Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 54(4); 2020 > Article
-
Review
Evolving pathologic concepts of serrated lesions of the colorectum -
Jung Ho Kim1,2
 , Gyeong Hoon Kang,1,2
, Gyeong Hoon Kang,1,2
-
Journal of Pathology and Translational Medicine 2020;54(4):276-289.
DOI: https://doi.org/10.4132/jptm.2020.04.15
Published online: June 26, 2020
1Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
2Laboratory of Epigenetics, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- Corresponding Author: Gyeong Hoon Kang, MD, PhD, Department of Pathology, Seoul National University College of Medicine, 103 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-740-8263, Fax: +82-2-765-5600, E-mail: ghkang@snu.ac.kr
© 2020 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Clinical and endoscopic characteristics of colorectal traditional serrated adenomas with dysplasia/adenocarcinoma in a Korean population
Ki-Hyun Kim, Eun Myung, Hyung Hoon Oh, Chan-Muk Im, Young-Eun Seo, Je-Seong Kim, Chae-June Lim, Ga-Ram You, Sung-Bum Cho, Wan-Sik Lee, Myung-Giun Noh, Kyung-Hwa Lee, Young-Eun Joo
World Journal of Gastrointestinal Oncology.2025;[Epub] CrossRef - Impact of AI-aided colonoscopy in clinical practice: a prospective randomised controlled trial
Johanna Schöler, Marko Alavanja, Thomas de Lange, Shunsuke Yamamoto, Per Hedenström, Jonas Varkey
BMJ Open Gastroenterology.2024; 11(1): e001247. CrossRef - The histologic features, molecular features, detection and management of serrated polyps: a review
Jin-Dong Wang, Guo-Shuai Xu, Xin-Long Hu, Wen-Qiang Li, Nan Yao, Fu-Zhou Han, Yin Zhang, Jun Qu
Frontiers in Oncology.2024;[Epub] CrossRef - Serrated polyps <10 mm cannot reliably be characterized by i-Scan without magnification at routine colonoscopy
Sabrina G.G. TESTONI, Chiara NOTARISTEFANO, Giuliano F. BONURA, Maria NAPOLITANO, Dario ESPOSITO, Edi VIALE, Lorella FANTI, Francesco AZZOLINI, Giulia M. CAVESTRO, PierAlberto TESTONI
Minerva Gastroenterology.2024;[Epub] CrossRef - Interobserver variability in the histopathological classification and grading of dysplasia in elevated colon lesions in the city of Lima
Guido Gallegos-Serruto, Aldo Gutiérrez, César Chian García, Isthvan Torres Perez
Revista de Gastroenterología del Perú.2024; 44(3): 239. CrossRef - Comparison of adenoma detection rate and proximal serrated polyp detection rate and their effect on post-colonoscopy colorectal cancer mortality in screening patients
Jasmin Zessner-Spitzenberg, Elisabeth Waldmann, Lena Jiricka, Lisa-Maria Rockenbauer, Anna Hinterberger, Jeremy Cook, Arno Asaturi, Aleksandra Szymanska, Barbara Majcher, Michael Trauner, Monika Ferlitsch
Endoscopy.2023; 55(05): 434. CrossRef - The yield of dysplasia and serrated lesions in a single-centre tertiary inflammatory bowel disease cohort
Fiona Yeaman, Lena Thin
Therapeutic Advances in Gastroenterology.2023;[Epub] CrossRef -
The BEETS (JACCRO CC-18) Trial: An Observational and Translational Study of
BRAF
-Mutated Metastatic Colorectal Cancer
Chiaki Inagaki, Ryo Matoba, Satoshi Yuki, Manabu Shiozawa, Akihito Tsuji, Eisuke Inoue, Kei Muro, Wataru Ichikawa, Masashi Fujii, Yu Sunakawa
Future Oncology.2023; 19(17): 1165. CrossRef - A retrospective analysis of the histology of resected polyps and colonoscopy quality parameters in Belgium
E Macken, S Van Dongen, G Van Hal
