Perspectives
Romain Brixtel, Sebastien Bougleux, Olivier Lezoray, Yann Caillot, Benoit Lemoine, Mathieu Fontaine, Dalal Nebati, Arnaud Renouf
IEEE Access.2022; 10: 131005. CrossRef - Generalizability of Deep Learning System for the Pathologic Diagnosis of Various Cancers
Hyun-Jong Jang, In Hye Song, Sung Hak Lee
Applied Sciences.2021; 11(2): 808. CrossRef - Recent advances in the use of stimulated Raman scattering in histopathology
Martin Lee, C. Simon Herrington, Manasa Ravindra, Kristel Sepp, Amy Davies, Alison N. Hulme, Valerie G. Brunton
The Analyst.2021; 146(3): 789. CrossRef - Preference and Demand for Digital Pathology and Computer-Aided Diagnosis among Korean Pathologists: A Survey Study Focused on Prostate Needle Biopsy
Soo Jeong Nam, Yosep Chong, Chan Kwon Jung, Tae-Yeong Kwak, Ji Youl Lee, Jihwan Park, Mi Jung Rho, Heounjeong Go
Applied Sciences.2021; 11(16): 7380. CrossRef - An SVM approach towards breast cancer classification from H&E-stained histopathology images based on integrated features
M. A. Aswathy, M. Jagannath
Medical & Biological Engineering & Computing.2021; 59(9): 1773. CrossRef - Diagnosis prediction of tumours of unknown origin using ImmunoGenius, a machine learning-based expert system for immunohistochemistry profile interpretation
Yosep Chong, Nishant Thakur, Ji Young Lee, Gyoyeon Hwang, Myungjin Choi, Yejin Kim, Hwanjo Yu, Mee Yon Cho
Diagnostic Pathology.2021;[Epub] CrossRef - Deep Learning for Automatic Subclassification of Gastric Carcinoma Using Whole-Slide Histopathology Images
Hyun-Jong Jang, In-Hye Song, Sung-Hak Lee
Cancers.2021; 13(15): 3811. CrossRef - A novel evaluation method for Ki-67 immunostaining in paraffin-embedded tissues
Eliane Pedra Dias, Nathália Silva Carlos Oliveira, Amanda Oliveira Serra-Campos, Anna Karoline Fausto da Silva, Licínio Esmeraldo da Silva, Karin Soares Cunha
Virchows Archiv.2021; 479(1): 121. CrossRef - Assessment of Digital Pathology Imaging Biomarkers Associated with Breast Cancer Histologic Grade
Andrew Lagree, Audrey Shiner, Marie Angeli Alera, Lauren Fleshner, Ethan Law, Brianna Law, Fang-I Lu, David Dodington, Sonal Gandhi, Elzbieta A. Slodkowska, Alex Shenfield, Katarzyna J. Jerzak, Ali Sadeghi-Naini, William T. Tran
Current Oncology.2021; 28(6): 4298. CrossRef - Prediction of genetic alterations from gastric cancer histopathology images using a fully automated deep learning approach
Hyun-Jong Jang, Ahwon Lee, Jun Kang, In Hye Song, Sung Hak Lee
World Journal of Gastroenterology.2021; 27(44): 7687. CrossRef - Clustered nuclei splitting based on recurrent distance transform in digital pathology images
Lukasz Roszkowiak, Anna Korzynska, Dorota Pijanowska, Ramon Bosch, Marylene Lejeune, Carlos Lopez
EURASIP Journal on Image and Video Processing.2020;[Epub] CrossRef - Current Trends of Artificial Intelligence for Colorectal Cancer Pathology Image Analysis: A Systematic Review
Nishant Thakur, Hongjun Yoon, Yosep Chong
Cancers.2020; 12(7): 1884. CrossRef - A bird’s-eye view of deep learning in bioimage analysis
Erik Meijering
Computational and Structural Biotechnology Journal.2020; 18: 2312. CrossRef - Pathomics in urology
Victor M. Schuettfort, Benjamin Pradere, Michael Rink, Eva Comperat, Shahrokh F. Shariat
Current Opinion in Urology.2020; 30(6): 823. CrossRef - Model Fooling Attacks Against Medical Imaging: A Short Survey
Tuomo Sipola, Samir Puuska, Tero Kokkonen
Information & Security: An International Journal.2020; 46(2): 215. CrossRef - Recommendations for pathologic practice using digital pathology: consensus report of the Korean Society of Pathologists
Yosep Chong, Dae Cheol Kim, Chan Kwon Jung, Dong-chul Kim, Sang Yong Song, Hee Jae Joo, Sang-Yeop Yi
Journal of Pathology and Translational Medicine.2020; 54(6): 437. CrossRef - A machine-learning expert-supporting system for diagnosis prediction of lymphoid neoplasms using a probabilistic decision-tree algorithm and immunohistochemistry profile database
