Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(4); 2022 > Article
-
Case Study
Primary pulmonary epithelioid inflammatory myofibroblastic sarcoma: a rare entity and a literature review -
Priyanka Singh1
 , Aruna Nambirajan1
, Aruna Nambirajan1 , Manish Kumar Gaur2
, Manish Kumar Gaur2 , Rahul Raj1
, Rahul Raj1 , Sunil Kumar2
, Sunil Kumar2 , Prabhat Singh Malik3
, Prabhat Singh Malik3 , Deepali Jain1
, Deepali Jain1
-
Journal of Pathology and Translational Medicine 2022;56(4):231-237.
DOI: https://doi.org/10.4132/jptm.2022.05.08
Published online: July 7, 2022
1Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
2Department of Surgical Oncology, Dr. BRA Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi, India
3Department of Medical Oncology, Dr. BRA Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi, India
- Corresponding Author: Deepali Jain, MD, FIAC, Department of Pathology, All India Institute of Medical Sciences, New Delhi 110029, India Tel: +91-1126549200, E-mail: deepalijain76@gmail.com
© 20222020 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Epithelioid inflammatory myofibroblastic sarcoma (EIMS) is an aggressive subtype of inflammatory myofibroblastic tumor (IMT) harboring anaplastic lymphoma kinase (ALK) gene fusions and is associated with high risk of local recurrence and poor prognosis. Herein, we present a young, non-smoking