Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(4); 2022 > Article
-
Case Study
Clinically undetected plasmacytoid urothelial carcinoma of the urinary bladder with non-mass-forming metastases in multiple organs: an autopsy case -
Yuya Asano1,2
 , Kosuke Miyai2,3
, Kosuke Miyai2,3 , Shinya Yoshimatsu4
, Shinya Yoshimatsu4 , Makoto Sasaki5
, Makoto Sasaki5 , Katsunori Ikewaki5
, Katsunori Ikewaki5 , Susumu Matsukuma2,4
, Susumu Matsukuma2,4
-
Journal of Pathology and Translational Medicine 2022;56(4):217-224.
DOI: https://doi.org/10.4132/jptm.2022.03.15
Published online: May 3, 2022
1National Defense Medical College, Saitama, Japan
2Department of Pathology and Laboratory Medicine, National Defense Medical College, Saitama, Japan
3Department of Pathology, Japan Self-Defense Forces Central Hospital, Tokyo, Japan
4Department of Laboratory Medicine, National Defense Medical College Hospital, Saitama, Japan
5Department of Anti-Aging and Vascular Medicine, National Defense Medical College, Saitama, Japan
- Corresponding Author: Kosuke Miyai, MD, PhD, Department of Pathology, Japan Self-Defense Forces Central Hospital, 1-2-24 Ikejiri, Setagaya-ku, Tokyo 154-8532, Japan, Tel: +81-3-3411-0151, Fax: +81-4-2996-5193, E-mail: mykusu228@nifty.com
© 2022 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- This case report outlines a clinically undetected urinary bladder plasmacytoid urothelial carcinoma (PUC) with multiple metastases detected at autopsy. An 89-year-old man presented with edema in the lower limbs. Pleural fluid cytology revealed discohesive carcinomatous cells, although imaging studies failed to identify the primary site of tumor. The patient died