Yosep Chong, Ji Young Lee, Yejin Kim, Jingyun Choi, Hwanjo Yu, Gyeongsin Park, Mee Yon Cho, Nishant Thakur
Journal of Pathology and Translational Medicine.2020; 54(6): 462. CrossRef
- Deep learning model to diagnose cardiac amyloidosis from haematoxylin/eosin-stained myocardial tissue
Original Articles
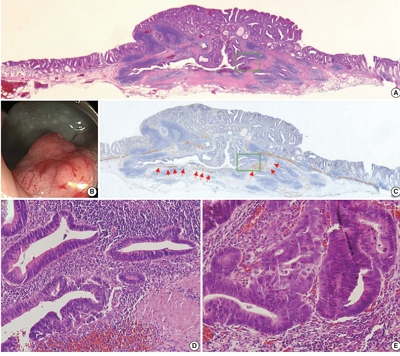
- Colorectal epithelial neoplasm associated with gut-associated lymphoid tissue
- Yo Han Jeon, Ji Hyun Ahn, Hee Kyung Chang
- J Pathol Transl Med. 2020;54(2):135-145. Published online January 29, 2020
- DOI: https://doi.org/10.4132/jptm.2019.11.06
- 7,758 View
- 245 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Colorectal epithelial neoplasm extending into the submucosal gut-associated lymphoid tissue (GALT) can cause difficulties in the differential diagnosis. Regarding GALT-associated epithelial neoplasms, a few studies favor the term “GALT carcinoma” while other studies have mentioned the term “GALT-associated pseudoinvasion/epithelial misplacement (PEM)”.
Methods
The clinicopathologic characteristics of 11 cases of colorectal epithelial neoplasm associated with submucosal GALT diagnosed via endoscopic submucosal dissection were studied.
Results
Eight cases (72.7%) were in males. The median age was 59 years, and age ranged from 53 to 73. All cases had a submucosal tumor component more compatible with GALT-associated PEM. Eight cases (72.7%) were located in the right colon. Ten cases (90.9%) had a non-protruding endoscopic appearance. Nine cases (81.8%) showed continuity between the submucosal and surface adenomatous components. Nine cases showed (81.8%) focal defects or discontinuation of the muscularis mucosae adjacent to the submucosal GALT. No case showed hemosiderin deposits in the submucosa or desmoplastic reaction. No case showed single tumor cells or small clusters of tumor cells in the submucosal GALT. Seven cases (63.6%) showed goblet cells in the submucosa. No cases showed oncocytic columnar cells lining submucosal glands.
Conclusions
Our experience suggests that pathologists should be aware of the differential diagnosis of GALT-associated submucosal extension by colorectal adenomatous neoplasm. Further studies are needed to validate classification of GALT-associated epithelial neoplasms. -
Citations
Citations to this article as recorded by- Family adenomatous polyposis come across dome type adenocarcinoma: a case report and literature review
Ying-Ying Chang, Xiao-Long Zhang, Yao-Hui Wang, Ting-Sheng Ling
Diagnostic Pathology.2025;[Epub] CrossRef - Radiation-induced injury and the gut microbiota: insights from a microbial perspective
Qiaoli Wang, Guoqiang Xu, Ouying Yan, Shang Wang, Xin Wang
Therapeutic Advances in Gastroenterology.2025;[Epub] CrossRef
- Family adenomatous polyposis come across dome type adenocarcinoma: a case report and literature review
- Double cocktail immunostains with high molecular weight cytokeratin and GATA-3: useful stain to discriminate in situ involvement of prostatic ducts or acini from stromal invasion by urothelial carcinoma in the prostate
- Junghye Lee, Youngeun Yoo, Sanghui Park, Min-Sun Cho, Sun Hee Sung, Jae Y. Ro
- J Pathol Transl Med. 2020;54(2):146-153. Published online February 10, 2020
- DOI: https://doi.org/10.4132/jptm.2019.11.12
- 7,008 View
- 128 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Distinguishing prostatic stromal invasion (PSI) by urothelial carcinoma (UC) from in situ UC involving prostatic ducts or acini with no stromal invasion (in situ involvement) may be challenging on hematoxylin and eosin stained sections. However, the distinction between them is important because cases with PSI show worse prognosis. This study was performed to assess the utility of double cocktail immunostains with high molecular weight cytokeratin (HMWCK) and GATA-3 to discriminate PSI by UC from in situ UC involvement of prostatic ducts or acini in the prostate.