male who presented with complaints of cough and dyspnoea and was found to harbor a large right lower lobe lung mass. Biopsy showed a high-grade epithelioid to rhabdoid tumor with ALK and desmin protein expression. The patient initially received 5 cycles of crizotinib and remained stable for 1 year; however, he then developed multiple bony metastases, for which complete surgical resection was performed. Histopathology confirmed the diagnosis of EIMS, with ALK gene rearrangement demonstrated by fluorescence in situ hybridization. Postoperatively, the patient is asymptomatic with stable metastatic disease on crizotinib and has been started on palliative radiotherapy. EIMS is a very rare subtype of IMT that needs to be included in the differential diagnosis of ALKexpressing lung malignancies in young adults.
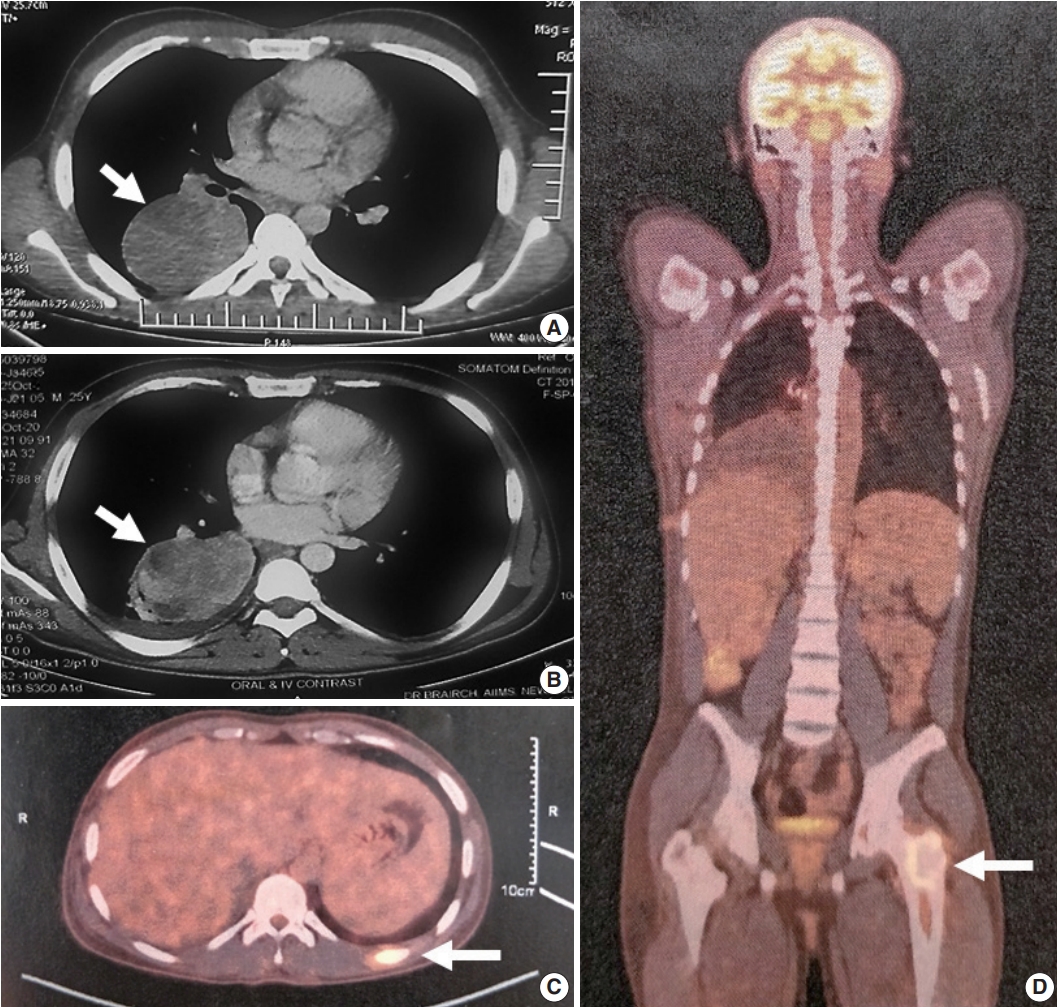
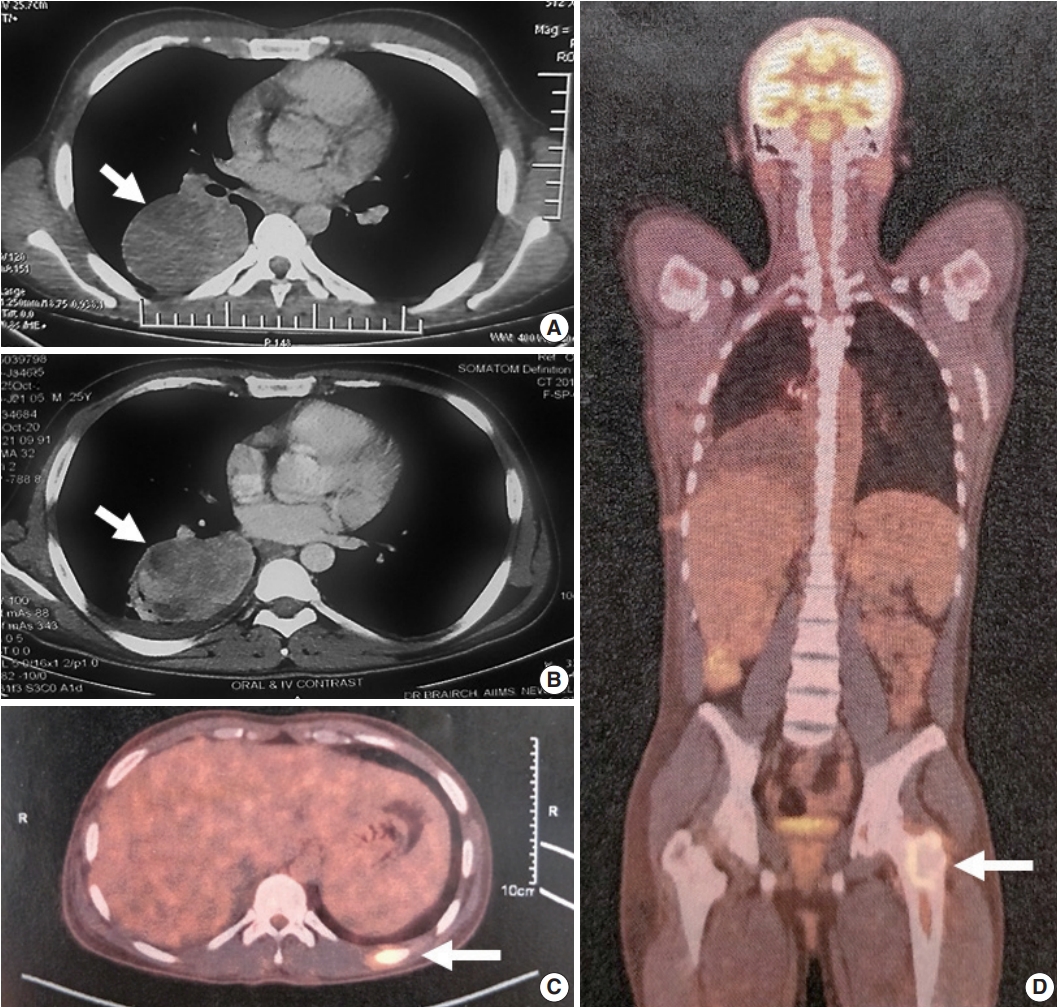
- A 25-year-old, non-smoking male presented with complaints of cough, significant weight loss, intermittent high-grade fever, and difficulty breathing for longer than 4 months. Imaging revealed a well-defined homogenous fluorodeoxyglucose (FDG)-avid mass of 6.9 × 4.2 × 6.1 cm involving the superior segment of the right lower lung lobe with no other pulmonary/pleural lesions or lymphadenopathy (Fig. 1A). Positron emission tomography scan showed another FDG-avid well-defined