of respiratory failure. Autopsy disclosed a prostate tumor and diffusely thickened urinary bladder and rectum without distinct tumorous lesions. Histologically, the tumor consisted of acinar-type prostate adenocarcinoma with no signs of metastasis. Additionally, small, plasmacytoid tumor cells were observed in the urinary bladder/rectum as isolated or small clustering fashions. These metastasized to the lungs, intestine, generalized lymph nodes in a non-mass-forming manner. Combined with immunohistochemical studies, these tumor cells were diagnosed PUC derived from the urinary bladder. Both clinicians and pathologists should recognize PUC as an aggressive histological variant, which can represent a rapid systemic progression without mass-forming lesions.
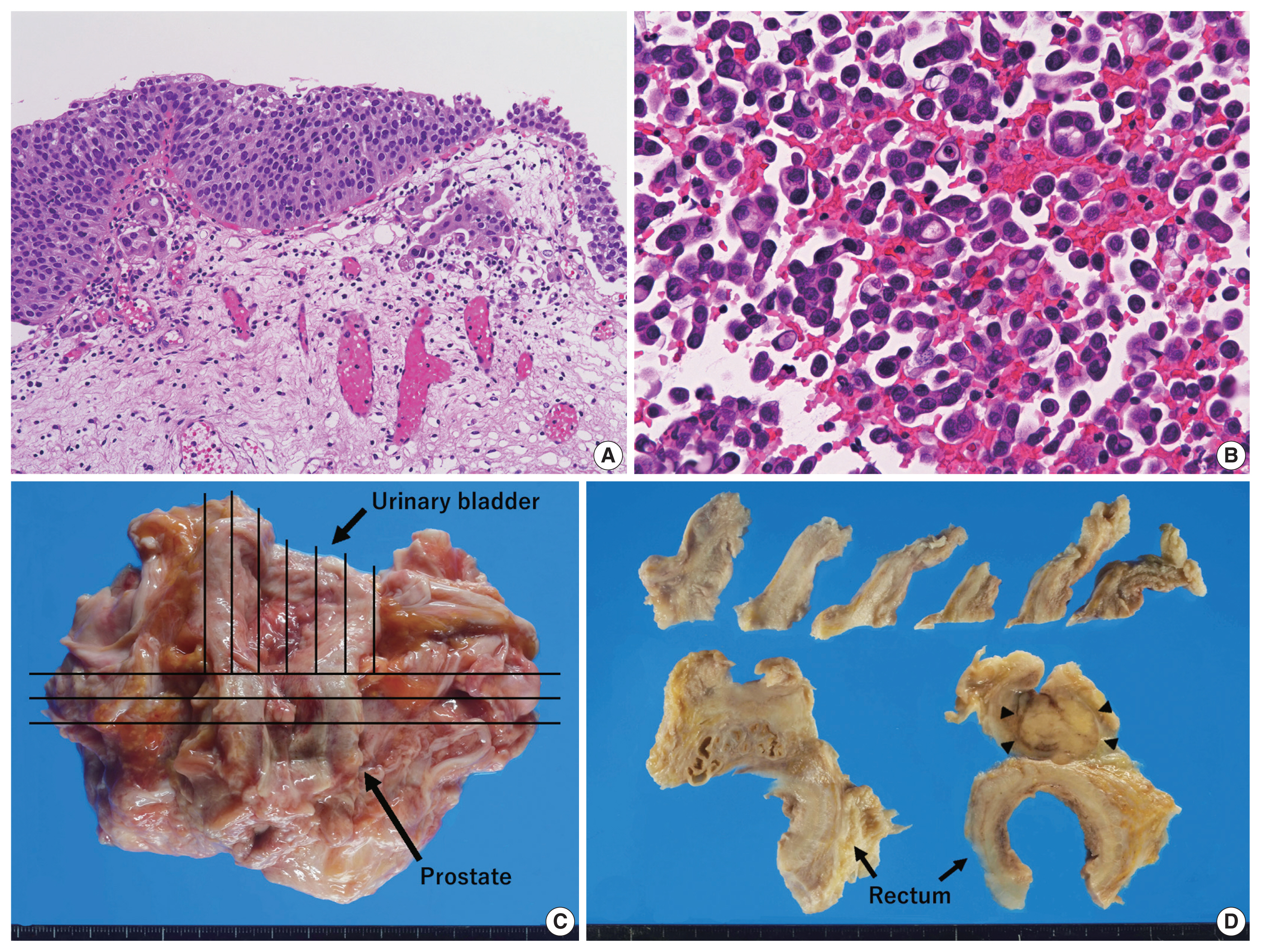
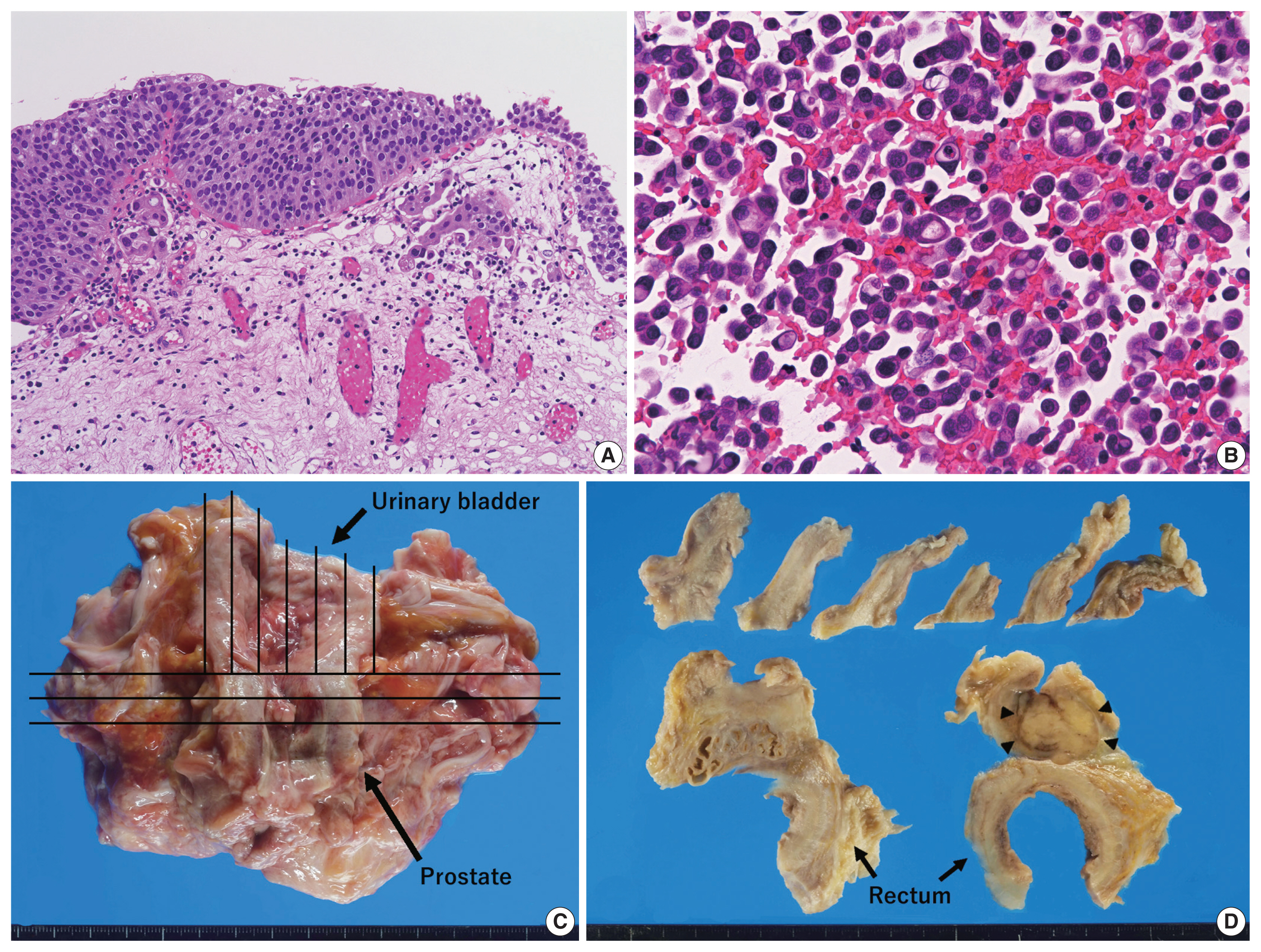
- An 89-year-old Japanese man was admitted to our hospital with severely edematous lower limbs. The patient was diagnosed with prostate cancer at the age of 80 and received hormone therapy for 8 years. Because of atypical urothelial cells in urine smear cytology, he underwent transurethral resection of bladder tumor (TURBT) 1 year and 4 months before admission, that revealed UC in situ with a stromal microinvasion (Fig. 1A). A follow-up abdominal computed tomography (CT) scan taken 3 months before admission showed thickening of the rectal wall; however, no significant deformation was found in the urinary bladder. At the time of admission, a high level of carbohydrate antigen 19-9 (2,771 U/mL; normal, < 37 U/mL) was detected in serum, whereas levels of carcinoembryonic antigen and prostate-specific antigen (PSA) were normal.