Methods
Among 117 radical cystoprostatectomy specimens for bladder UCs, 25 cases showed secondary involvement of bladder UC in prostatic ducts/acini only or associated stromal invasion and of these 25 cases, seven cases revealed equivocal PSI. In these seven cases with equivocal PSI, HMWCK, and GATA-3 double immunohistochemical stains were performed to identify whether this cocktail stain is useful to identify the stromal invasion.
Results
In all cases, basal cells of prostate glands showed strong cytoplasmic staining for HMWCK and UC cells showed strong nuclear staining for GATA-3. In cases with stromal invasion of UC, GATA-3-positive tumor cells in the prostatic stroma without surrounding HMWCK-positive basal cells were highlighted and easily recognized. Among seven equivocal cases, two cases showed PSI and five in situ UC in the prostate. In two cases, the original diagnoses were revised.
Conclusions
Our study suggested that HMWCK and GATA-3 double stains could be utilized as an adjunct method in the distinction between PSI by UC from in situ UC involving prostatic ducts or acini. -
Citations
Citations to this article as recorded by- Aberrant expression of GATA3 in metastatic adenocarcinoma of the prostate: an important pitfall
João Lobo, Nazario P Tenace, Sofia Cañete‐Portillo, Isa Carneiro, Rui Henrique, Roberta Lucianò, Lara R Harik, Cristina Magi‐Galluzzi
Histopathology.2024; 84(3): 507. CrossRef - Utility of D2-40, Cytokeratin 5/6, and High–Molecular-weight Cytokeratin (Clone 34βE12) in Distinguishing Intraductal Spread of Urothelial Carcinoma From Prostatic Stromal Invasion
Oleksii A. Iakymenko, Laurence M. Briski, Katiana S. Delma, Merce Jorda, Oleksandr N. Kryvenko
American Journal of Surgical Pathology.2022; 46(4): 454. CrossRef
- Aberrant expression of GATA3 in metastatic adenocarcinoma of the prostate: an important pitfall
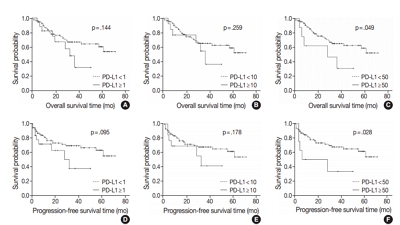
- Programmed death-ligand 1 expression and its correlation with clinicopathological parameters in gallbladder cancer
- Ji Hye Kim, Kyungbin Kim, Misung Kim, Young Min Kim, Jae Hee Suh, Hee Jeong Cha, Hye Jeong Choi
- J Pathol Transl Med. 2020;54(2):154-164. Published online February 10, 2020
- DOI: https://doi.org/10.4132/jptm.2019.11.13
- 8,380 View
- 167 Download
- 15 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Immunomodulatory therapies targeting the interaction between programmed cell death protein 1 and programmed death-ligand 1 (PD-L1) have become increasingly important in anticancer treatment. Previous research on the subject of this immune response has established an association with tumor aggressiveness and a poor prognosis in certain cancers. Currently, scant information is available on the relationship between PD-L1 expression and gallbladder cancer (GBC).
Methods
We investigated the expression of PD-L1 in 101 primary GBC cases to determine the potential association with prognostic impact. PD-L1 expression was immunohistochemically assessed using a single PD-L1 antibody (clone SP263). Correlations with clinicopathological parameters, overall survival (OS), or progression- free survival (PFS) were analyzed.
Results
PD-L1 expression in tumor cells at cutoff levels of 1%, 10%, and 50% was present in 18.8%, 13.8%, and 7.9% of cases. Our study showed that positive PD-L1 expression at any cutoff was significantly correlated with poorly differentiated histologic grade and the presence of lymphovascular invasion (p < .05). PD-L1 expression at cutoff levels of 10% and 50% was significantly positive in patients with perineural invasion, higher T categories, and higher pathologic stages (p < .05). Additionally, there was a significant association noted between PD-L1 expression at a cutoff level of 50% and worse OS or PFS (p = .049 for OS, p = .028 for PFS). Other poor prognostic factors included histologic grade, T category, N category, pathologic stage, lymphovascular invasion, perineural invasion, growth pattern, and margin of resection (p < .05).