hypodense lesion of size 1.1 × 1.2 cm in segment IVA of the liver, likely metastasis. Biochemistry including serum tumor biomarkers was within normal range. A computed tomography–guided biopsy of the lung mass showed a poorly differentiated malignant tumor immunonegative for epithelial (pan-cytokeratin, epithelial membrane antigen), hematopoietic (CD45, myeloperoxidase, CD30), pneumocytic (thyroid transcription factor 1), melanocytic (human melanoma black 45, S-100), and mesothelial (calretinin, Wilms tumor protein 1) markers with retained INI-1 and BRG-1 expression. Tumor cells showed cytoplasmic ALK (VENTANA anti-ALK [D5F3] antibody developed by Roche Diagnostics [one 5 mL dispenser of VENTANA anti-ALK (D5F3) antibody contains approximately 70 μg of the rabbit monoclonal (D5F3) antibody; the antibody is diluted in 0.08 M phosphate buffered saline with 3% carrier protein and 0.05% Pro-Clin 300, a preservative]) and cytoplasmic desmin. Myogenin was negative. The possibility of EIMS was suggested, and resection/ re-biopsy was advised for molecular confirmation due to limited remaining biopsy material.
- After multidisciplinary thoracic oncology tumor board discussion, the patient was planned for surgical excision of the lung tumor and radiofrequency ablation of the liver lesion because of the young age of the patient with good performance status, solitary