Acta Gastro Enterologica Belgica.2023; 86(2): 277. CrossRef - Prognostic Biomarkers of Cell Proliferation in Colorectal Cancer (CRC): From Immunohistochemistry to Molecular Biology Techniques
Aldona Kasprzak
Cancers.2023; 15(18): 4570. CrossRef - Assimilating Epigenetics and Transcriptomics for the Identification
of Prognostic Novel Biomarkers and Imminent Targets in
Colorectal Carcinoma with Therapeutic Potential
Suman Kumar Ray, Sukhes Mukherjee
Current Molecular Medicine.2023; 23(8): 784. CrossRef - Multitarget Stool RNA Test for Colorectal Cancer Screening
Erica K. Barnell, Elizabeth M. Wurtzler, Julie La Rocca, Thomas Fitzgerald, Jessica Petrone, Yansheng Hao, Yiming Kang, Faith L. Holmes, David A. Lieberman
JAMA.2023; 330(18): 1760. CrossRef - Microbiome in Colonic Carcinogenesis
Jun Sun, Yinglin Xia
Comprehensive Physiology.2023; 13(3): 4685. CrossRef - Impact of comprehensive optical diagnosis training using Workgroup serrAted polypS and Polyposis classification on detection of adenoma and sessile serrated lesion
Jooyoung Lee, Jung Ho Bae, Su Jin Chung, Hae Yeon Kang, Seung Joo Kang, Min‐Sun Kwak, Ji Yeon Seo, Ji Hyun Song, Sun Young Yang, Jong In Yang, Seon Hee Lim, Jeong Yoon Yim, Joo Hyun Lim, Goh Eun Chung, Eun Hyo Jin, Ji Min Choi, Yoo Min Han, Joo Sung Kim
Digestive Endoscopy.2022; 34(1): 180. CrossRef - Clinicopathological and molecular analyses of hyperplastic lesions including microvesicular variant and goblet cell rich variant hyperplastic polyps and hyperplastic nodules—Hyperplastic nodule is an independent histological entity
Noriyuki Uesugi, Yoichi Ajioka, Tomio Arai, Yoshihito Tanaka, Tamotsu Sugai
Pathology International.2022; 72(2): 128. CrossRef - Comprehensive clinicopathologic, molecular, and immunologic characterization of colorectal carcinomas with loss of three intestinal markers, CDX2, SATB2, and KRT20
Ji Ae Lee, Mi-Kyoung Seo, Seung-Yeon Yoo, Nam-Yun Cho, Yoonjin Kwak, Kyoungbun Lee, Jung Ho Kim, Gyeong Hoon Kang
Virchows Archiv.2022; 480(3): 543. CrossRef - Serrated Colorectal Lesions: An Up-to-Date Review from Histological Pattern to Molecular Pathogenesis
Martino Mezzapesa, Giuseppe Losurdo, Francesca Celiberto, Salvatore Rizzi, Antonio d’Amati, Domenico Piscitelli, Enzo Ierardi, Alfredo Di Leo
International Journal of Molecular Sciences.2022; 23(8): 4461. CrossRef - Arterial stiffness is associated with high-risk colorectal adenomas and serrated lesions: A cross-sectional study in a Taiwanese population
Hung-Yu Chen, Wen-Huang Lee, Hung-Lung Hsu, Yu-Tsung Chou, Fei-Lin Su, I-Hsuan Wu, Ting-Hsing Chao
Journal of Cardiology.2022; 80(2): 139. CrossRef - Morphological and molecular characterization of colorectal sessile serrated lesions with dysplasia
Filippo Cappello, Valentina Angerilli, Luca Dal Santo, Giada Munari, Marianna Sabbadin, Marcello Lo Mele, Gianmaria Pennelli, Claudio Luchini, Paola Parente, Stefano Lazzi, Matteo Fassan
Pathology - Research and Practice.2022; 240: 154214. CrossRef - Serrated polyposis: an overview
Jonathan Fawkes
Gastrointestinal Nursing.2022; 20(9): 24. CrossRef - Sessile serrated lesion presenting as large pedunculated polyp in the rectum: A case report
Shin Ju Oh, Jung-Wook Kim, Chi Hyuk Oh
Medicine.2022; 101(51): e32287. CrossRef - WHICH LESIONS ARE AT HIGHER RISK OF DEVELOPING COLORECTAL CARCINOMAS: SUPERFICIALLY ELEVATED SERRATED LESIONS OR DEPRESSED LESIONS?