Conclusions
The expression of PD-L1 in GBC varies according to cutoff level but is valuably associated with poor prognostic parameters and survival. Our study indicates that the overexpression of PD-L1 in GBC had a negative prognostic impact. -
Citations
Citations to this article as recorded by- PD-L1 Expression in Biliary Tract Cancer: Comparison Across Antibody Clones and Role as a Predictor of Response to Chemoimmunotherapy: A Meta-Analysis
Juan J. Juarez-Vignon Whaley, Soravis Osataphan, Ben Ponvilawan, Nipith Charoenngam, Mary Linton Peters
JCO Precision Oncology.2025;[Epub] CrossRef - Lacking Immunotherapy Biomarkers for Biliary Tract Cancer: A Comprehensive Systematic Literature Review and Meta-Analysis
Giorgio Frega, Fernando P. Cossio, Jesus M. Banales, Vincenzo Cardinale, Rocio I. R. Macias, Chiara Braconi, Angela Lamarca
Cells.2023; 12(16): 2098. CrossRef - Gallbladder carcinomas: review and updates on morphology, immunohistochemistry, and staging
Whayoung Lee, Vishal S. Chandan
Human Pathology.2023; 132: 149. CrossRef - Prognostic Relevance of PDL1 and CA19-9 Expression in Gallbladder Cancer vs. Inflammatory Lesions
Neetu Rawal, Supriya Awasthi, Nihar Ranjan Dash, Sunil Kumar, Prasenjit Das, Amar Ranjan, Anita Chopra, Maroof Ahmad Khan, Sundeep Saluja, Showket Hussain, Pranay Tanwar
Current Oncology.2023; 30(2): 1571. CrossRef - Identification of genes associated with gall bladder cell carcinogenesis: Implications in targeted therapy of gall bladder cancer
Ishita Ghosh, Ruma Dey Ghosh, Soma Mukhopadhyay
World Journal of Gastrointestinal Oncology.2023; 15(12): 2053. CrossRef - CD73 and PD-L1 as Potential Therapeutic Targets in Gallbladder Cancer
Lu Cao, Kim R. Bridle, Ritu Shrestha, Prashanth Prithviraj, Darrell H. G. Crawford, Aparna Jayachandran
International Journal of Molecular Sciences.2022; 23(3): 1565. CrossRef - Evolving Role of Immunotherapy in Advanced Biliary Tract Cancers
Sandra Kang, Bassel F. El-Rayes, Mehmet Akce
Cancers.2022; 14(7): 1748. CrossRef - Novel immune scoring dynamic nomograms based on B7-H3, B7-H4, and HHLA2: Potential prediction in survival and immunotherapeutic efficacy for gallbladder cancer
Chao Lv, Shukun Han, Baokang Wu, Zhiyun Liang, Yang Li, Yizhou Zhang, Qi Lang, Chongli Zhong, Lei Fu, Yang Yu, Feng Xu, Yu Tian
Frontiers in Immunology.2022;[Epub] CrossRef - PD-1 inhibitors plus nab-paclitaxel-containing chemotherapy for advanced gallbladder cancer in a second-line setting: A retrospective analysis of a case series
Sirui Tan, Jing Yu, Qiyue Huang, Nan Zhou, Hongfeng Gou
Frontiers in Oncology.2022;[Epub] CrossRef - Expression of HER2 and Mismatch Repair Proteins in Surgically Resected Gallbladder Adenocarcinoma
You-Na Sung, Sung Joo Kim, Sun-Young Jun, Changhoon Yoo, Kyu-Pyo Kim, Jae Hoon Lee, Dae Wook Hwang, Shin Hwang, Sang Soo Lee, Seung-Mo Hong
Frontiers in Oncology.2021;[Epub] CrossRef - Programmed Death Ligand-1 (PD-L1) Is an Independent Negative Prognosticator in Western-World Gallbladder Cancer
Thomas Albrecht, Fritz Brinkmann, Michael Albrecht, Anke S. Lonsdorf, Arianeb Mehrabi, Katrin Hoffmann, Yakup Kulu, Alphonse Charbel, Monika N. Vogel, Christian Rupp, Bruno Köhler, Christoph Springfeld, Peter Schirmacher, Stephanie Roessler, Benjamin Goep
Cancers.2021; 13(7): 1682. CrossRef - Immune Microenvironment in Gallbladder Adenocarcinomas
Pallavi A. Patil, Kara Lombardo, Weibiao Cao
Applied Immunohistochemistry & Molecular Morphology.2021; 29(8): 557. CrossRef - Molecular Targets and Emerging Therapies for Advanced Gallbladder Cancer
Matteo Canale, Manlio Monti, Ilario Giovanni Rapposelli, Paola Ulivi, Francesco Giulio Sullo, Giulia Bartolini, Elisa Tiberi, Giovanni Luca Frassineti
Cancers.2021; 13(22): 5671. CrossRef - Overview of current targeted therapy in gallbladder cancer
Xiaoling Song, Yunping Hu, Yongsheng Li, Rong Shao, Fatao Liu, Yingbin Liu
Signal Transduction and Targeted Therapy.2020;[Epub] CrossRef
- PD-L1 Expression in Biliary Tract Cancer: Comparison Across Antibody Clones and Role as a Predictor of Response to Chemoimmunotherapy: A Meta-Analysis
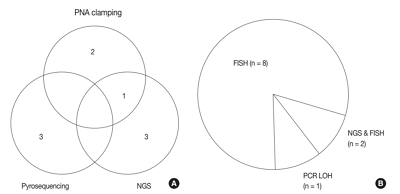
- Adjunctive markers for classification and diagnosis of central nervous system tumors: results of a multi-center neuropathological survey in Korea
- Yoon Jin Cha, Se Hoon Kim, Na Rae Kim
- J Pathol Transl Med. 2020;54(2):165-170. Published online February 20, 2020
- DOI: https://doi.org/10.4132/jptm.2020.02.04
- 7,101 View
- 216 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
The revised 4th 2016 World Health Organization (WHO) classification of tumors of the central nervous system (CNS) classification has adopted integrated diagnosis encompassing the histology and molecular features of CNS tumors. We aimed to investigate the immunohistochemistry, molecular testing, and testing methods for diagnosis of CNS tumors in pathological labs of tertiary centers in Korea, and evaluate the adequacy of tests for proper diagnosis in daily practice.
