Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(2); 2022 > Article
-
Original Article
Fatty acid synthetase expression in triple-negative breast cancer -
Jin Hee Park1
 , Hye Seung Han1
, Hye Seung Han1 , So Dug Lim1
, So Dug Lim1 , Wook Youn Kim1
, Wook Youn Kim1 , Kyoung Sik Park2
, Kyoung Sik Park2 , Young Bum Yoo2
, Young Bum Yoo2 , Seung Eun Lee1
, Seung Eun Lee1 , Wan-Seop Kim1
, Wan-Seop Kim1
-
Journal of Pathology and Translational Medicine 2022;56(2):73-80.
DOI: https://doi.org/10.4132/jptm.2021.10.27
Published online: January 21, 2022
1Department of Pathology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea
2Department of Surgery, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea
-
Corresponding Author: Seung Eun Lee, MD, PhD, Department of Pathology, Konkuk University Medical Center, Konkuk University School of Medicine, 120-1 Neungdong-ro, Gwangjin-gu, Seoul 05030, Korea, Tel: +82-2-2030-5644, Fax: +82-2-2030-5629, E-mail: 20150063@kuh.ac.kr
Corresponding Author: Wan-Seop Kim, MD, PhD, Department of Pathology, Konkuk University Medical Center, Konkuk University School of Medicine, 120-1 Neungdong-ro, Gwangjin-gu, Seoul 05030, Korea, Tel: +82-2-2030-5642, Fax: +82-2-2030-5629, E-mail: wskim@kuh.ac.kr
