Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 55(1); 2021 > Article
-
Review
DNA-protein biomarkers for immunotherapy in the era of precision oncology -
Binnari Kim1,2
 , So Young Kang1
, So Young Kang1 , Kyoung-Mee Kim1,3
, Kyoung-Mee Kim1,3
-
Journal of Pathology and Translational Medicine 2021;55(1):26-32.
DOI: https://doi.org/10.4132/jptm.2020.09.23
Published online: December 3, 2020
1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
2Department of Pathology, Ulsan University Hospital, Ulsan, Korea
3Center of Clinical Genomics, Samsung Medical Center, Seoul, Korea
- Corresponding Author: Kyoung-Mee Kim, MD, Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea, Tel: +82-2-3410-2807, Fax: +82-2-3410-6396, E-mail: kkmkys@skku.edu
© 2021 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- NCKAP1 as a prognostic and immunological biomarker: pan-cancer analysis and validation in renal clear cell carcinoma
Xiao Liang
American Journal of Translational Research.2024; 16(8): 4083. CrossRef - Biomarkers for Predicting Response to Personalized Immunotherapy in Gastric Cancer
Moonsik Kim, Ji Yun Jeong, An Na Seo
Diagnostics.2023; 13(17): 2782. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


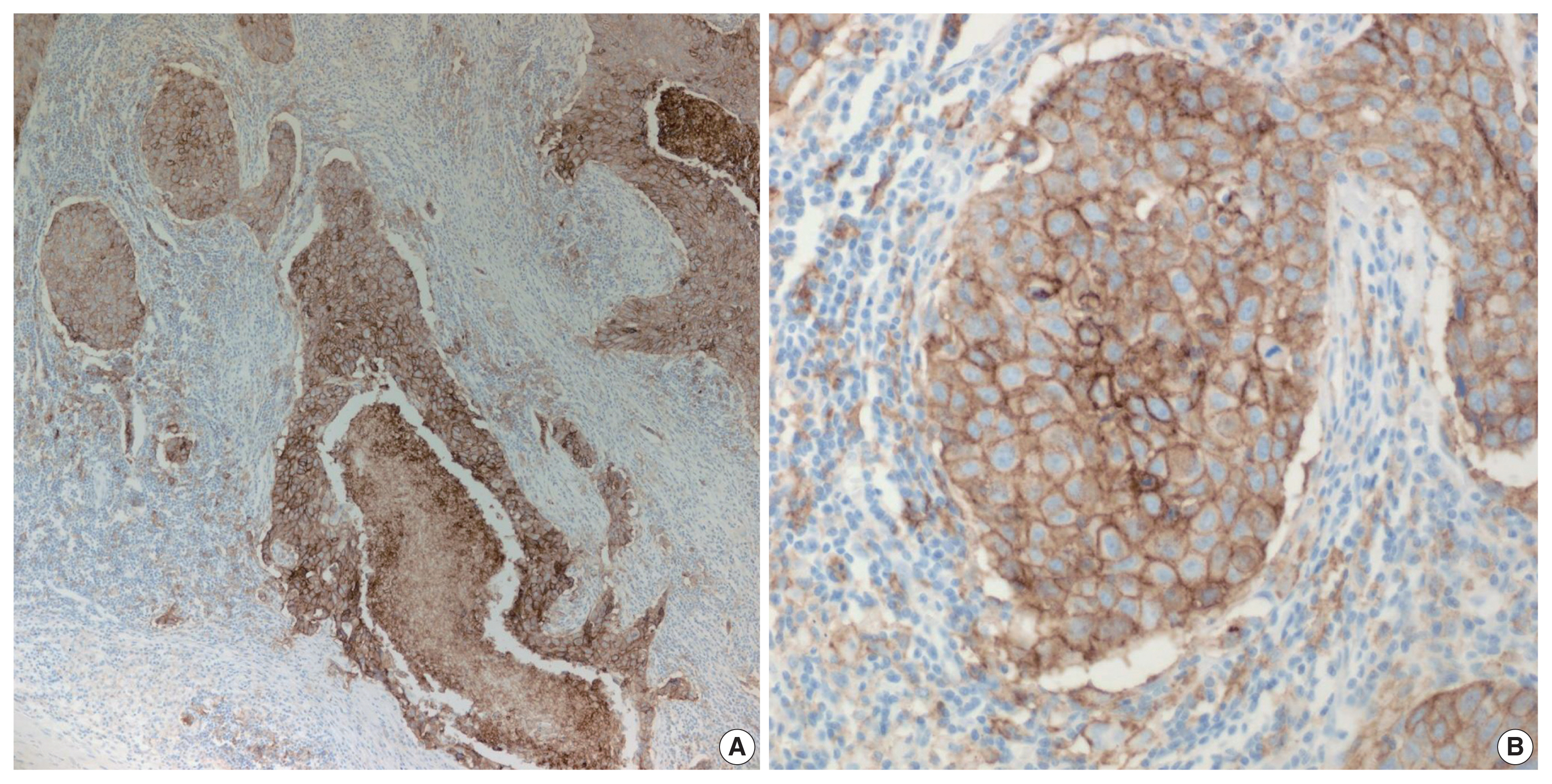
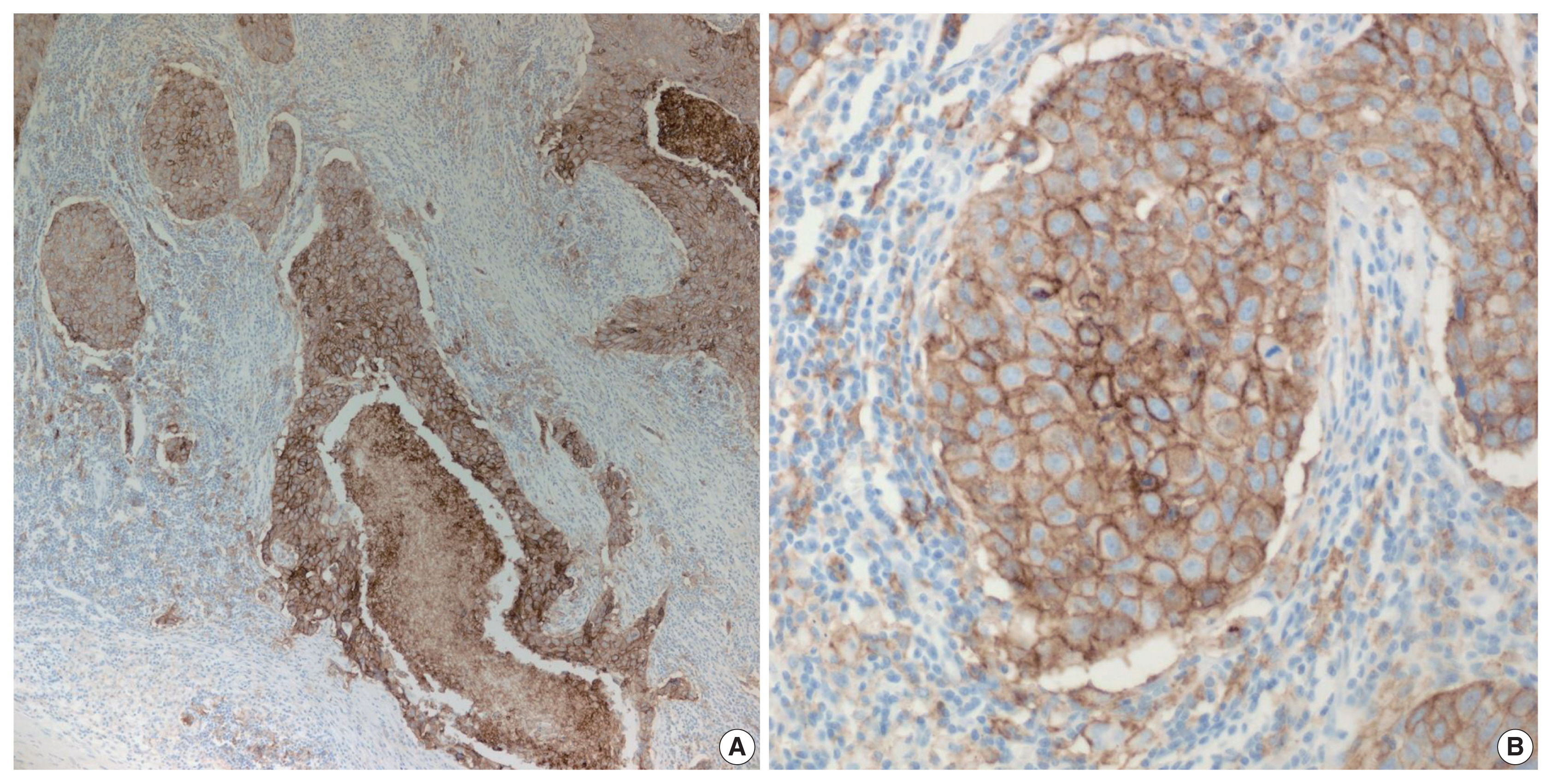
Fig. 1
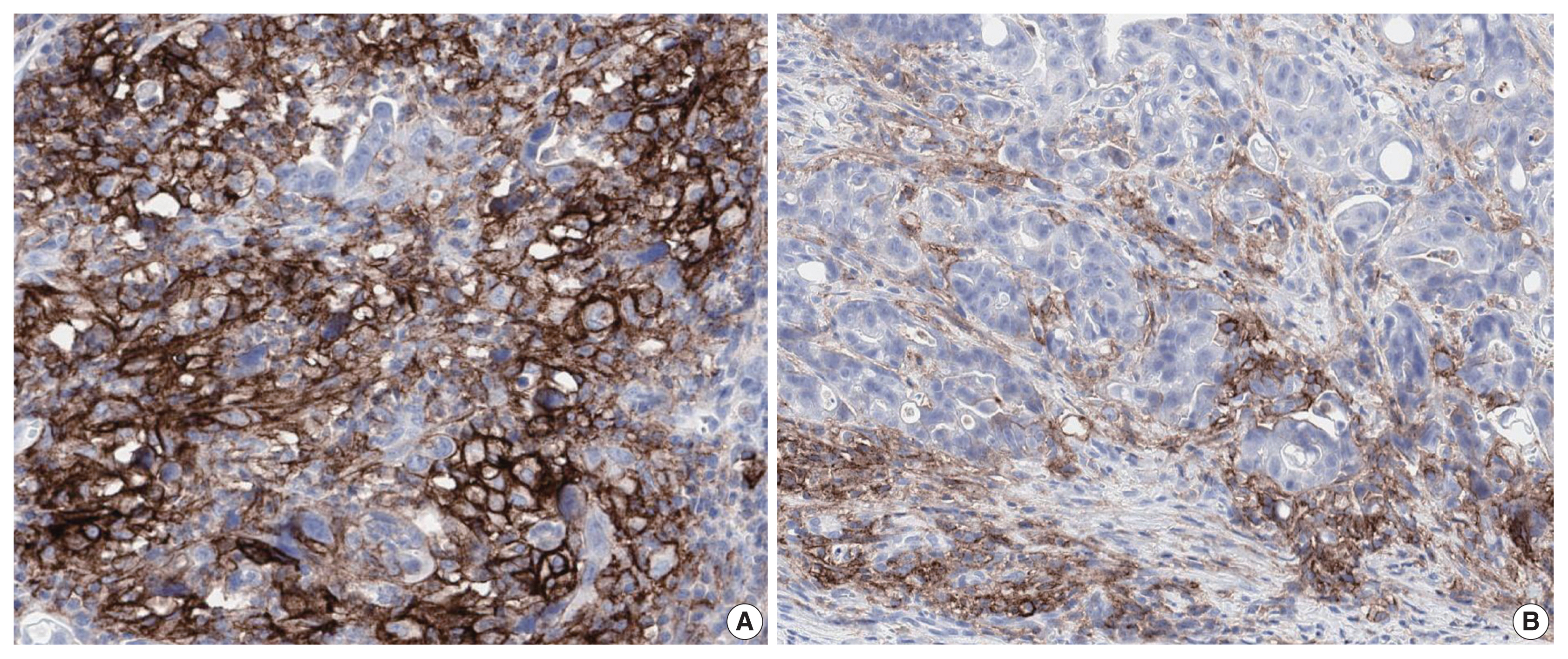
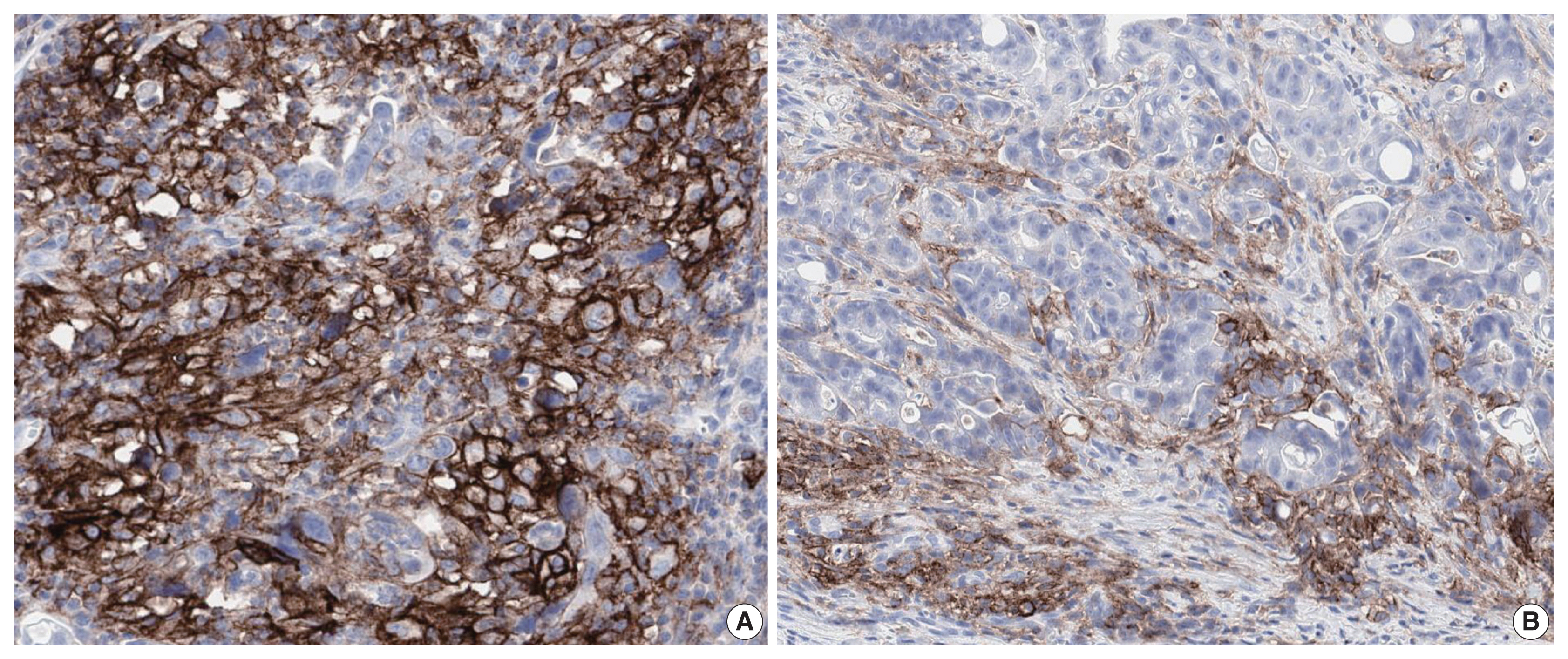
Fig. 2
| Cancer type | No. of patients | Methods | Name of panel | No. of genes | Cut-off (mt/Mb) | Cut-off (percentile) | ICI responses | Study |
|---|---|---|---|---|---|---|---|---|
| Pan-cancer | 1,638 | Targeted sequencing | FoundationOne | ~315 | 20 | 90 | Yes | Goodman et al. [ |