Methods
A survey, composed of eight questions concerning molecular testing for diagnosis of CNS tumors, was sent to 10 neuropathologists working in tertiary centers in Korea.
Results
For diagnosis of astrocytic and oligodendroglial tumors, all 10 centers performed isocitrate dehydrogenase mutations testing and 1p/19q loss of heterozygosity. For glioneuronal tumors, immunohistochemistry (IHC) assays for synaptophysin (n = 9), CD34 (n = 7), BRAF(VE1) (n = 5) were used. For embryonal tumors, particularly in medulloblastoma, four respondents used IHC panel (growth factor receptor bound protein 2-associated protein 1, filamin A, and yes-associated protein 1) for molecular subclassification. Regarding meningioma, all respondents performed Ki-67 IHC and five performed telomerase reverse transcriptase promoter mutation.
Conclusions
Most tertiary centers made proper diagnosis in line with 2016 WHO classification. As classification of CNS tumors has evolved to be more complex and more ancillary tests are required, these should be performed considering the effect of necessity and justification. -
Citations
Citations to this article as recorded by- Exploring the role of epidermal growth factor receptor variant III in meningeal tumors
Rashmi Rana, Vaishnavi Rathi, Kirti Chauhan, Kriti Jain, Satnam Singh Chhabra, Rajesh Acharya, Samir Kumar Kalra, Anshul Gupta, Sunila Jain, Nirmal Kumar Ganguly, Dharmendra Kumar Yadav, Timir Tripathi
PLOS ONE.2021; 16(9): e0255133. CrossRef
- Exploring the role of epidermal growth factor receptor variant III in meningeal tumors
- Contribution of cytologic examination to diagnosis of poorly differentiated thyroid carcinoma
- Na Rae Kim, Jae Yeon Seok, Yoo Seung Chung, Joon Hyop Lee, Dong Hae Chung
- J Pathol Transl Med. 2020;54(2):171-178. Published online February 5, 2020
- DOI: https://doi.org/10.4132/jptm.2019.12.03
- 7,206 View
- 201 Download
- 3 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
The cytologic diagnosis of poorly differentiated thyroid carcinoma (PDTC) is difficult because it lacks salient cytologic findings and shares cytologic features with more commonly encountered neoplasms. Due to diverse cytologic findings and paucicellularity of PDTC, standardization of cytologic diagnostic criteria is limited. The purpose of this study is to investigate and recognize diverse thyroid findings of fine needle aspiration (FNA) cytology and frozen smear cytology in diagnosis of this rare but aggressive carcinoma.
Methods
The present study included six cases of FNA cytology and frozen smears of histologically diagnosed PDTCs.
Results
PDTC showed cytologic overlap with well-differentiated thyroid carcinomas (WDTCs). Five of six cases showed dedifferentiation arising from well differentiated thyroid carcinomas. Only one de novo PDTC showed highly cellular smears composed of discohesive small cells, high nuclear/cytoplasmic (N/C) ratio, prominent micronucleoli, and irregular nuclei. Retrospectively reviewed, these findings are highly suspicious for PDTC. Cytologic findings of nuclear atypia, pleomorphism, and irregularity were frequently found, whereas scattered small cells were seen only in the de novo case.