- Following admission, bilateral pleural effusion progressed, and pleural fluid smear/cell block cytology revealed discohesive carcinomatous cells (Fig. 1B) that were immunoreactive for cytokeratin (CK) 7 and CK20 and negative for PSA and thyroid transcription factor-1. Various organs, including the stomach, bile duct, pancreas, and urinary tract, were speculated to be the origin of tumor. However, fluorodeoxyglucose positron emission tomography/CT scan taken 20 days after admission failed to detect any primary lesions (i.e., carcinoma of unknown origin). The patient’s respiratory function gradually deteriorated because of aspiration pneumonia. The patient was treated with only palliative therapy and died 27 days after admission. An autopsy was performed on the same day (postmortem, 7 hours).
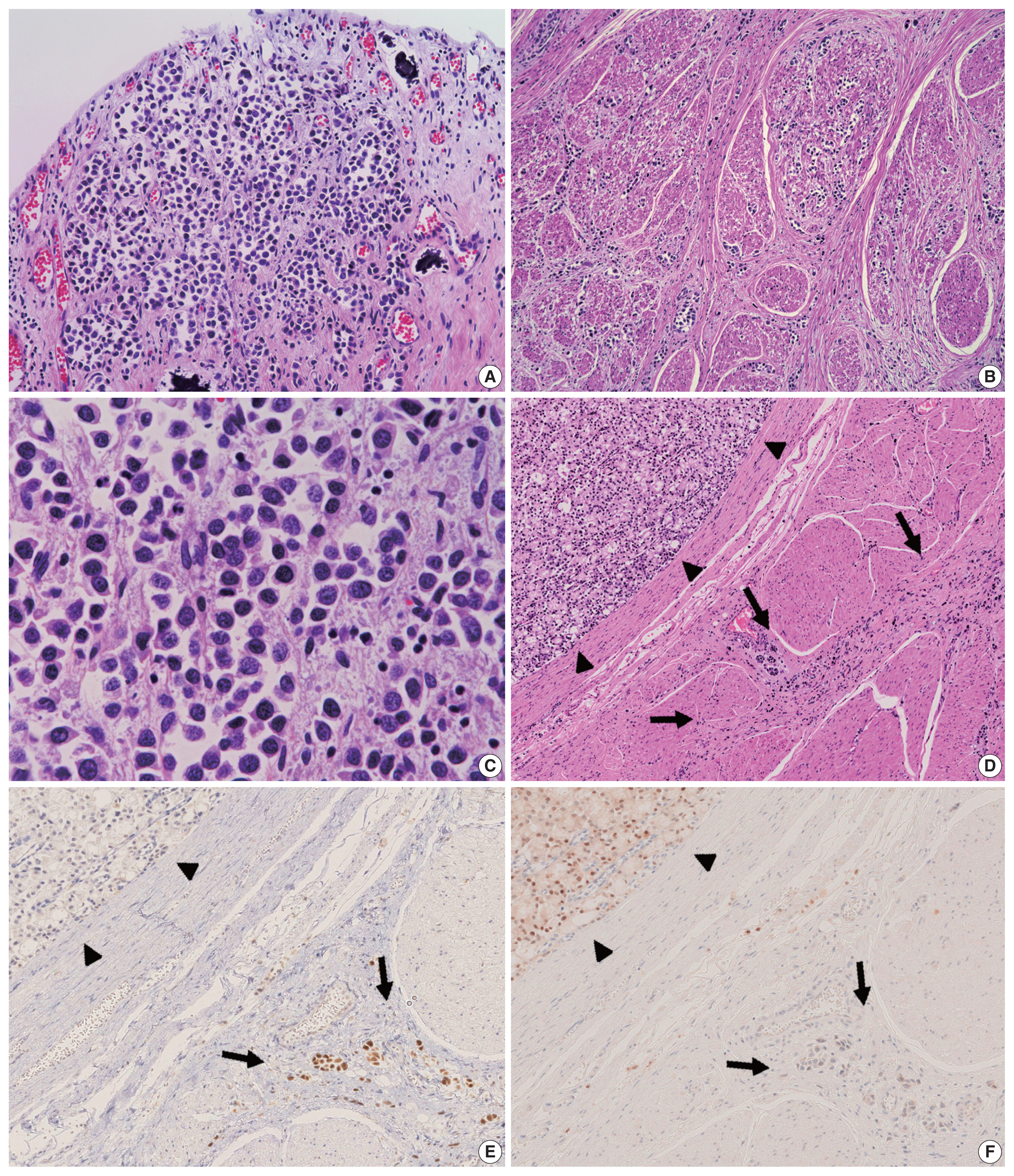
- Grossly, a prostate tumor measuring 3.5 cm in the largest diameter, protruding into the urethra, was detected. Around this tumor, the rectal wall and posterior wall of the urinary bladder were diffusely thickened, but no other mass-forming lesions were observed in the whole body examined (Fig. 1C, D). Histologically, the prostate tumor showed fused-gland features of acinar-type adenocarcinoma, Gleason Grade Group 4, extending to the rectal wall and urethra. Examination of the thickened urinary bladder wall revealed diffuse infiltration of discohesive, plasmacytoid tumor cells in a single file pattern and small nests in the edematous lamina propria (Fig. 2A), muscularis propria (Fig. 2B), and perivesical adipose tissue. The individual tumor cells displayed oval-to-round eccentrically located nuclei and abundant densely eosinophilic and occasionally vacuolated/signet-ring-cell-like cytoplasm (Fig. 2C). The tumor was exposed to the serosal surface of the urinary bladder and massively extended into the thickened rectal wall, seminal vesicles, and periprostatic tissue, surrounding the prostate cancer (Fig. 2D). Autopsy failed to detect any colorectal intramucosal lesions of the tumor.