| Pan-cancer | 2,189 | Targeted sequencing | Custom Panel | 592 | 17 | 92.3 | Yes | Vanderwalde et al. [ |
| Pan-cancer | 1,662 | Targeted sequencing | MSK-IMPACT v3 | 468 | 8.8 | 80 | Yes | Samstein et al. [ |
| SCLC | 134 | Targeted sequencing | DFCI OncoPanel | 447 | 9.68 | 50 | Yes | Ricciuti et al. [ |
| Colorectal | 6,004 | Targeted sequencing | Comprehensive Genomic Profiling (CGP) | 315 | 11.7 | NA | No | Fabrizio et al. [ |
| NSCLC | 1,649 | Targeted sequencing | FoundationOne | 324 | 10 | 50 | Yes | Hellmann et al. [ |
| NSCLC | 98 | Targeted sequencing | FoundationOne | 324 | 10 | 50 | Yes | Ready et al. [ |
| Urothelial | 316 | Targeted sequencing | FoundationOne | 315 | 16 | 75 | Yes | Rosenberg et al. [ |
| Urothelial | 931 | Targeted sequencing | FoundationOne | NA | 9.65 | 50 | Yes | Powles et al. [ |
| Gastric | 330 | Targeted sequencing | CancerScan | 404 | 10.5 | 89 | No | Cho et al. [ |