© 2022 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Lipid metabolism involved in progression and drug resistance of breast cancer
Wenxiang Fu, Aijun Sun, Huijuan Dai
Genes & Diseases.2025; 12(4): 101376. CrossRef - Unveiling the impact of lipid metabolism on triple-negative breast cancer growth and treatment options
Xin-xian Cai, Zhe-zhong Zhang, Xiao-xiao Yang, Wen-rui Shen, Liu-wei Yuan, Xi Ding, Ying Yu, Wen-yu Cai
Frontiers in Oncology.2025;[Epub] CrossRef - Protein biomarkers for diagnosis of breast cancer
Emeka Eze Joshua Iweala, Doris Nnenna Amuji, Faith Chinasaokwu Nnaji
Scientific African.2024; 25: e02308. CrossRef - Microarray analysis points to LMNB1 and JUN as potential target genes for predicting metastasis promotion by etoposide in colorectal cancer
Jiafei Liu, Hongjie Yang, Peng Li, Yuanda Zhou, Zhichun Zhang, Qingsheng Zeng, Xipeng Zhang, Yi Sun
Scientific Reports.2024;[Epub] CrossRef - The signature of extracellular vesicles in hypoxic breast cancer and their therapeutic engineering
Baiheng Zhu, Kehao Xiang, Tanghua Li, Xin Li, Fujun Shi
Cell Communication and Signaling.2024;[Epub] CrossRef - NFYA promotes malignant behavior of triple-negative breast cancer in mice through the regulation of lipid metabolism