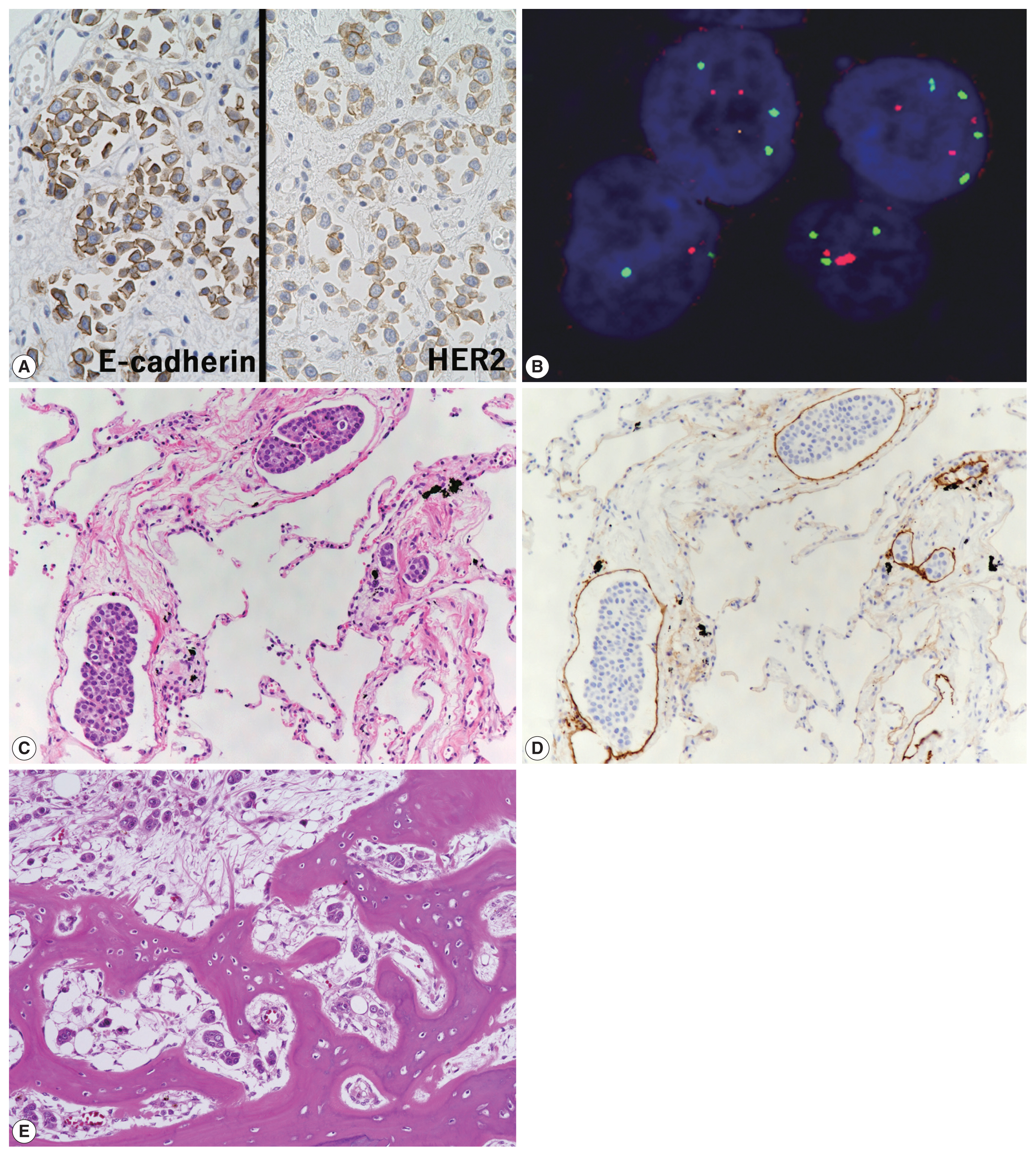
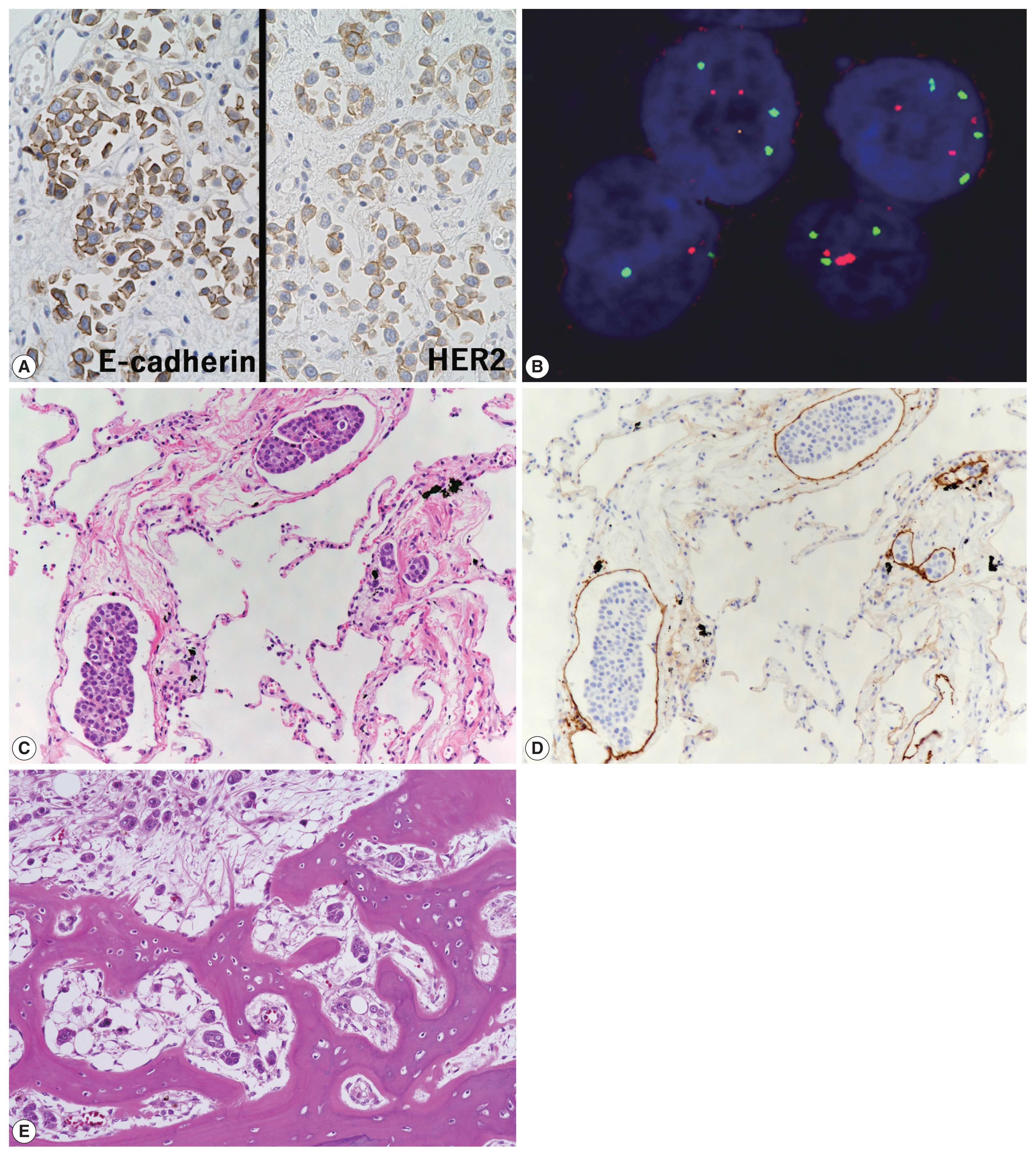
- Characteristics of primary antibodies used in immunohistochemical analysis are summarized in Table 1. Immunohistochemically, the plasmacytoid tumor cells were diffusely positive for CK7, CK20, p63, and GATA binding protein 3 (GATA3) and negative for NK3 homeobox 1 (Nkx3.1), while the opposite was observed in the prostate cancer cells (i.e., Nkx3.1+ and CK7/CK20/p63/GATA3−) (Fig. 2E, F). The plasmacytoid tumor cells were also negative for CDX2 and nuclear β-catenin. Immunoreactivity for E-cadherin was retained in the plasmacytoid tumor cells, and that for human epidermal growth factor receptor 2 (HER2) had a score of 2+ (Fig. 3A). PathVysion HER2 DNA probe kit (HER2 SpectrumOrange/CEP17 SpectrumGreen DNA probes, Abbott Molecular, Downers Grove, IL, USA) was used for fluorescence in situ hybridization analysis, as described previously [11]. Hybridization was performed between the denatured probes and denatured DNA on tissue sections at 37°C for 14–18 hours. The sections were counterstained with 4,6-diamidino-2-phenylindone. The number of fluorescence signals from both centromeric region of chromosome 17 (CEP17) and HER2 probes in the 40 interphase tumor cell nuclei was counted. The HER2/CEP17 ratio and mean HER2 signal were less than 2.0 and 4.0, respectively (Fig. 3B). PUC in the present case showed no HER2 amplification. Based on these findings, the urinary bladder tumor was diagnosed as PUC that histologically involved an area measuring 10 × 7 cm.
- Non-mass-forming multiple metastases of PUC were found in the lungs (bilateral), jejunum, sigmoid colon, sacrum, and generalized lymph nodes and in peritoneal and pleural disseminations, with or without lymphatic invasion (Fig. 3C–E). No features of conventional UC were found in the primary and metastatic sites. Autopsy failed to detect distant metastases of the prostate cancer.
CASE REPORT
- Emerging evidence demonstrates that PUC is associated with adverse clinicopathological features, advanced stage at cystectomy, and poor prognosis [3,12–16]. In a recent meta-analysis of eight case series, PUC of the bladder showed a higher frequency of pathological stage ≥ T3, increased risk of perivesical/ureteral margin positivity, and lymph node metastasis, and elevated overall mortality rate compared to conventional UC [4]. While early tumor detection and accurate