metastasis, and unconfirmed primary pathological diagnosis. However, the patient was started on ALK inhibitor (crizotinib), and surgical intervention was deferred due to coronavirus disease 2019. Six months later, repeat imaging showed stable pulmonary disease (Fig. 1B) and complete resolution of the liver lesion. Crizotinib was continued and the patient was re-assessed for definitive surgical management. Repeat imaging 1 year after presentation showed increase in the size of the pulmonary mass (7.2 × 5.3 × 4.2 cm), and bone scan revealed metastasis in the right humerus (mid-shaft), left 10th rib, and left proximal femur (Fig. 1C, D). Pleural effusion or mediastinal lymph node metastasis (endobronchial ultrasound-guided transbronchial needle aspiration showed reactive lymphoid hyperplasia) was absent. Despite new bony metastases, because of the young age of the patient, surgical intervention was carried out as planned by means of video-assisted thoracoscopy-assisted right lower lobectomy.
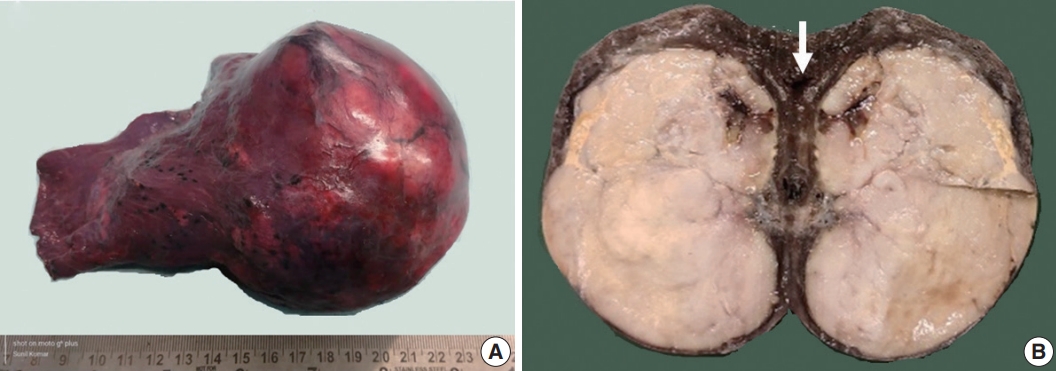
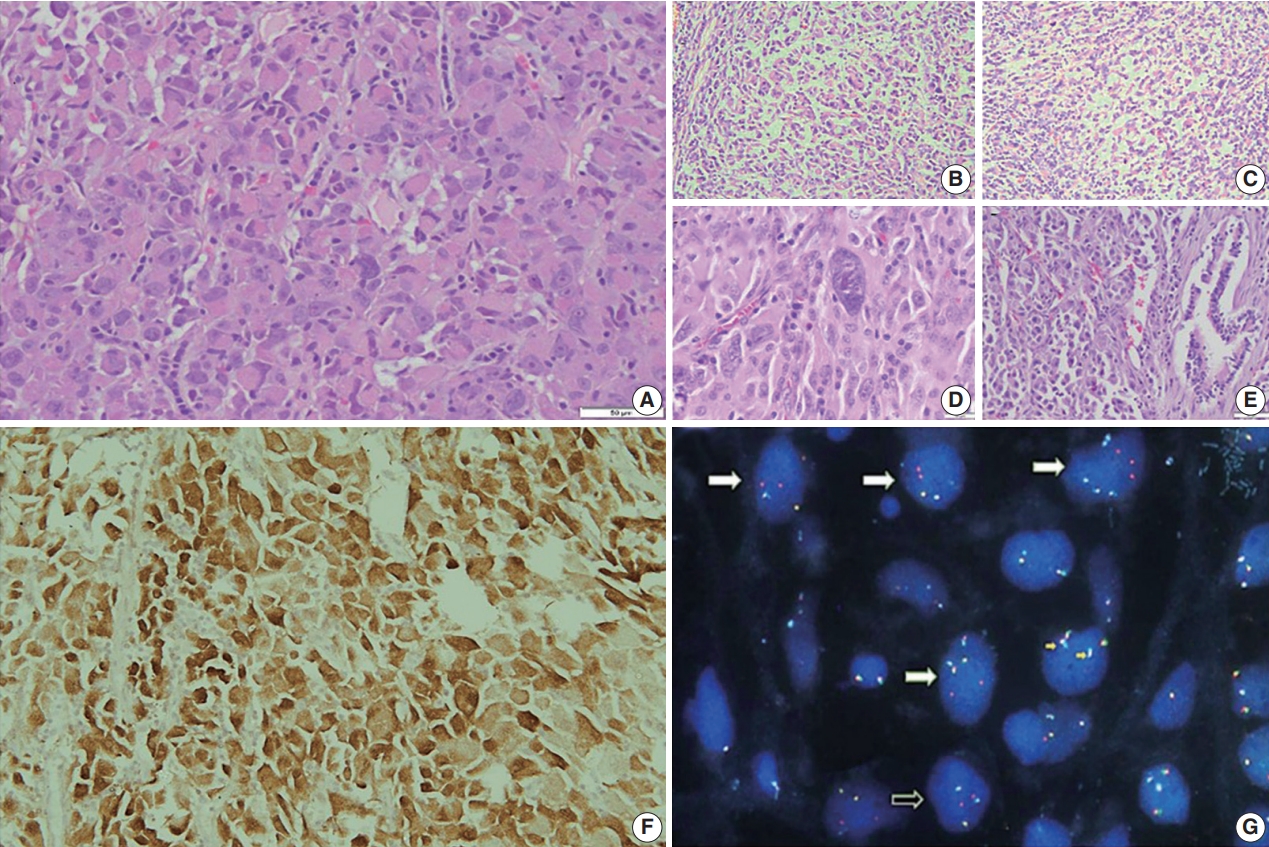
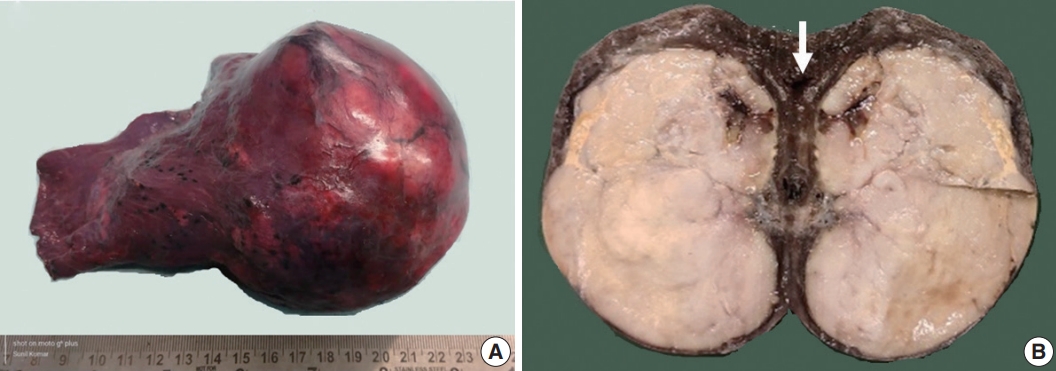
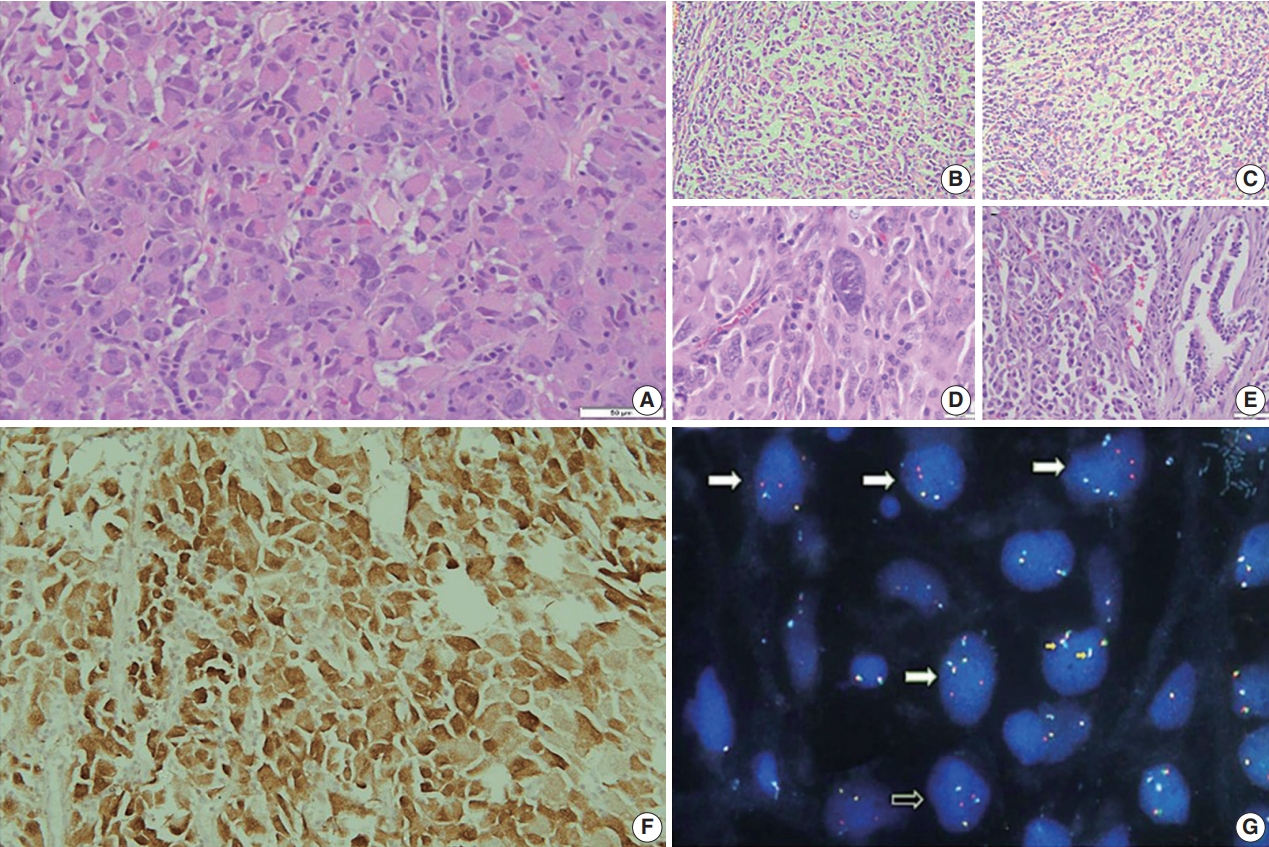