Nobuhiro Okada, Chihiro Ueki, Masahiro Shimazaki, Goki Tsujimoto, Susumu Kohno, Hayato Muranaka, Kiyotsugu Yoshikawa, Chiaki Takahashi
Communications Biology.2023;[Epub] CrossRef - Role of EGFR and FASN in breast cancer progression
Suchi Chaturvedi, Mainak Biswas, Sushabhan Sadhukhan, Avinash Sonawane
Journal of Cell Communication and Signaling.2023; 17(4): 1249. CrossRef - Bioinformatics Method Was Used to Analyze the Highly Expressed Gene FAM83A of Breast Cancer in Young Women
Yongzhe Tang, Hao Wang, Qi He, Yuanyuan Chen, Jie Wang, Fahd Abd Algalil
Applied Bionics and Biomechanics.2022; 2022: 1. CrossRef - NCAPH promotes proliferation as well as motility of breast cancer cells by activating the PI3K/AKT pathway
Ting Zhang, Peng Li, Wanying Guo, Qipeng Liu, Weiqiang Qiao, Miao Deng
Physiology International.2022;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
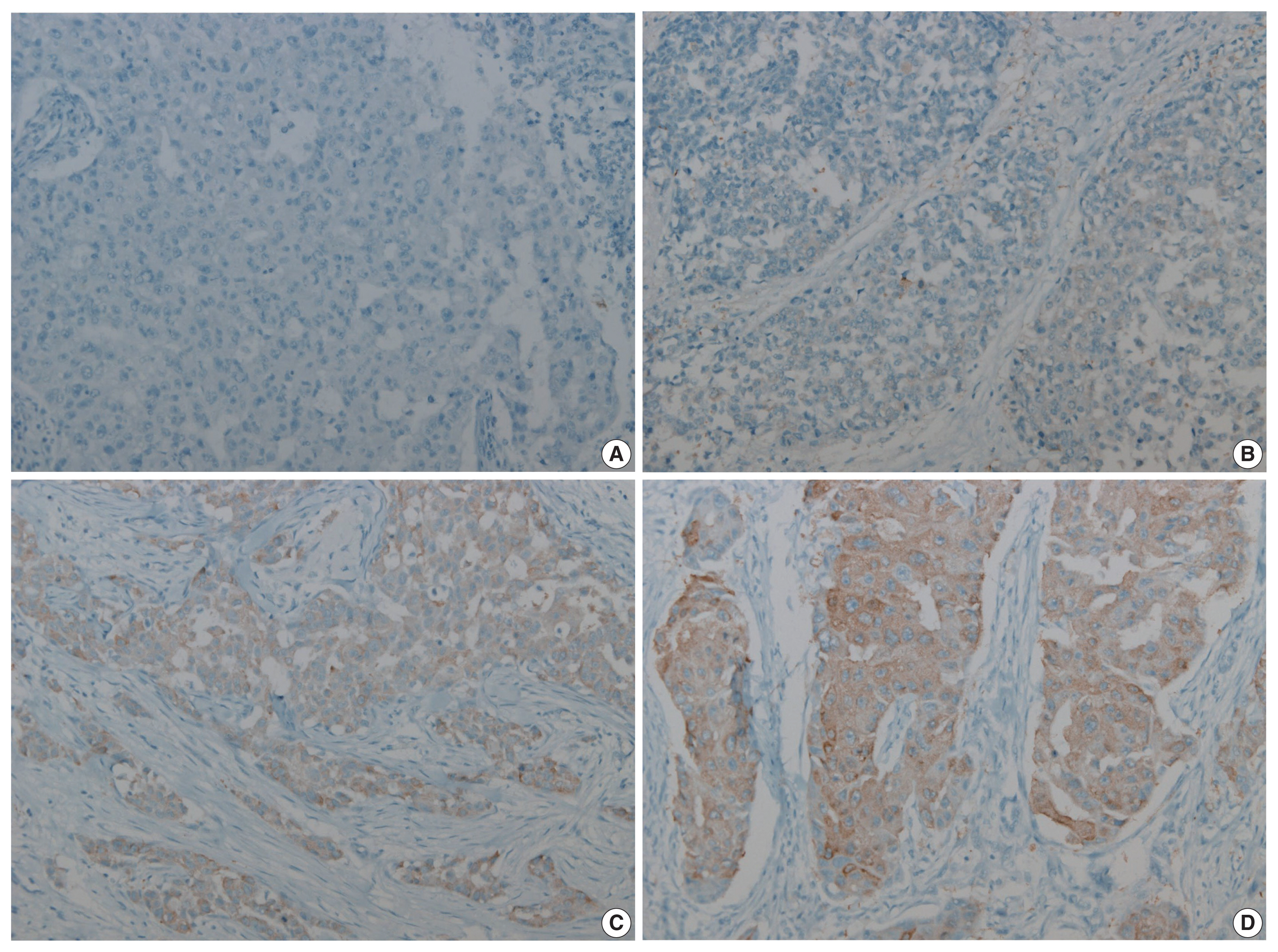
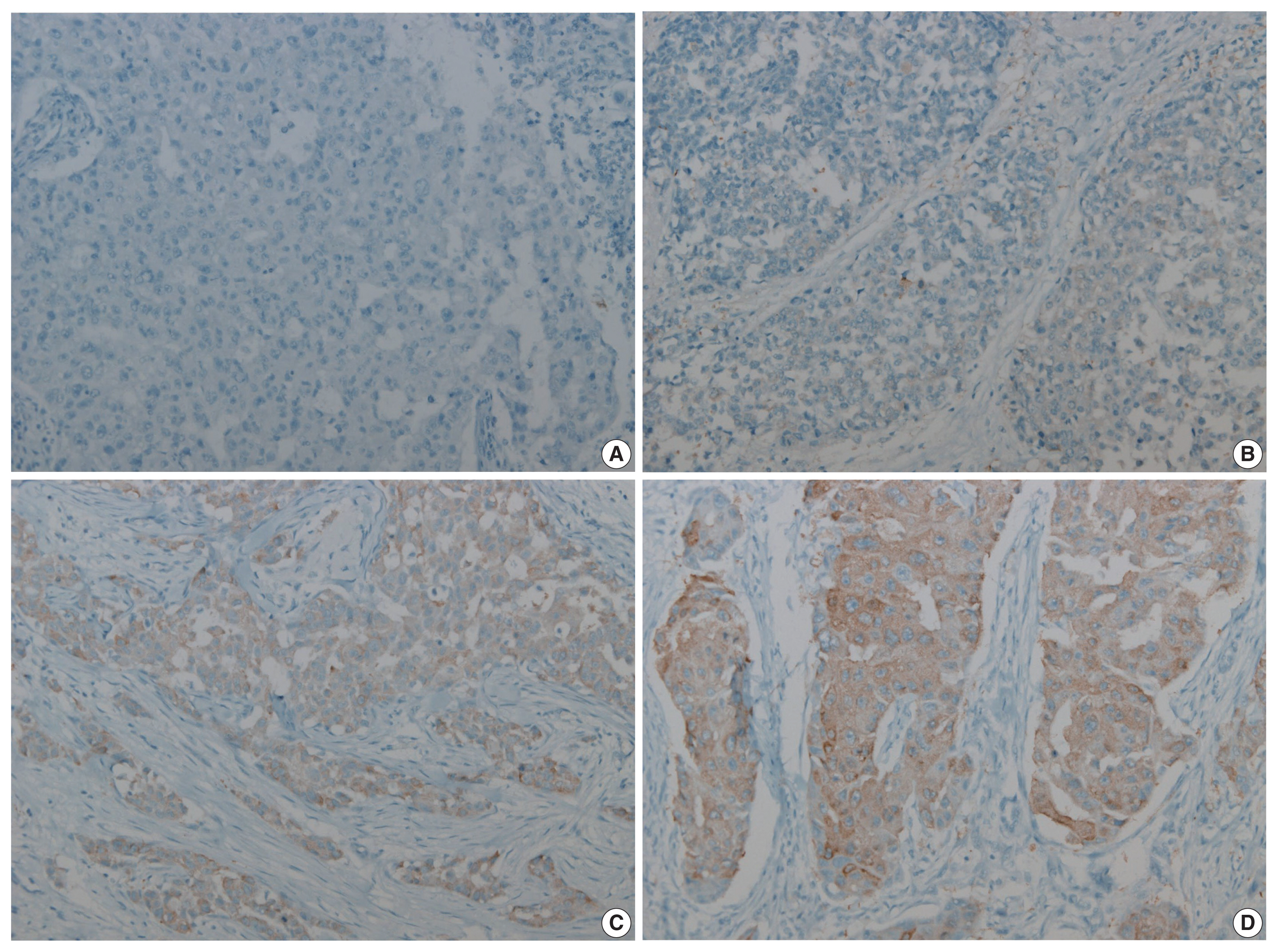
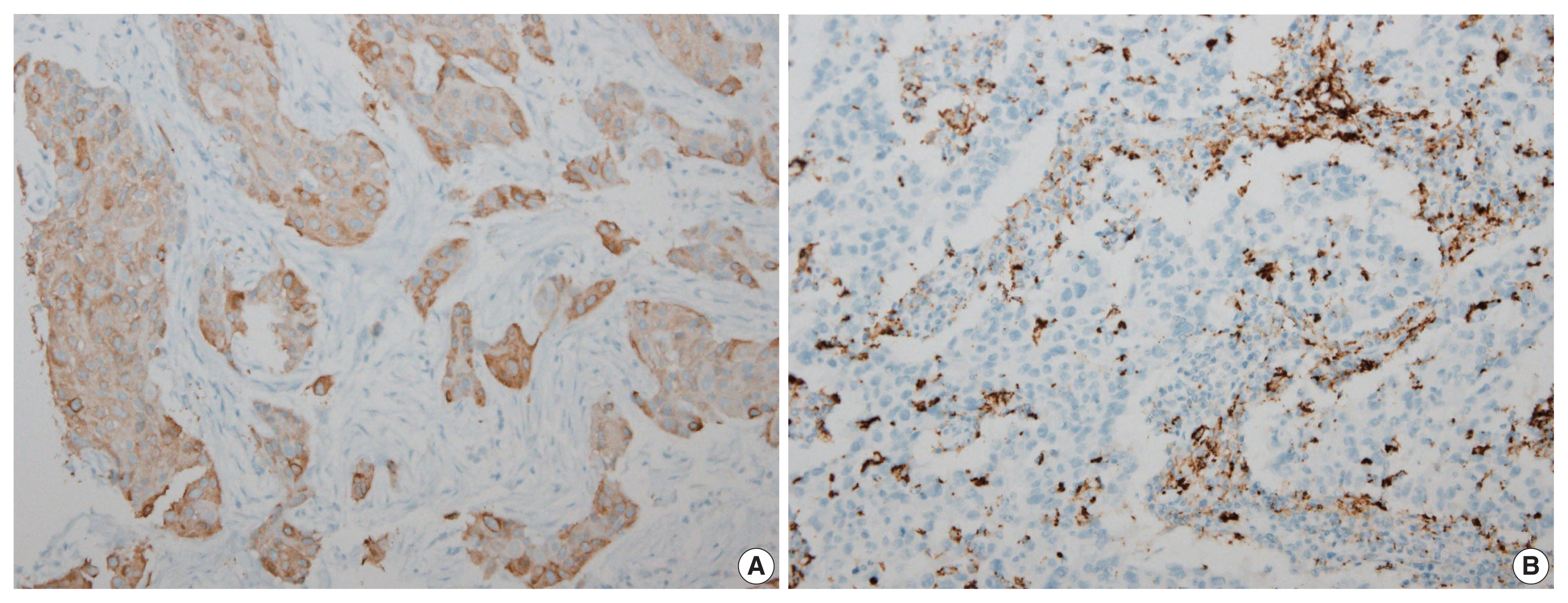
- Figure


Fig. 1
Fig. 2
| Characteristics | No. of patients |
|---|---|
| Age (yr) | 51 (28–83) |
| Age (yr) | |
| ≤ 50 | 81 (48.8) |
| > 50 | 85 (51.2) |
| Histologic type | |
| IDC, NOS | 154 (92.8) |
| Carcinoma with medullary feature | 8 (4.8) |