Conclusions
Heterogeneous cytologic findings of PDTCs are shared with those of WDTCs and contribute to difficult preoperative cytologic diagnoses. Most PDTCs show dedifferentiation from WDTCs. Albeit rare, de novo PDTC should be considered with cytology showing discohesive small cells with high N/C ratio. This will enable precise diagnosis and prompt treatment of this aggressive malignancy -
Citations
Citations to this article as recorded by- Non-papillary thyroid carcinoma diagnoses in The Bethesda System for Reporting Thyroid Cytopathology categories V and VI: An institutional experience
Myunghee Kang, Na Rae Kim, Jae Yeon Seok
Annals of Diagnostic Pathology.2024; 71: 152263. CrossRef - Cytologic features of differentiated high‐grade thyroid carcinoma: A multi‐institutional study of 40 cases
Vanda F. Torous, Tikamporn Jitpasutham, Zubair Baloch, Richard L. Cantley, Darcy A. Kerr, Xiaoying Liu, Zahra Maleki, Ross Merkin, Vania Nosé, Liron Pantanowitz, Isabella Tondi Resta, Esther D. Rossi, William C. Faquin
Cancer Cytopathology.2024; 132(8): 525. CrossRef - An Unexpected Finding of Poorly Differentiated Thyroid Carcinoma in a Toxic Thyroid Nodule
Kimberly Yuang, Huda Al-Bahadili, Alan Chang
JCEM Case Reports.2023;[Epub] CrossRef - Revisiting the cytomorphological features of poorly differentiated thyroid carcinoma: a comparative analysis with indeterminate thyroid fine-needle aspiration samples
Yazeed Alwelaie, Ali Howaidi, Mohammed Tashkandi, Ahmad Almotairi, Hisham Saied, Moammar Muzzaffar, Doaa Alghamdi
Journal of the American Society of Cytopathology.2023; 12(5): 331. CrossRef - Characterization of the genomic alterations in poorly differentiated thyroid cancer
Yeeun Lee, SeongRyeol Moon, Jae Yeon Seok, Joon-Hyop Lee, Seungyoon Nam, Yoo Seung Chung
Scientific Reports.2023;[Epub] CrossRef
- Non-papillary thyroid carcinoma diagnoses in The Bethesda System for Reporting Thyroid Cytopathology categories V and VI: An institutional experience
Case Studies
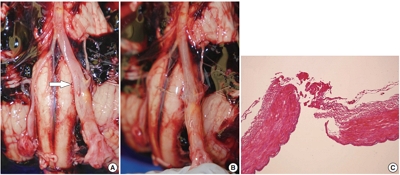
- Inconspicuous longitudinal tears of the intracranial vertebral artery in traumatic basal subarachnoid hemorrhage
- Seongho Kim
- J Pathol Transl Med. 2020;54(2):179-183. Published online November 8, 2019
- DOI: https://doi.org/10.4132/jptm.2019.10.15
- 8,747 View
- 197 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - Blunt force trauma to the head or neck region can cause traumatic basal subarachnoid hemorrhage (TBSAH), which can result in rapid loss of consciousness and death; however, detecting such a vascular injury is difficult. Posterior neck dissection was performed to investigate the bleeding focus in TBSAH cases 2018 and 2019. In all four cases, autopsies revealed a longitudinal tear in the midsection of the vertebral artery’s intracranial portion. The midportion of the intracranial vertebral artery appears to be most vulnerable to TBSAH. Interestingly, three of the cases showed only a vaguely visible longitudinal fissure in the artery without a grossly apparent tear; rupture was confirmed by microscopic examination. Longitudinal fissures of the intracranial vertebral artery, which are difficult to identify without detailed examination, may be overlooked in some cases of TBSAH. Thus, careful gross and microscopic examination of the vertebral artery is recommended in cases of TBSAH.