evaluation of tumor spreading are important for proper clinical management of PUC [3], its unique non-mass-forming progression makes it difficult to diagnose. Some reports indicate that PUCs tend to invade along the perirectal/perivesical fascial plane [12,15,16], which is usually challenging to detect using conventional CT/magnetic resonance imaging [6]. In the present case, autopsy showed an extensive local spreading to the perivesical fascial plane and generalized metastases of the tumor; however, it could not be detected via clinical imaging. PUC of the bladder is a rare disease; most histological data in previous case series were only from TURBT or cystectomy specimens. We suggest that the lack of data regarding precise histological evaluation of such generalized disease (i.e., autopsy study) is one of the major factors that makes clinical/imaging analysis of PUC difficult.
- Only four other autopsy cases of PUC have been reported to date [7–10]. The clinicopathological features of these cases (cases 1–4) and the present case (case 5) are summarized in Table 2. In all five cases, the primary tumor location was the bladder, and four patients were men. The percentage of PUC in the total UC area was described in only one case (case 1); the tumor was a pure type PUC, similar to that in the present case. Immunohistochemically, three of four cases (75%) were positive for CK7 and CK20, three of three cases (100%) were positive for GATA3, and loss of E-cadherin staining was detected in three of five cases (60%). In cases 1, 2, and 4, PUC was diagnosed using TURBT specimens. In the present case, TURBT revealed only a minor amount of stromal invasion of cancer cells. However, no obvious detrusor muscle was detected in the whole specimen, indicating a high risk of residual tumor. In this context, if re-TURBT had been performed, the PUC invading the muscle layer might have been disclosed. All cases showed direct invasion of tumor to adjacent organs, and cases 1 and 3 showed progression along the perirectal fascial plane, similar to that seen in the present case. In all cases except case 2, multiple distant metastases were found in the intra-abdominal organs (cases 1, 3–5), thoracic cavity (cases 1 and 5), and bone (cases 4 and 5). PUC was the main cause of death in all cases except for case 4 (myocardial infarction), and all five patients died within 2 years from the initial diagnosis of the tumor.