| Pleomorphic carcinoma | 3 (1.8) |
| Metaplastic carcinoma | 1 (0.6) |
| T category | |
| 1 | 66 (39.8) |
| 2 | 88 (53.0) |
| 3 | 11 (6.6) |
| 4 | 1 (0.6) |
| N category | |
| 0 | 109 (65.7) |
| 1 | 40 (24.1) |
| 2 | 9 (5.4) |
| 3 | 4 (2.4) |
| NA | 4 (2.4) |
| Histologic grade | |
| 1 | 0 |
| 2 | 24 (14.5) |
| 3 | 142 (85.5) |
| AJCC stage | |
| 1 | 33 (19.9) |
| 2 | 110 (66.3) |
| 3 | 19 (11.4) |
| N/A | 4 (2.4) |
| Neoadjuvant CTx | |
| Yes | 10 (6.0) |
| No | 156 (94.0) |
| CTx | |
| Yes | 154 (92.8) |
| No | 12 (7.2) |
| RTx | |
| Yes | 140 (84.3) |
| No | 26 (15.7) |
| Recur | |
| Yes | 26 (15.7) |
| No | 140 (84.3) |
| Low (n = 126) | High (n = 40) | p-value | |
|---|---|---|---|
| Age (yr) | .018 | ||
| < 50 | 58 (46.0) | 27 (67.5) | |
| > 50 | 68 (54.0) | 13 (32.5) | |
| Histologic type | .319 | ||
| IDC, NOS | 116 (92.1) | 38 (95.0) | |
| Carcinoma with medullary feature | 7 (5.6) | 1 (2.5) | |
| Pleomorphic carcinoma | 3 (2.4) | 0 | |
| Metaplastic carcinoma | 0 | 1 (2.5) | |
| T category | .080 | ||
| 1 | 44 (66.7) | 22 (33.3) | |
| 2 | 73 (83.0) | 15 (17.0) | |
| 3 | 8 (72.7) | 3 (27.3) | |
| 4 | 1 (100) | 0 | |
| N category | .614 | ||
| 0 | 79 (72.5) | 30 (27.5) | |
| 1 | 33 (82.5) | 7 (17.5) | |
| 2 | 8 (88.9) | 1 (11.1) | |