- Grossly, a well circumscribed, soft, fleshy tumor was identified in the lung parenchyma with focal areas of haemorrhage and necrosis (Fig. 2). Microscopy (Fig. 3) revealed a homogeneous tumor composed of ovoid to polygonal cells arranged in fascicles and sheets. The cells showed moderate to abundant eosinophilic cytoplasm with markedly pleomorphic nuclei, prominent nucleoli, and fine granular cytoplasm showing frequent mitoses (8 per 2 mm2). Necrosis was present that had not been seen in the biopsy. Focal areas of myxoid change and lymphoplasmacytic infiltrate were noted. The immuno-profile of the tumor was similar to that of the biopsy except for focal cytokeratin expression in the resection, while histomorphology of the tumor was similar to that of the biopsy. The Ki-67 labeling index was ~30%–35% in the highest labeled areas. There was diffuse strong cytoplasmic staining for ALK protein. Fluorescence in situ hybridization assay using the FlexISH ALK/ROS1 DistinguISH Probe (ZytoVision, GmbH, Bremerhaven, Germany) showed one or more isolated 3' signals (extra orange signals) of the ALK gene without corresponding 5' signals (green signals) in the majority of tumor cells, indicative of ALK gene rearrangements [10], confirming the diagnosis of primary pulmonary EIMS. The ROS1 gene represented by fused aqua-green-orange signals was intact (Fig. 3). The postoperative period was unremarkable, and the patient is currently asymptomatic 4-month post-surgery. He is continuing on crizotinib and is planned for palliative radiotherapy for the bony metastases.