- In the present case, although plasmacytoid carcinomatous cells were detected during pleural fluid cytology, we could not consider those in relation to the conventional UC in the previous TURBT specimens. PUC is often observed at advanced stages with metastasis [6], and its differential diagnosis includes a wide variety of diseases such as signet-ring cell adenocarcinoma (especially colorectal origin due to the perirectal spreading pattern of PUC), carcinoma with rhabdoid features, lymphoma/myeloma, and melanoma [13]. The first case of PUC was also identified in a bone metastatic lesion and was reported to mimic a myeloma [1]. In such situations, the possibility of PUC should be recognized initially, and effective immunohistochemical analysis should be performed. GATA3 is the most sensitive marker for tumors derived from urinary epithelium, and CDX2/β-catenin might be useful to distinguish PUC from colorectal signet-ring adenocarcinoma [17]; hence, these markers should have been included in our immunohistochemical panel for pleural fluid cytology examination for diagnosing the present case. Although loss of E-cadherin expression is observed in 70%–80% of cases and is thought to be one of the diagnostic hallmarks of PUC [18], its frequency was found to be 57% in another case series [17]. Retention of cytoplasmic expression of E-cadherin is not sufficient to rule out PUCs.
- Although there was moderate immunoreactivity for HER2, no gene amplification was detected in the tumor cells in the present case. A recent molecular study of 1005 UC cases revealed that HER2 gene amplification was found in approximately 10% of the cases, and concluded that anti-HER2 antibody is expected to be applied to the novel standard treatment of UC [19]. Interestingly, HER2 amplification is one of the features characterizing “luminal unstable” type in the recent consensus molecular classification, which is enriched in plasmacytoid histology of the bladder UC [20]. Further investigations directed towards HER2 status and the effectiveness of HER2-targeted therapy for this aggressive variant of UC are required.
- In summary, we described a case of clinically undetected PUC that was initially identified as a “cancer of unknown primary” through pleural fluid cytology but autopsy revealed broad local spreading and multiple organ metastasis. This rare form of UC should be considered in the differential diagnosis of aggressive occult tumors. Data accumulation and analysis of additional autopsy case reports are essential to clarify the peculiar non-mass-forming spread of PUC, which can help in its early detection.
DISCUSSION
Acknowledgments
Acknowledgments
Ethics Statement
This single case report in exempted submission to Institutional Review Board and subsequent informed consent by the National Defense Medical College, Tokorozawa, Japan (Registration number, 4007; decision date, August 8, 2021).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: YA, KM. Data curation: YA, KM. Formal analysis: KM. Investigation: YA, KM, SY. Methodology: KM. Resources: SM. Supervision: MS, KI, SM. Visualization: KM. Writing—original draft: YA, KM. Writing—review & editing: SY, MS, KI, SM. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.

| Case No. | Author | Age (yr)/Sex | Primary tumor size/Location | Operative procedure | Chemoradiotherapy | % PUC/Total UC | Immunohistochemical analysis | Direct invasion | Distant metastasis | Prognosis | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| CK7 | CK20 | E-cad | GATA3 | HER2 | ||||||||||
| 1 | Simon et al. [7] | 65/M | 7 cm per gross/bladder | TURBT and cysto-prostatectomy | MVAC (3 cycles), Atezolizumab | 100 | + | + | − | + | N/A | Rectal wall | Lungs, pleura, diaphragm, small/large intestine, gallbladder, thoracic and abdominal lymph nodes | DOD 9 moa |
| 2 | Kohada et al. [8] | 75/F | N/A/bladder | TURBT | GC (2 cycles), Pembrolizumab | N/A | − | − | + | + | + | Uterus, retroperitoneum | None | DOD 4 moa |
| 3 | Ando et al. [9] | 83/M | N/A/bladder | Nephro-ureterectomy | None | N/A | N/A | N/A | − | N/A | N/A | Rectal wall, prostate gland | Peritoneal dissemination and retroperitoneal lymphatic permeation | DOD 2 mob |
| 4 | Tanaka et al. [10] | 85/M | 8 × 6 cmc/ bladder | TURBT | None | N/A | + | + | − | N/A | N/A | Prostate gland | Liver, spleen, kidneys, adrenal glands, bone marrow | DOC (AMI) 37 daysa |
| 5 | Present case | 89/M | 10 × 7 cmc/ bladder | TURBT | None | 100 | + | + | + | + | − | Rectal wall, seminal vesicle, paraprostatic tissue | Lungs, right kidney and ureter, left testis, pancreas, liver hilum, right adrenal gland, small/large intestine, pleural/peritoneal dissemination, retroperitoneal tissue, bone marrow, thoracic and abdominal lymph nodes | DOD 1 year and 5 moa |
PUC, plasmacytoid urothelial carcinoma; UC, urothelial carcinoma; CK, cytokeratin; E-cad, E-cadherin; HER2, human epidermal growth factor receptor 2; M, male; TURBT, transurethral resection of bladder tumor; MVAC, methotrexate, vinblastine, doxorubicin and cisplatin; N/A, not available; DOD, death of disease; F, female; GC, gemcitabine and cisplatin; DOC, death of other cause; AMI, acute myocardial infarction.
aPeriod after TURBT;
bPeriod after para-duodenum tissue biopsy, by which carcinoma of unknown origin was firstly detected;
cAt the time of autopsy.