Artur Adolfo PARADA, Filadelfio Euclydes VENCO, Miguel Reynaldo VARCA-NETO, Roberto EL IBRAHIM, Paula Bechara POLETTI, Helcio Pedrosa BRITO, Heloisa de Fátima SARE, Osvaldo MALAFAIA
ABCD. Arquivos Brasileiros de Cirurgia Digestiva (São Paulo).2022;[Epub] CrossRef - WNT5a in Colorectal Cancer: Research Progress and Challenges
Guangshun Sun, Liangliang Wu, Guoqiang Sun, Xuesong Shi, Hongyong Cao, Weiwei Tang
Cancer Management and Research.2021; Volume 13: 2483. CrossRef - Endoscopic diagnosis for colorectal sessile serrated lesions
Toshihiro Nishizawa, Shuntaro Yoshida, Akira Toyoshima, Tomoharu Yamada, Yoshiki Sakaguchi, Taiga Irako, Hirotoshi Ebinuma, Takanori Kanai, Kazuhiko Koike, Osamu Toyoshima
World Journal of Gastroenterology.2021; 27(13): 1321. CrossRef - NTRK oncogenic fusions are exclusively associated with the serrated neoplasia pathway in the colorectum and begin to occur in sessile serrated lesions
Jung Ho Kim, Jeong Hoon Hong, Yoon‐La Choi, Ji Ae Lee, Mi‐kyoung Seo, Mi‐Sook Lee, Sung Bin An, Min