CASE REPORT
- Primary pulmonary EIMS is extremely rare, with only three cases reported previously in the English literature to the best of our knowledge (Table 1) [7-9]. Including the present case, primary pulmonary EIMS occurs across a wide age range (21–71 years at diagnosis) with a male preponderance. The tumor presents as a solitary parenchymal mass or as pleural mass with a relatively short symptom duration (< 6 months) and is not uncommonly metastatic at presentation, with brain and skeleton appearing to be the preferred sites of metastases (Table 1).
- Diagnosis of EIMS is challenging. The poorly differentiated epithelioid morphology can mimic anaplastic large cell lymphoma (ALCL), malignant melanoma, alveolar rhabdomyosarcoma, alveolar soft part sarcoma, epithelioid leiomyosarcoma, undifferentiated sarcoma, malignant mesothelioma, poorly differentiated carcinoma, and pulmonary pleomorphic carcinoma (containing a minimum 10% spindle and/or giant cells) and needs to be differentiated by relevant immunohistochemical markers (Table 2). Conventional IMTs are more common in the lung but are differentiated from EIMS by their bland spindle morphology, prominent lymphoplasmacytic cellular infiltrate, and absence of mitoses and pleomorphism. Rare cases of otherwise conventional IMTs that show increased mitotic activity, necrosis, and aggressive biological behavior are reported [11,12]; these should not be designated as EIMS in the absence of distinctive morphology of round to epithelioid cells, myxoid stroma, and mixed inflammatory infiltrate, as was observed in the present case. ALK protein expression is essential for diagnosis but is not specific as many other lung tumors express ALK protein due to underlying ALK gene rearrangements, as in ALCL, conventional IMTs, and non-small cell lung carcinoma (NSCLC), or to ALK copy number alterations as in rhabdomyosarcomas [13]. ALK-rearranged lung adenocarcinoma/NSCLC shows a variety of histological characteristics ranging from cribriform pattern, mucincontaining cells, presence of psammoma bodies, and solid signetring cells. None of the histological parameters are specific for a particular genotype [14-19]. Fluorescence in situ hybridization using break-apart probes is useful to confirm ALK gene rearrangements; however, polymerase chain reaction and/or sequencing of RNA transcripts is required for delineating ALK fusion partners. ALK protein expression in a nuclear membranous or perinuclear cytoplasmic pattern gives a clue to the presence of underlying RANBP2 or RRBP1 fusion partners with ALK, which are the most common fusions in EIMS; however, these fusions can also be observed in IMTs and ALCLs [20]. On the other hand, other fusion partners including EML4, the most common fusion partner in ALK-rearranged NSCLC, have also been reported in EIMS associated with the more common cytoplasmic ALK staining pattern [26]. The various ALK gene rearrangement seen in NSCLC are EML4, TFG, KIF5B, KCL1, and multiple EML4 isoforms [27].
- Thus, due to considerable genetic overlap with other ALKomas, EIMS is essentially a histopathological diagnosis, and definitive diagnosis can be difficult on a small biopsy, even with ancillary testing.