- 1. Sahin AA, Myhre M, Ro JY, Sneige N, Dekmezian RH, Ayala AG. Plasmacytoid transitional cell carcinoma: report of a case with initial presentation mimicking multiple myeloma. Acta Cytol 1991; 35: 277-80. PubMed
- 2. Zukerberg LR, Harris NL, Young RH. Carcinomas of the urinary bladder simulating malignant lymphoma: a report of five cases. Am J Surg Pathol 1991; 15: 569-76. PubMed
- 3. Ro JY, Shen SS, Lee HI, et al. Plasmacytoid transitional cell carcinoma of urinary bladder: a clinicopathologic study of 9 cases. Am J Surg Pathol 2008; 32: 752-7. PubMed
- 4. Kim DK, Kim JW, Ro JY, et al. Plasmacytoid variant urothelial carcinoma of the bladder: a systematic review and meta-analysis of clinicopathological features and survival outcomes. J Urol 2020; 204: 215-23. ArticlePubMed
- 5. Sood S, Paner GP. Plasmacytoid urothelial carcinoma: an unusual variant that warrants aggressive management and critical distinction on transurethral resections. Arch Pathol Lab Med 2019; 143: 1562-7. ArticlePubMedPDF
- 6. Chung AD, Schieda N, Flood TA, et al. Plasmacytoid urothelial carcinoma (PUC): imaging features with histopathological correlation. Can Urol Assoc J 2017; 11: E50-7. ArticlePubMedPMCPDF
- 7. Simon CT, Skala SL, Killen PD, et al. Plasmacytoid urothelial carcinoma: a rapid autopsy case report with unique clinicopathologic and genomic profile. Diagn Pathol 2019; 14: 113.ArticlePubMedPMCPDF
- 8. Kohada Y, Kaiho Y, Ito J, et al. Progressive plasmacytoid variant bladder cancer with retroperitoneal dissemination: an autopsy case report. IJU Case Rep 2020; 3: 166-9. ArticlePubMedPMCPDF
- 9. Ando T, Watanabe K, Takahashi K, Mizusawa T, Sakai T, Katagiri A. Duodenal and rectal obstructions due to urothelial cancer infiltration from recurrent renal pelvic cancer in the bladder wall: an autopsy case. Urol Case Rep 2019; 27: 100903.ArticlePubMedPMC
- 10. Tanaka A, Ohori M, Hashimoto T, et al. A case of plasmacytoid urothelial carcinoma of the bladder: rapid progression after transurethral resection. Hinyokika Kiyo 2012; 58: 101-3. PubMed
- 11. Miyai K, Ito K, Matsukuma S, Tsuda H. Frequent EGFR expression/EGFR amplification and lack of activating mutation in testicular choriocarcinoma. Pathol Int 2020; 70: 262-9. ArticlePubMedPDF
- 12. Dayyani F, Czerniak BA, Sircar K, et al. Plasmacytoid urothelial carcinoma, a chemosensitive cancer with poor prognosis, and peritoneal carcinomatosis. J Urol 2013; 189: 1656-61. ArticlePubMedPMC
- 13. Lopez-Beltran A, Requena MJ, Montironi R, Blanca A, Cheng L. Plasmacytoid urothelial carcinoma of the bladder. Hum Pathol 2009; 40: 1023-8. ArticlePubMed
- 14. Monn MF, Kaimakliotis HZ, Pedrosa JA, et al. Contemporary bladder cancer: variant histology may be a significant driver of disease. Urol Oncol 2015; 33: 18.Article
- 15. Cockerill PA, Cheville JC, Boorjian SA, et al. Outcomes following radical cystectomy for plasmacytoid urothelial carcinoma: defining the need for improved local cancer control. Urology 2017; 102: 143-7. ArticlePubMed
- 16. Li Q, Assel M, Benfante NE, et al. The impact of plasmacytoid variant histology on the survival of patients with urothelial carcinoma of bladder after radical cystectomy. Eur Urol Focus 2019; 5: 104-8. ArticlePubMedPMC
- 17. Perrino CM, Eble J, Kao CS, et al. Plasmacytoid/diffuse urothelial carcinoma: a single-institution immunohistochemical and molecular study of 69 patients. Hum Pathol 2019; 90: 27-36. ArticlePubMed
- 18. Sangoi AR, Chan E, Stohr BA, Kunju LP. Invasive plasmacytoid urothelial carcinoma: a comparative study of E-cadherin and P120 catenin. Hum Pathol 2020; 102: 54-9. ArticlePubMed
- 19. Lae M, Couturier J, Oudard S, Radvanyi F, Beuzeboc P, Vieillefond A. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann Oncol 2010; 21: 815-9. ArticlePubMedPMC
- 20. Kamoun A, de Reynies A, Allory Y, et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol 2020; 77: 420-33. PubMed
REFERENCES
Figure & Data
References
Citations

- Severe Rectal Stenosis as the First Clinical Appearance of a Metastasis Originating from the Bladder: A Case Report and Literature Review
Claudiu Daha, Eugen Brătucu, Ioan Burlănescu, Virgiliu-Mihail Prunoiu, Hortensia-Alina Moisă, Ștefania Ariana Neicu, Laurențiu Simion
Life.2025; 15(5): 682. CrossRef - Carcinomatous Meningitis and Hydrocephalus in Plasmacytoid Urothelial Carcinoma of the Urinary Bladder With Extremely Elevated CA19-9 Levels
Fumiaki Henmi, Kayako Ukai, Atsuhito Nakayama, Yutaka Takazawa, Yoshikazu Uesaka
Cureus.2024;[Epub] CrossRef - Current Advances in the Management of Nonurothelial Subtypes of Bladder Cancer
Evangelia Vlachou, Burles Avner Johnson, Ezra Baraban, Rosa Nadal, Jean Hoffman-Censits
American Society of Clinical Oncology Educational Book.2024;[Epub] CrossRef - Plasmacytoid urothelial carcinoma: a multidisciplinary approach to the diagnosis and management
Marcus Zorovich, Jude Khatib, Aysha Mubeen, Katie Gardner, Nayana Patel
Abdominal Radiology.2024;[Epub] CrossRef - Divergent Histology in Bladder Cancer: What We Need to Know?