| 3 | 2 (50.0) | 2 (50.0) | |
| AJCC stage | .075 | ||
| 1 | 18 (54.5) | 15 (45.5) | |
| 2 | 91 (82.7) | 19 (17.3) | |
| 3 | 13 (68.4) | 6 (31.6) | |
| N/A | 4 (100) | 0 | |
| Histologic grade | .097 | ||
| 2 | 15 (11.9) | 9 (22.5) | |
| 3 | 111 (88.1) | 31 (77.5) | |
| Neoadjuvant CTx | 10 (7.94) | 0 | .120 |
| CTx | 119 (94.4) | 35 (87.5) | .140 |
| RTx | 105 (83.3) | 35 (87.5) | .528 |
| Recur | 23 (18.3) | 3 (7.5) | .135 |
| Mann-Whitney U test | Ki-67 | p-value |
|---|---|---|
| Low FASN, median (range) | 60 (40–80) | .003 |
| High FASN, median (range) | 72.75 (55.13–87.7) | |
| Spearman’s correlation | ||
| FASN expression level | ρ = 0.257 | .001 |
Values are presented as median (interquartile range) or number (%). TNBC, triple-negative breast cancer; IDC, invasive ductal carcinoma; NOS, not otherwise specified; NA, not applicable; AJCC, American Joint Committee on Cancer; CTx, chemotherapy; RTx, radiation therapy.
FASN, fatty acid synthetase; IDC, invasive ductal carcinoma; NOS, not otherwise specified; N/A, not applicable; AJCC, American Joint Committee on Cancer; CTx, chemotherapy; RTx, radiation therapy. Linear by linear association; Fisher’s exact test.
FASN, fatty acid synthetase.

 E-submission
E-submission