| Gastric | 581 | Targeted sequencing | Custom Panel | 592 | 17 | 93.1 | No | Weinberg et al. [ |
| Gastric | 80 | Targeted sequencing | Oncomine Comprehensive Assay v3 | 161 | 10 | 41 | Yes | Mishima et al. [ |
| Gastric | 63 | Targeted sequencing | Oncomine Tumor Mutation Load Assay | 409 | 10.6 | 80 | Yes | Kim et al. [ |
| Factor |
|---|
| Type of tumor (organ) |
| Indications including types of drug |
| Pre-analytic factors (input DNA amount, tumor cell percentages, quality and quantity of DNA) |
| Method (type of panel sequencing including size and number of genes, read depth and coverage) |
| Bioinformatics (limit of detection, threshold for allele frequency and definition of mutation, filter settings for germline variants and deamination artifacts) |
| Advantages | Shortcomings | Solutions for shortcomings |
|---|---|---|
| Simple | Issues with reproducibility and false positive/negative results | Strict interpretation criteria, validation, quality control, and accuracy |
| Inexpensive | Suffer from inter-observer variation or subjective interpretation | Digital microscopy and precision image analysis technologies |
| Processed slides can be stored for years and reassessed | Fixation can affect results | Standardization of analytical and pre-analytical variables |
| Cell morphology can be viewed in parallel | Staining quality affects results | Assay optimization with best-in-class primary antibody selection |
| Usually only 1–2 proteins can be analyzed per a section | Multiplex immunohistochemistry |
ICI, immune checkpoint inhibitors; SCLC, small cell lung cancer; NA, not available; NSCLC, non-small cell lung cancer.

 E-submission
E-submission