Shashank Agrawal, Arun Ramdas Menon, Ginil Kumar Pooleri
UroCancer Clinics of India.2024; 2(2): 100. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1
Fig. 2
Fig. 3
| Protein | Clone | Manufacturer, catalog number | Host | Antigen retrieval | Dilution |
|---|---|---|---|---|---|
| β-catenin | Monoclonal, β-catenin-1 | Dako, Glostrup, Denmark, M3539 | Mouse | Boil, 95°C 30 min, EDTA pH = 9 | 1:200 |
| CK7 | Monoclonal, OV-TL 12/30 | Dako, M7018 | Mouse | Proteinase K, 37°C 15 min | 1:200 |
| CK20 | Monoclonal, Ks20.8 | Dako, M7019 | Mouse | Proteinase K, 37°C 15 min | 1:100 |
| D2-40 | Monoclonal, D2-40 | Dako, M3619 | Mouse | None | 1:50 |
| E-cadherin | Monoclonal, 4A2c7 | Invitrogen, Carlsbad, CA, USA, 18-0223 | Mouse | Boil, 95°C 30 min, EDTA pH = 9 | 1:100 |
| GATA3 | Monoclonal, L50-823 | Biocare Medical, Concord, CA, USA, 1-800-799-9499 | Mouse | Boil, 95°C 30 min, EDTA pH = 9 | 1:300 |
| HER2 | Polyclonal | Dako, K5204 (HercepTest II) | Rabbit | Boil, 95°C 40 min, specialized buffer | Ready-to-use |
| Nkx3.1 | Monoclonal, EP356 | Nichirei, Tokyo, Japan, 418281 | Rabbit | Autoclave, 121°C 10 min, EDTA pH = 9 | Ready-to-use |
| p63 | Monoclonal, 7JUL | Leica Biosystems, Newcastle Upon Tyne, UK, NCL-p63 | Mouse | Boil, 95°C 30 min, EDTA pH = 9 | 1:50 |
| PSA | Monoclonal, ER-PR8 | Dako, M0750 | Mouse | None | 1:100 |
| TTF-1 | Monoclonal, SPT24 | Novocastra, Newcastle Upon Tyne, UK, NCL-TTF-1 | Mouse | Boil, 95°C 30 min, EDTA pH = 9 | 1:200 |
| Case No. | Author | Age (yr)/Sex | Primary tumor size/Location | Operative procedure | Chemoradiotherapy | % PUC/Total UC | Immunohistochemical analysis | Direct invasion | Distant metastasis | Prognosis | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| CK7 | CK20 | E-cad | GATA3 | HER2 | ||||||||||
| 1 | Simon et al. [ |
65/M | 7 cm per gross/bladder | TURBT and cysto-prostatectomy | MVAC (3 cycles), Atezolizumab | 100 | + | + | − | + | N/A | Rectal wall | Lungs, pleura, diaphragm, small/large intestine, gallbladder, thoracic and abdominal lymph nodes | DOD 9 mo |
| 2 | Kohada et al. [ |
75/F | N/A/bladder | TURBT | GC (2 cycles), Pembrolizumab | N/A | − | − | + | + | + | Uterus, retroperitoneum | None | DOD 4 mo |
| 3 | Ando et al. [ |
83/M | N/A/bladder | Nephro-ureterectomy | None | N/A | N/A | N/A | − | N/A | N/A | Rectal wall, prostate gland | Peritoneal dissemination and retroperitoneal lymphatic permeation | DOD 2 mo |
| 4 | Tanaka et al. [ |
85/M | 8 × 6 cm |
TURBT | None | N/A | + | + | − | N/A | N/A | Prostate gland | Liver, spleen, kidneys, adrenal glands, bone marrow | DOC (AMI) 37 days |
| 5 | Present case | 89/M | 10 × 7 cm |
TURBT | None | 100 | + | + | + | + | − | Rectal wall, seminal vesicle, paraprostatic tissue | Lungs, right kidney and ureter, left testis, pancreas, liver hilum, right adrenal gland, small/large intestine, pleural/peritoneal dissemination, retroperitoneal tissue, bone marrow, thoracic and abdominal lymph nodes | DOD 1 year and 5 mo |
EDTA, ethylenediaminetetraacetic acid; CK, cytokeratin; GATA3, GATA binding protein 3; HER2, human epidermal growth factor receptor 2; Nkx3.1, NK3 homeobox 1; PSA, prostate-specific antigen; TTF-1, thyroid transcription factor-1.
PUC, plasmacytoid urothelial carcinoma; UC, urothelial carcinoma; CK, cytokeratin; E-cad, E-cadherin; HER2, human epidermal growth factor receptor 2; M, male; TURBT, transurethral resection of bladder tumor; MVAC, methotrexate, vinblastine, doxorubicin and cisplatin; N/A, not available; DOD, death of disease; F, female; GC, gemcitabine and cisplatin; DOC, death of other cause; AMI, acute myocardial infarction. Period after TURBT; Period after para-duodenum tissue biopsy, by which carcinoma of unknown origin was firstly detected; At the time of autopsy.

 E-submission
E-submission