Jung Sung, Nam‐Yun Cho, Sung‐Su Kim, Young Kee Shin, Sangwoo Kim, Gyeong Hoon Kang
The Journal of Pathology.2021; 255(4): 399. CrossRef - Differential pre-malignant programs and microenvironment chart distinct paths to malignancy in human colorectal polyps
Bob Chen, Cherie’ R. Scurrah, Eliot T. McKinley, Alan J. Simmons, Marisol A. Ramirez-Solano, Xiangzhu Zhu, Nicholas O. Markham, Cody N. Heiser, Paige N. Vega, Andrea Rolong, Hyeyon Kim, Quanhu Sheng, Julia L. Drewes, Yuan Zhou, Austin N. Southard-Smith, Y
Cell.2021; 184(26): 6262. CrossRef - Molecular Insights Into Colorectal Carcinoma
Domenika Ortiz Requena, Monica Garcia-Buitrago
Archives of Medical Research.2020; 51(8): 839. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
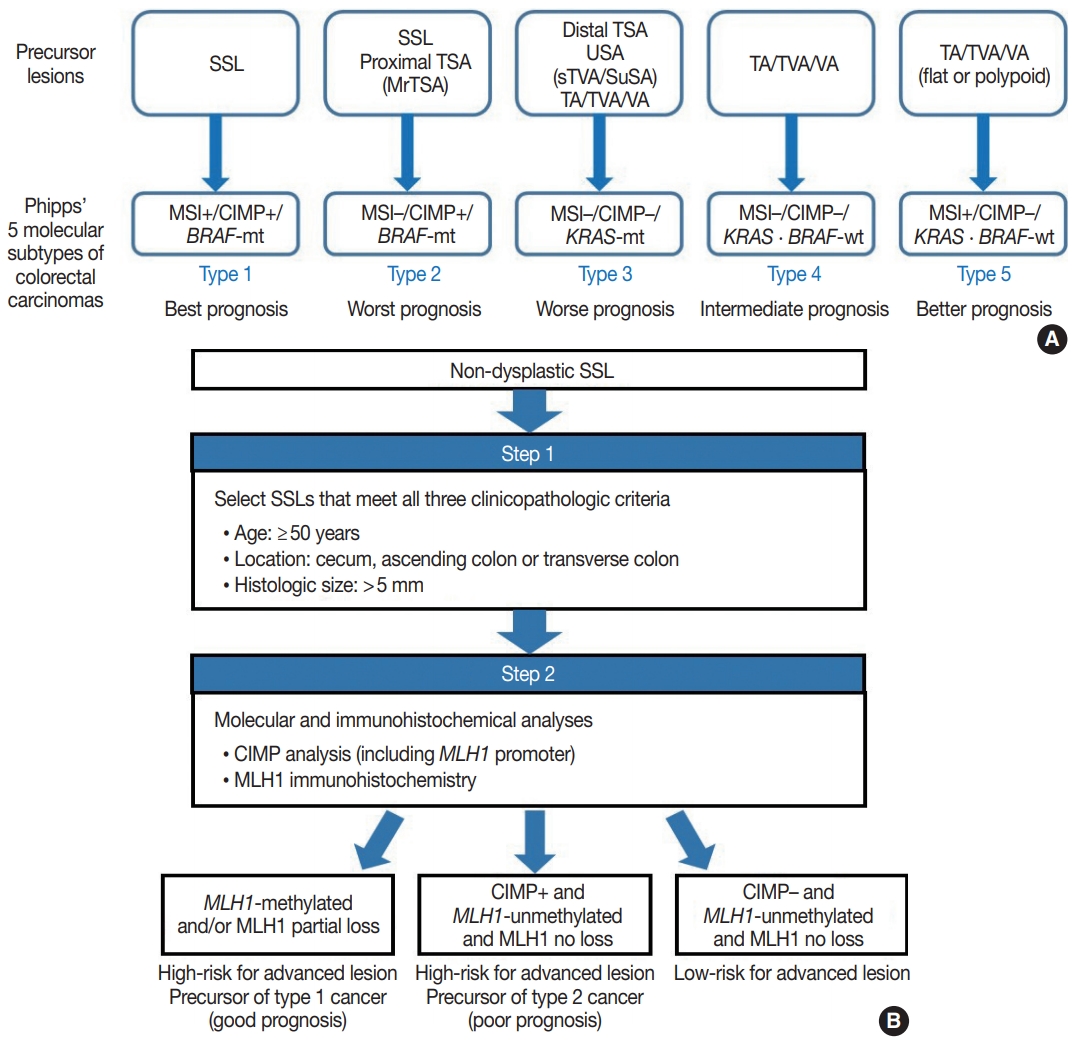
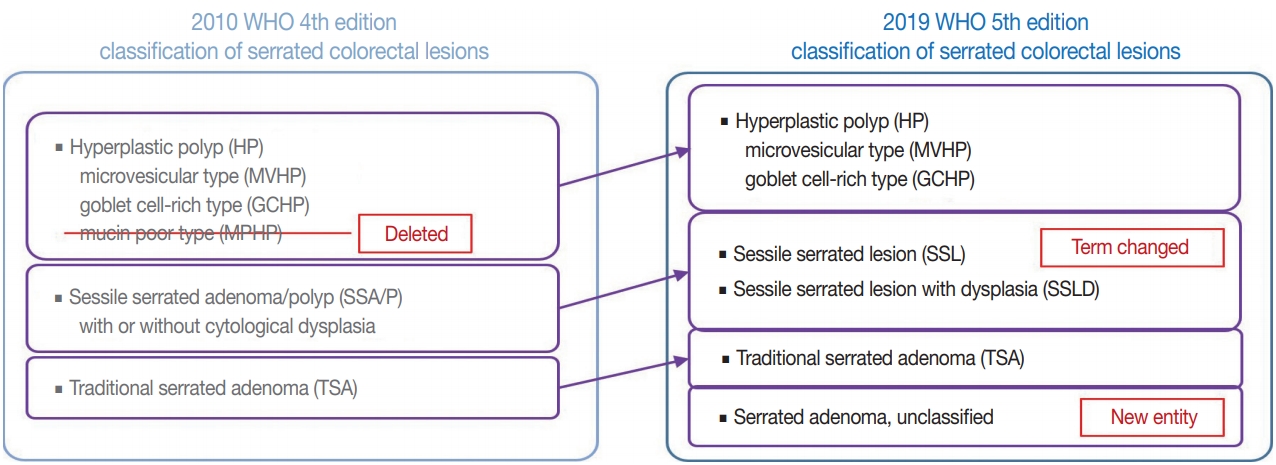
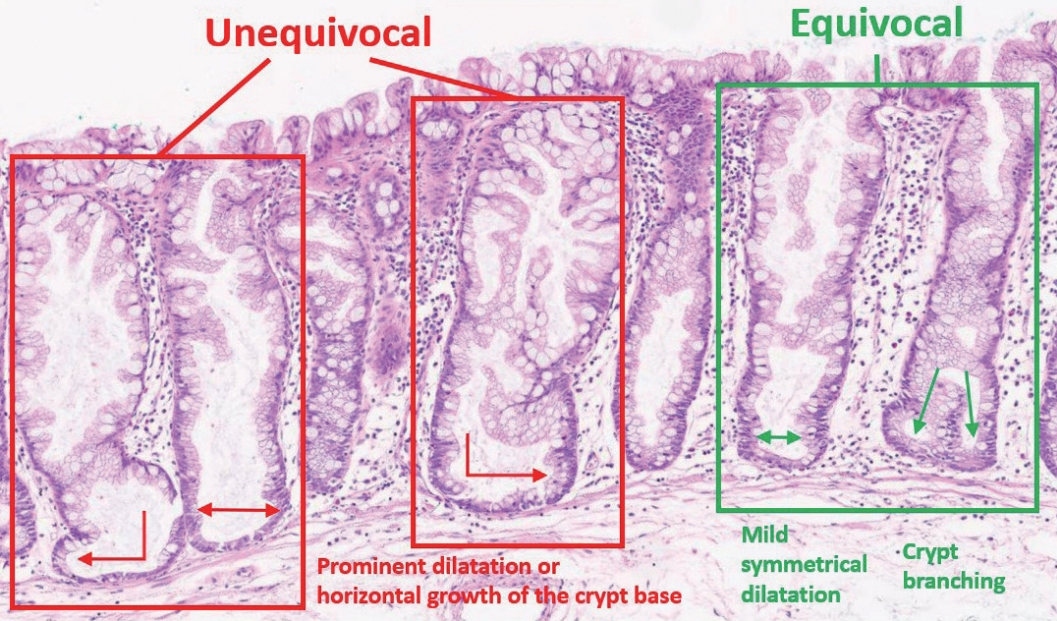
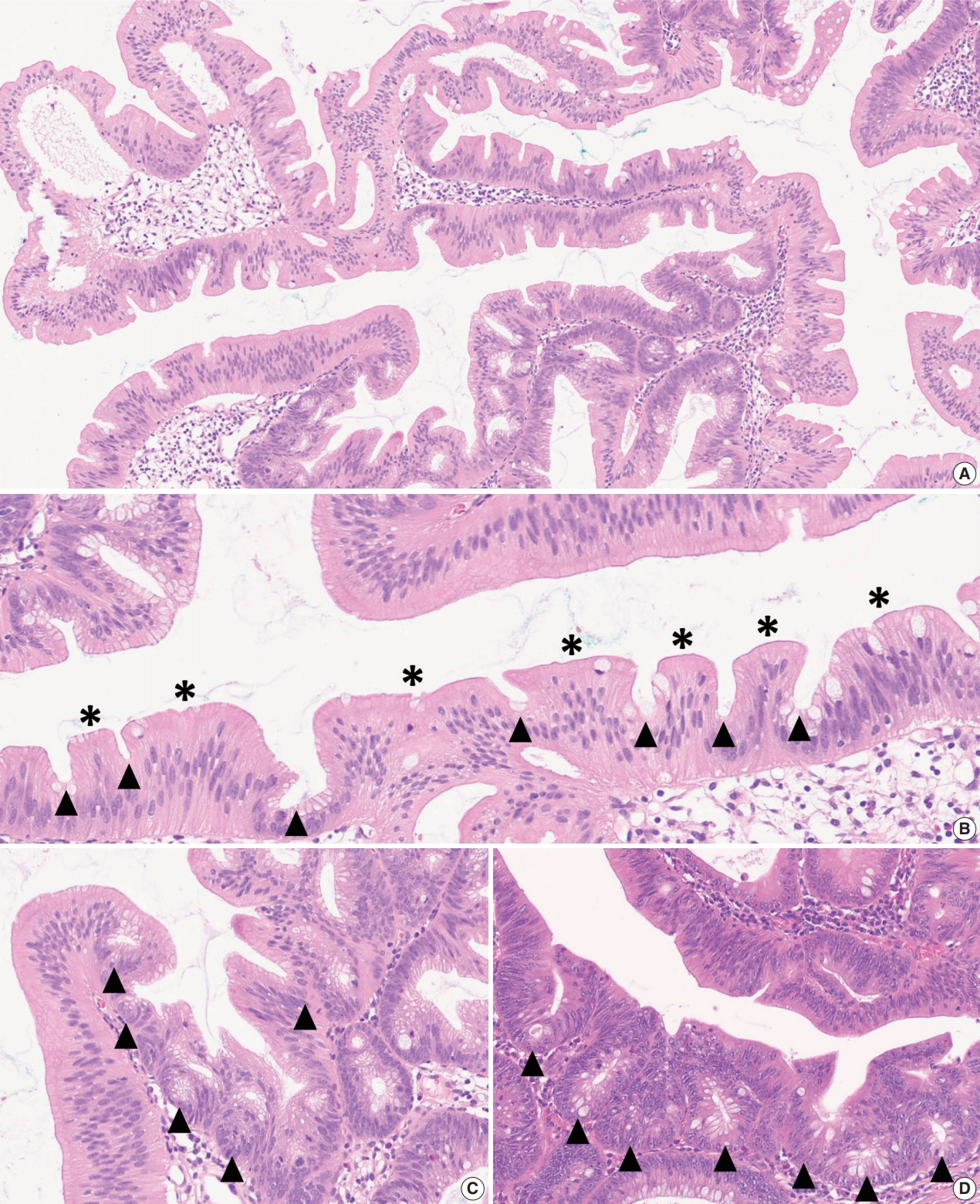
- Figure





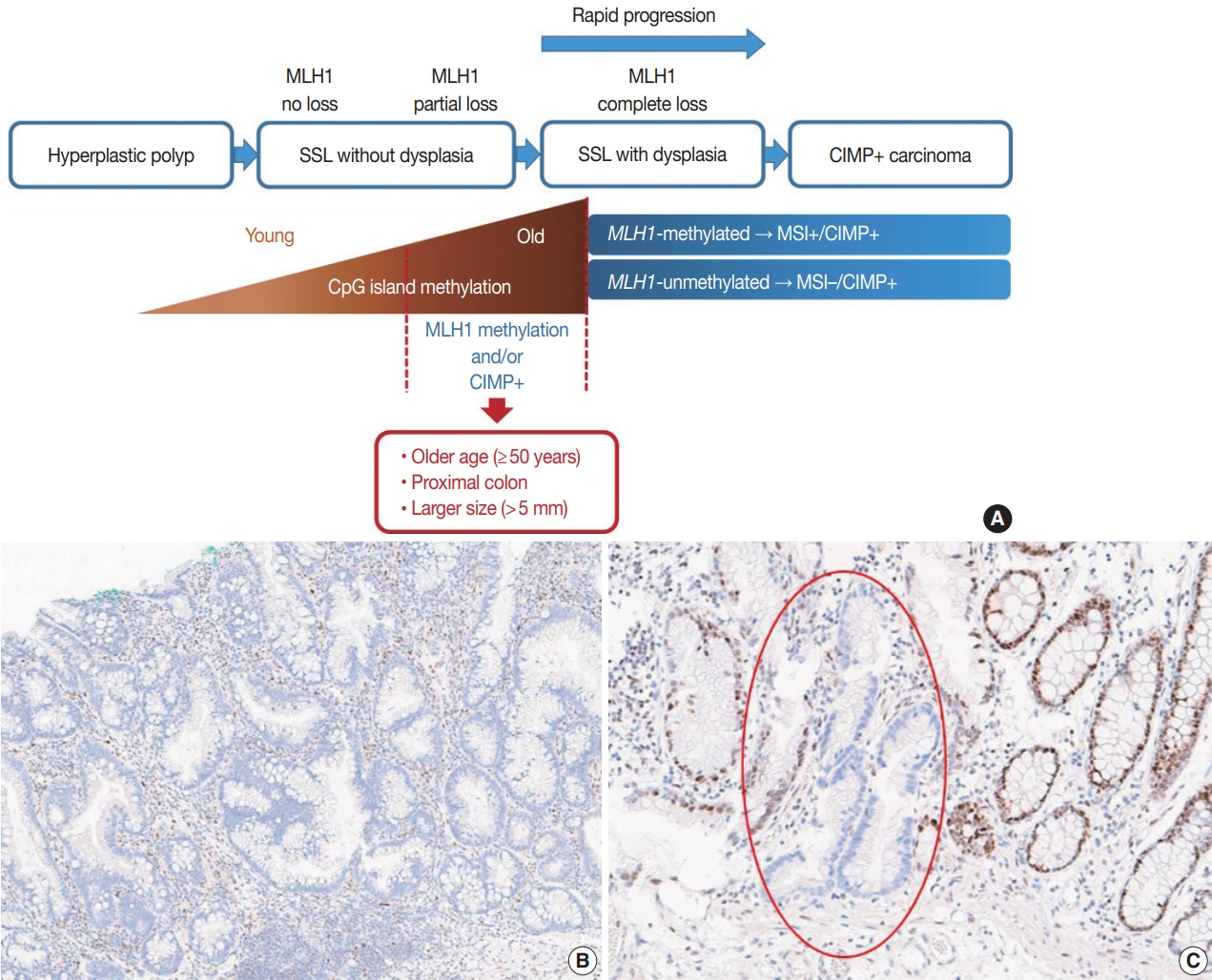
Fig. 1.
Fig. 2.
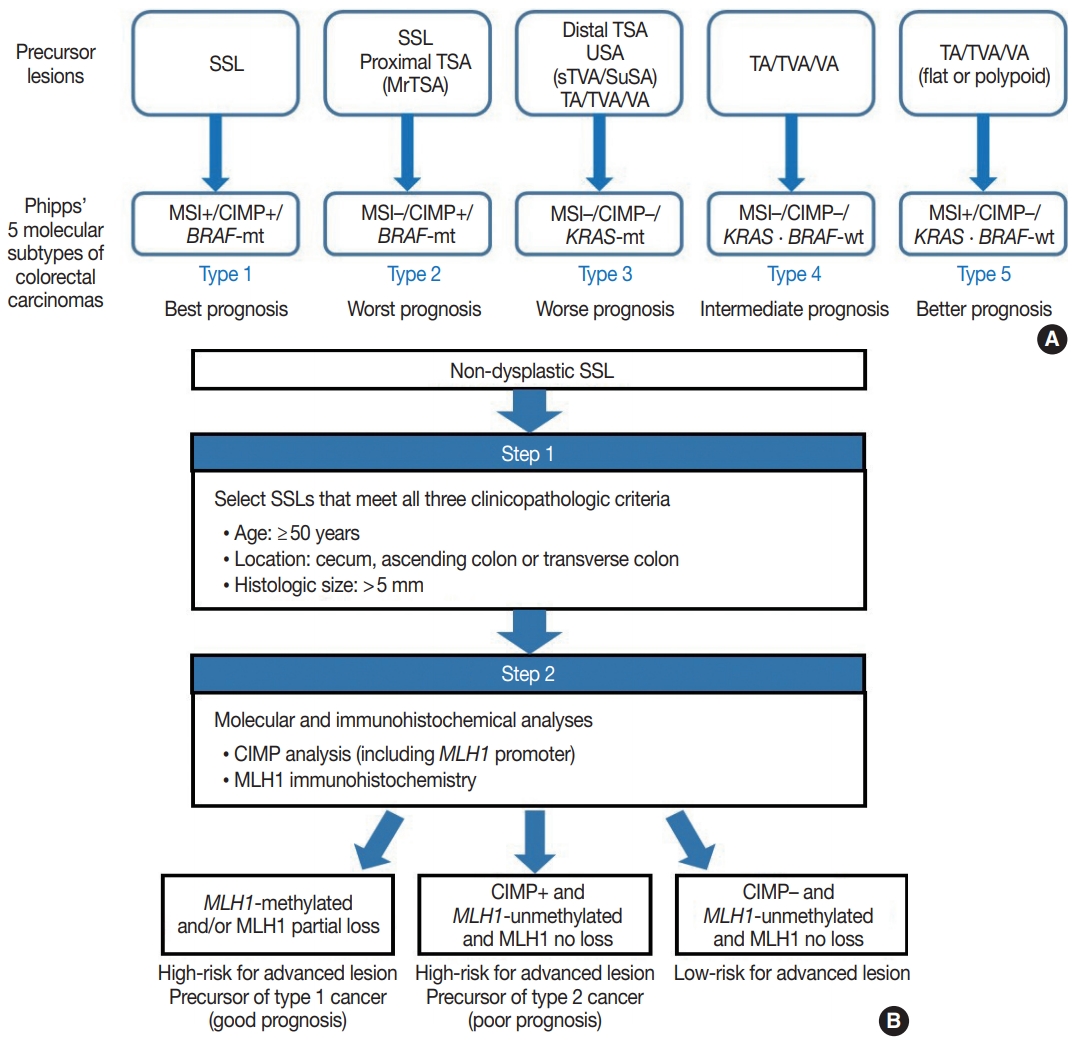
Fig. 3.