- EIMS is an aggressive tumor with poor prognosis and local recurrence [1,3]. Review of previous reported pulmonary EIMS showed that surgical resection remained the mainstay of treatment when feasible (Table 1). These tumors do not respond to chemotherapy or radiotherapy [28,29]. Most ALK fusion proteins including those resulting from RANBP2-ALK fusions are sensitive to ALK tyrosine kinase inhibitor (TKI) inhibition in pre-clinical models [30], suggesting a role for targeted therapy. From the limited data available, EIMS does appear to respond to the ALK TKI crizotinib, as in our patient who experienced complete resolution of liver metastases. However, long-term responses or remissions are rare, with most EIMS patients progressing with metastatic disease within 3 to 6 months of crizotinib monotherapy [7-9], likely due to secondary resistance mechanisms [10]. A recent study demonstrated prolonged survival with a combination of ALK and CD30 targeted therapies [31]; however, CD30 expression appears to be uncommon in pulmonary EIMS (Table 1). EIMS is a very rare subtype of IMT that presents as a primary lung mass in young non-smokers, posing a considerable diagnostic challenge due to aggressive behavior with frequent metastases. There are limited data on treatment protocols; in the present case, targeted ALK inhibitor therapy combined with surgery achieved considerable disease control.
DISCUSSION
Ethics Statement
This study was approved by the Institutional Review Board with a waiver of informed consent (IRB No IEC-404/02.09.2016) and performed in accordance with the principles of the Declaration of Helsinki.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
Author contributions
Conceptualization: PS. Data curation: PS. Formal analysis: PS. Supervision: DJ. Visualization: DJ. Writing—original draft: PS. Writing—review & editing: PS, AN, MKG, RR, SK, PSM, DJ. Approval of final manuscript: all authors.
Conflicts of Interest
D.J., a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
No funding to declare.
| No. | Study | Age (yr)/Sex | Anatomic site | Size (cm) | Clinical Presentation | Metastasis at presentation | Immunoprofile | Molecular confirmation (method) | Primary treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Fu et al. [7] | 21/M | Left lower lobe lung | 10 | Weight loss, fatigue | None | Desmin+, ALK (c), CD30– | ALK rearrangement (FISH) | Lobectomy | Bone metastases (pelvis, vertebrae) and underwent laminectomy; death 4 mo after resection |
| 2 | Sarmiento et al. [8] | 71/M | Pleura based, left lower lobe | 12.5 | Dyspnoea, pleural effusion | None | NA | ALK rearrangement (FISH) | Lobectomy and adjuvant crizotinib | Progressed on crizotinib after 2 mo; near complete response to second line ALK inhibitor; remission after 1 yr at last follow-up |
| 3 | Kozu et al. [9] | 57/M | Pleural cavity/chest wall | NA | Pleural effusion and dyspnoea | N/A | Desmin+, cytokeratin+, ALK+ (c), CD30– | RANBP2-ALK fusion (PCR) | Crizotinib | NA |
| 4 | Present case | 25/M | Lung | 7 | Cough, loss of weight, fever, dyspnoea | Liver (solitary) | Desmin+, focal cytokeratin+, ALK+ (c), CD30– | ALK rearrangement (FISH) | Crizotinib | Complete resolution of liver metastases and stable pulmonary disease for 6 mo; bony metastases developed at 10 mo; lobectomy done and stable metastatic disease on crizotinib for 3 mo |
| Right lower lobe |
| Entity | Incidence as lung mass | Epithelial markers | Mesenchymal markers | Other markers | ALK protein expression | Commonest ALK fusions |
|---|---|---|---|---|---|---|
| IMT [2] | Common | Pan CK+/– | SMA+, Desmin+ | CD30– | Cytoplasmic (50%–60%) | TPM3-ALK, TPM4-ALK |