-
Citations
Citations to this article as recorded by- Effect of Ginseng Extract Ginsenoside Rg1 on Mice with Intracerebral Injury
Zixin Zhuang, Jinman Chen, Hao Xu, Yongjun Wang, Qianqian Liang
Chinese Medicine and Culture.2023;[Epub] CrossRef
- Effect of Ginseng Extract Ginsenoside Rg1 on Mice with Intracerebral Injury
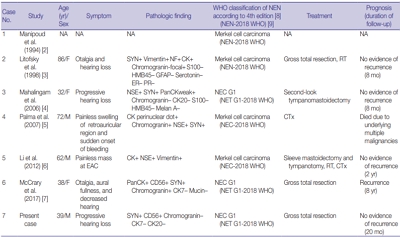
- Primary carcinoid tumor in the external auditory canal
- Dong Hae Chung, Gyu Cheol Han, Na Rae Kim
- J Pathol Transl Med. 2020;54(2):184-187. Published online November 13, 2019
- DOI: https://doi.org/10.4132/jptm.2019.11.07
- 7,788 View
- 166 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF - A 39-year-old man visited the department of otolaryngology due to an ongoing hearing disturbance that had lasted for 1 year. Temporal bone computed tomography revealed soft tissue density nearly obliterating the left external auditory canal (EAC). The mass was composed of sheets of round tumor cells containing moderate amounts of fine granular cytoplasm and salt and pepper chromatin. Neither mitosis nor necrosis was found. The Ki-67 proliferation index was less than 2%. Cells were positive for CD56 and synaptophysin but negative for chromogranin, cytokeratin (CK) 20, and CK7. Based on these findings, the tumor was diagnosed as a carcinoid tumor, well differentiated neuroendocrine carcinoma, grade 1 (G1) according to current World Health Organization (WHO) classification of head and neck tumors; and a neuroendocrine tumor, G1 according to neuroendocrine neoplasm (NEN)-2018 WHO standard classification. He remained free of local recurrence and metastasis after 20 months of follow up. To date, only six cases of primary NENs in the EAC have been reported. Metastatic tumor should be included in the differential diagnoses. Because of its rarity, the prognosis and treatment have not yet been clarified.
-
Citations
Citations to this article as recorded by- First Report on a Rare Poorly Differentiated Neuroendocrine Tumour of the External Auditory Canal Involving Pinna
Akash Varshney, Amit Kumar Tyagi, Prashant Durgapal, Kajal Mahto, Akhilesh Chandra Yadav, Ankita Semwal
Indian Journal of Otolaryngology and Head & Neck Surgery.2025; 77(4): 1922. CrossRef - Incidental finding of a neuroendocrine neoplasm in a suspected ear canal exostosis
Alexander Wieck Fjaeldstad, Gerda Elisabeth Villadsen, Gitte Dam, Stephen Jacques Hamilton-Dutoit, Thomas Winther Frederiksen
Otolaryngology Case Reports.2022; 22: 100394. CrossRef - 68Ga-DOTATATE Uptake in Well-Differentiated Neuroendocrine Tumor of the External Auditory Canal
Özge Erol Fenercioğlu, Ediz Beyhan, Rahime Şahin, Mehmet Can Baloğlu, Tevfik Fikret Çermik
Clinical Nuclear Medicine.2022; 47(8): e552. CrossRef
- First Report on a Rare Poorly Differentiated Neuroendocrine Tumour of the External Auditory Canal Involving Pinna
Brief Case Reports
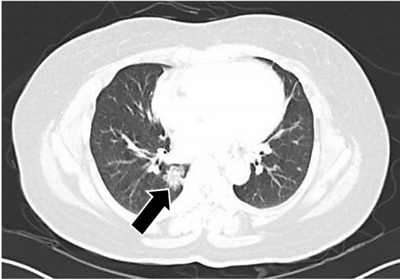
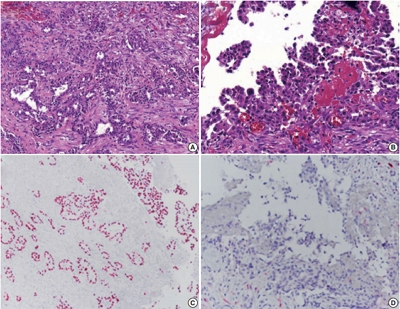
- Tumor-to-tumor metastasis: metastatic invasive lobular carcinoma of the breast within adenocarcinoma of the lung
- Myoung Jae Kang, Ae Ri An, Myoung Ja Chung, Kyoung Min Kim
- J Pathol Transl Med. 2020;54(2):188-191. Published online September 16, 2019
- DOI: https://doi.org/10.4132/jptm.2019.09.07
- 5,355 View
- 159 Download
- 7 Web of Science
- 7 Crossref
-
 PDF
PDF -
Citations
Citations to this article as recorded by- Tumor-in-Tumor-Metastase: Ein seltenes Phänomen
Felix Elsner, Katharina Keller, Florian Fuchs, Michael Uder, Arndt Hartmann
Die Pathologie.2025; 46(3): 139. CrossRef - Unraveling Tumor-to-Tumor Metastasis: Insights into Pathogenesis, Diagnostic Challenges, and Treatment Modalities
Wennei Mei, Dongdong Zhang