Fig. 4.
Fig. 5.
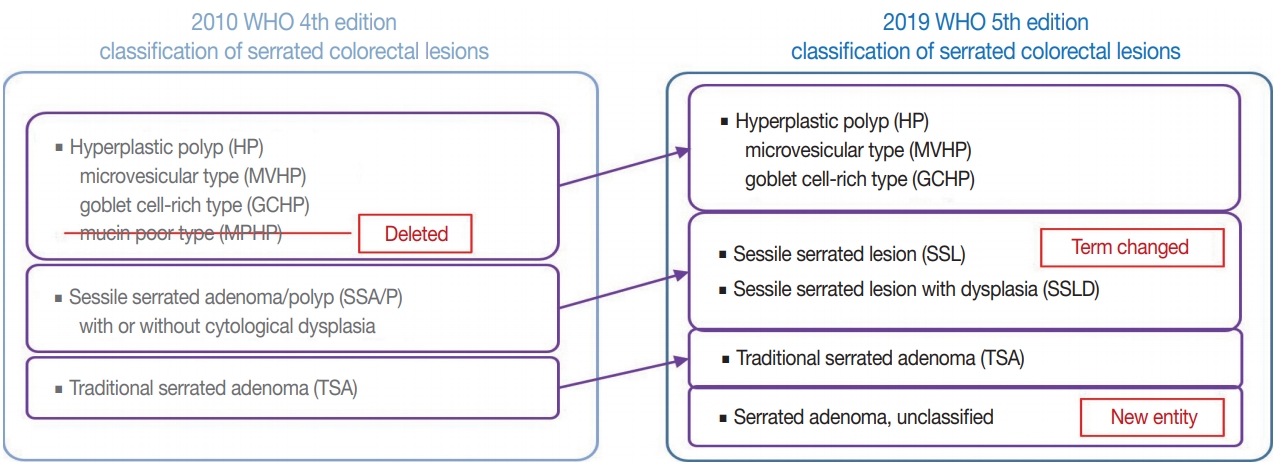
| Summary | ||
|---|---|---|
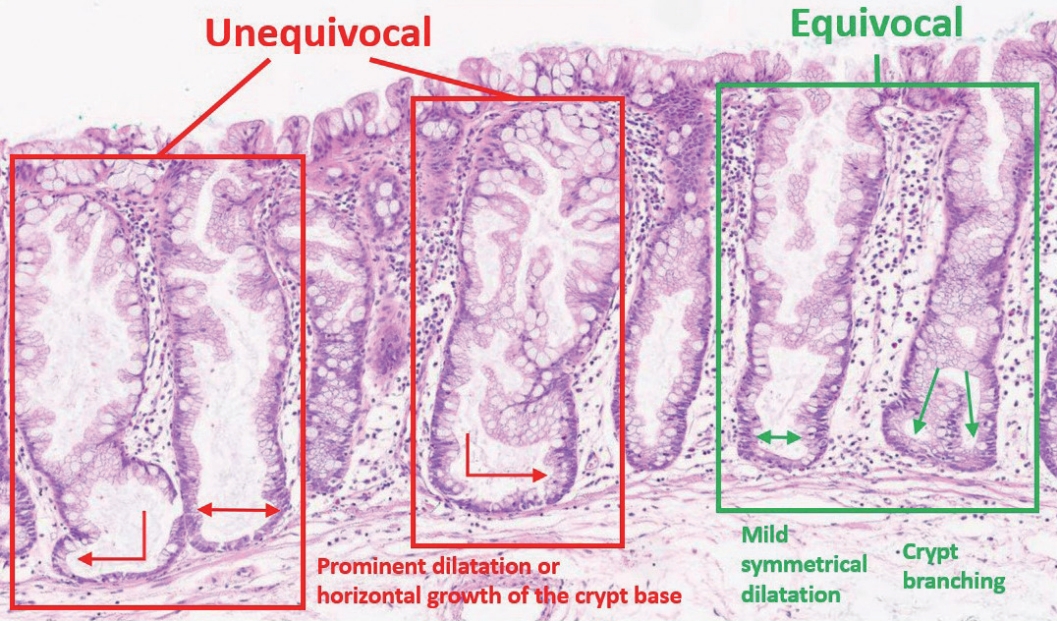
| Definition of an “architecturally distorted serrated crypt” that is typical in SSLs | A crypt showing at least one of the following histologic features: | |
| Horizontal growth along the muscularis mucosa (L-shaped or inverted T-shaped crypt) | ||
| Dilation of the crypt base (basal one-third of the crypt) | ||
| Serrations extending into the crypt base | ||
| Asymmetrical proliferation (shift of the proliferation zone from the base to the lateral side) | ||
| Diagnostic criteria of SSL | The presence of at least one unequivocal “architecturally distorted serrated crypt” (defined above) | |