Biologics: Targets and Therapy.2025; Volume 19: 43. CrossRef - Case Report: Tumor-to-tumor metastasis: a rare case of prostate adenocarcinoma metastasis to lung squamous cell carcinoma in a patient with multiple primary malignancies
Baoxiang Pei, Jikuan Liu, Zhiliang Hu, Fen Pan
Frontiers in Oncology.2025;[Epub] CrossRef - Tumor-to-Tumor Metastases Involving Clear Cell Renal Cell Carcinomas: A Diagnostic Challenge for Pathologists Needing Clinical Correlation
Claudia Manini, Claudia Provenza, Leire Andrés, Igone Imaz, Rosa Guarch, Raffaelle Nunziata, José I. López
Clinics and Practice.2023; 13(1): 288. CrossRef - Metástasis tumor a tumor en pulmón: reporte de tres casos y revisión de la literatura
Paula Cristina Castro Quiroga, Blanca Viviana Fajardo Idrobo, Diana Marcela Caicedo Ruiz, Julieth Alexandra Franco Mira, Carlos Andrés Carvajal Fierro, Alfredo Ernesto Romero Rojas, Rafael Santiago Parra Medina
Revista Colombiana de Cancerología.2023; 27(1): 107. CrossRef - Lobular to Lobule: Metastatic Breast Carcinoma to Olfactory Neuroblastoma
Kent M. Swimley, Silvana Di Palma, Lester D. R. Thompson
Head and Neck Pathology.2021; 15(2): 642. CrossRef - A case of colorectal cancer with intratumoral metastasis to primary lung cancer
Yasushi Cho, Mitsuhito Kaji, Shunsuke Nomura, Yusuke Motohashi, Masaaki Sato, Motoya Takeuchi
The Journal of the Japanese Association for Chest Surgery.2021; 35(5): 576. CrossRef
- Tumor-in-Tumor-Metastase: Ein seltenes Phänomen
- Pseudomesotheliomatous carcinoma of the lung in the parietal pleura
- Ae Ri An, Kyoung Min Kim, Jong Hun Kim, Gong Yong Jin, Young Hoon Choe, Myoung Ja Chung
- J Pathol Transl Med. 2020;54(2):192-195. Published online January 29, 2020
- DOI: https://doi.org/10.4132/jptm.2019.11.14
- 5,995 View
- 145 Download
- 2 Web of Science
- 4 Crossref
-
 PDF
PDF -
Citations
Citations to this article as recorded by- Pseudomesotheliomatous lung cancer mimicking malignant pleural mesothelioma: A case report
Supakorn Chansaengpetch, Ruchira Ruangchira-urai, Nisa Muangman, Rathachai Kaewlai, Trongtum Tongdee, Teerapat Singwicha, Narongpon Dumavibhat
The ASEAN Journal of Radiology.2025; 26(1): 24. CrossRef - Pseudomesotheliomatous Carcinoma of the Lung with Morphological Characteristics of Signet Ring Cell Carcinoma: An Autopsy Case Report
Tetsu Hirakawa, Takuya Tanimoto, Yui Hattori, Ryo Katsura, Shinya Miyake, Suguru Fujita, Sayaka Ueno, Ken Masuda, Takashi Nishisaka, Nobuhisa Ishikawa
Internal Medicine.2024; 63(7): 979. CrossRef - Intrapulmonary Biphasic Mesothelioma Misdiagnosed as Adenocarcinoma: Case Report and a Potential Diagnostic Pitfall
Wenfeng Xu, XingYan Zhu, Hao Tang, Qijian Ying, Yujuan Xu, Deyu Guo
OncoTargets and Therapy.2024; Volume 17: 925. CrossRef -
A RARE CASE OF UNCLASSIFIED CARCINOMA OF THE LUNG: DIAGNOSTIC CHALLENGES
B.M. Fylenko, N.V. Royko, I.I. Starchenko, O.V. Starchenko, O.Y. Horodynska, S.A. Proskurnia
Azerbaijan Medical Journal.2024; (4): 182. CrossRef
- Pseudomesotheliomatous lung cancer mimicking malignant pleural mesothelioma: A case report
Corrigendum
- Correction of acknowledgments: PD-L1 testing in non-small cell lung cancer: past, present, and future
- Hyojin Kim, Jin-Haeng Chung
- J Pathol Transl Med. 2020;54(2):196-196. Published online March 10, 2020
- DOI: https://doi.org/10.4132/jptm.2020.02.17
- Corrects: J Pathol Transl Med 2019;53(4):199
- 4,491 View
- 82 Download
- 1 Crossref
-
 PDF
PDF -
Citations
Citations to this article as recorded by- ORC6 acts as an effective prognostic predictor for non‑small cell lung cancer and is closely associated with tumor progression
Letian Chen, Dongdong Zhang, Yujuan Chen, Huilan Zhu, Zhipeng Liu, Zhiping Yu, Junping Xie
Oncology Letters.2024;[Epub] CrossRef
- ORC6 acts as an effective prognostic predictor for non‑small cell lung cancer and is closely associated with tumor progression

 E-submission
E-submission






 First
First Prev
Prev