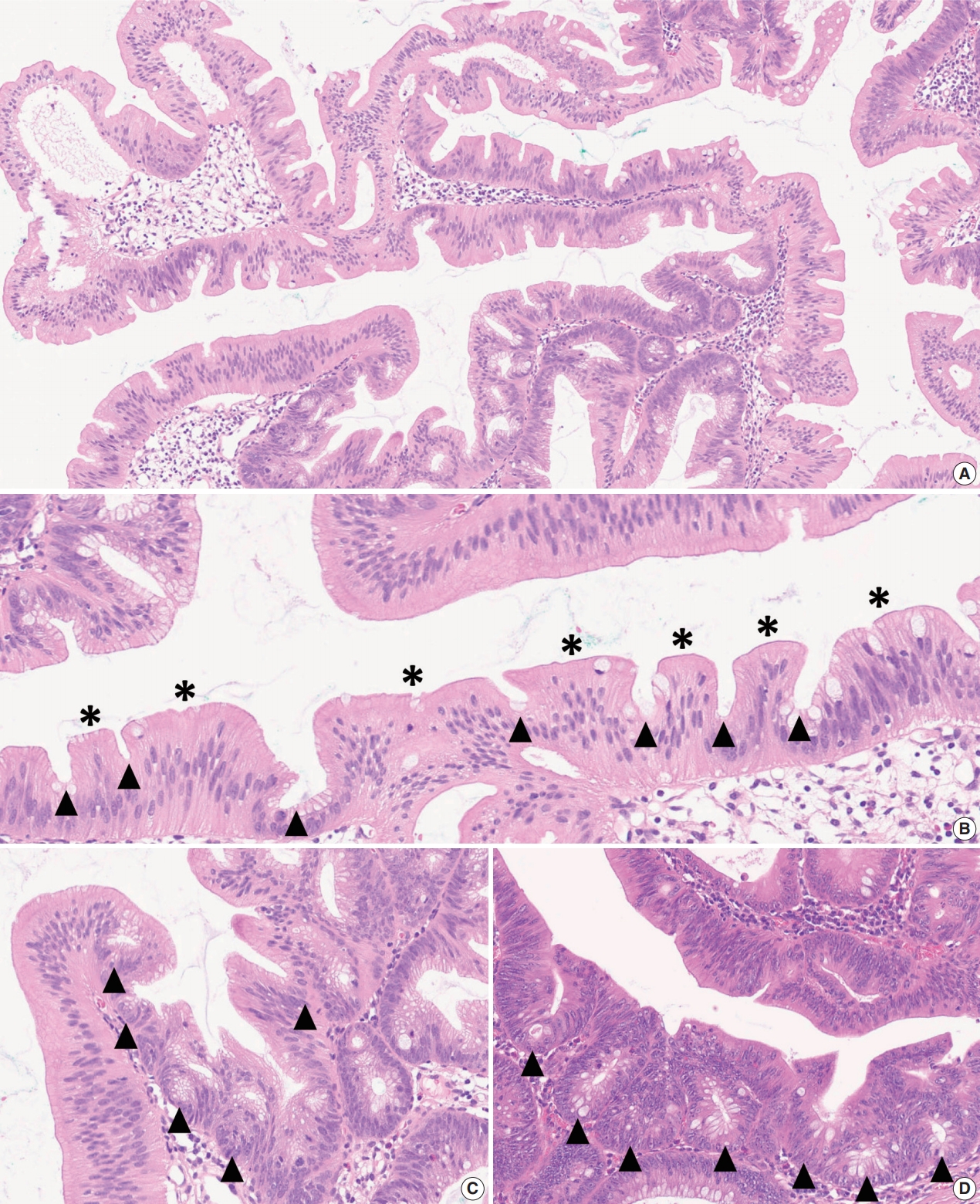
| SSLs in Western countries |
SSLs in Eastern countries |
p-value | |
|---|---|---|---|
| BRAF mutation | 932/1,028 (91) | 798/1,048 (76) | < .001 |
| KRAS mutation | 22/988 (2) | 65/1,053 (6) | < .001 |
WHO, World Health Organization; SSL, sessile serrated lesion.
Values are presented as number (%). Detailed frequency data from individual studies are listed in Supplementary Table S1. Western countries include United States, Canada, Australia, Germany, Austria, and Switzerland (total Eastern countries include South Korea, Japan, and China (total BRAF-tested samples n = 1,048; total KRAStested samples n = 1,053).

 E-submission
E-submission