| ALCL [21] | Uncommon | EMA+ | SMA+, Desmin– | CD30+ | Nuclear and cytoplasmic | NPM-ALK |
| ALK+LBL [21] | Rare | EMA+ | – | CD138+, CD38+, Mum1/IRF1+, IgA+, Bob1+ | Cytoplasmic, nuclear and nucleolar | CLTC-ALK |
| ALK-SEC31A | ||||||
| ALK-rearranged NSCLC | Common | CK+ | – | Cytoplasmic | EML4-ALK [14] | |
| Melanoma | Extremely rare | CK– | – | S-100+, Melan A+, HMB45+, SOX10+ | ALKATI (seen in cutaneous melanoma) [22] | EML4-ALK [23] |
| Epithelioid mesothelioma [2] | Uncommon | CK5/6+/–, EMA+/– | Desmin– | Calretinin+/–, WT+, D2-40+ | – | – |
| Rhabdomyosarcoma | Rare | CK/EMA– | Desmin+, Actin+ | MyoD1+, Myogenin+ CD30– | Cytoplasmic | NPM-ALK [24], EML4-ALK [25] |
| Epithelioid leiomyosarcoma [1] | Rare | EMA+ | SMA+, Desmin+, h-caldesmon+ | CD34+/–CK+/–, EMA+/– | – | – |
| EIMS [2] | Rare | CK or EMA–/+ | Desmin+, SMA+ | CD30+ | Nuclear membrane or cytoplasmic with perinuclear accentuation | ALK-RRBP1 [3-5], ALK-RANBP2 |
| Pleomorphic carcinoma [2] | < 1% | NSCC component CK+, TTF-1 +, EMA+ | Vimentin+ | Surfactant protein A+, p53+ | Rare | – |
ALK, anaplastic lymphoma kinase; ALK ATI, anaplastic lymphoma kinase with alternative transcription initiation; IMT, inflammatory myofibroblastic tumor; CK/PanCK, cytokeratin; SMA, smooth muscle actin; TPM, tropomyosin; ALCL, anaplastic large cell lymphoma; EMA, epithelial membrane antigen; NPM, nucleophosmin; Mum1/IRF1, multiple myeloma 1/interferon regulatory factor 4 protein; CLTC, clathrin heavy chain; NSCLC, non–small cell lung carcinoma; EML4, echinoderm microtubule-associated protein-like 4; HMB45, human melanoma black 45; EIMS, epithelioid Inflammatory myofibroblastic sarcoma; RRBP1, ribosome binding protein 1; RANBP, Ran-binding protein; NSCC, non-small cell lung cancer; TTF-1, thyroid transcription factor 1.
- 1. Yamamoto H. Inflammatory myofibroblastic tumor. WHO Classification of Tumours Editorial Board. WHO classification of tumors: soft tissue and bone tumours. Lyon: IARC Press, 2020; 109-11.
- 2. Tavora F, Glass C, Hornick JL, Jain D, Sheppared MN, Yi ES. Inflammatory myofibroblastic tumor. WHO Classification of Tumours Editorial Board. WHO classification of tumors: thoracic tumours. Lyon: IARC Press, 2021; 288-9.
- 3. Marino-Enriquez A, Wang WL, Roy A, et al. Epithelioid inflammatory myofibroblastic sarcoma: an aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol 2011; 35: 135-44. PubMed
- 4. Xu P, Shen P, Jin Y, Wang L, Wu W. Epithelioid inflammatory myofibroblastic sarcoma of stomach: diagnostic pitfalls and clinical characteristics. Int J Clin Exp Pathol 2019; 12: 1738-44. PubMedPMC
- 5. Lee JC, Li CF, Huang HY, et al. ALK oncoproteins in atypical inflammatory myofibroblastic tumours: novel RRBP1-ALK fusions in epithelioid inflammatory myofibroblastic sarcoma. J Pathol 2017; 241: 316-23. ArticlePubMedPMCPDF
- 6. Garg R, Kaul S, Arora D, Kashyap V. Posttransplant epithelioid inflammatory myofibroblastic sarcoma: a case report. Indian J Pathol Microbiol 2019; 62: 303-5. ArticlePubMed
- 7. Fu X, Jiang J, Tian XY, Li Z. Pulmonary epithelioid inflammatory myofibroblastic sarcoma with multiple bone metastases: case report and review of literature. Diagn Pathol 2015; 10: 106.ArticlePubMedPMCPDF
- 8. Sarmiento DE, Clevenger JA, Masters GA, Bauer TL, Nam BT. Epithelioid inflammatory myofibroblastic sarcoma: a case report. J Thorac Dis 2015; 7: E513-6. PubMedPMC
- 9. Kozu Y, Isaka M, Ohde Y, Takeuchi K, Nakajima T. Epithelioid inflammatory myofibroblastic sarcoma arising in the pleural cavity. Gen Thorac Cardiovasc Surg 2014; 62: 191-4. ArticlePubMedPDF
- 10. Yoshida A, Bubendorf L, Varella-Garcia M. ALK testing with FISH. In: Tsao MS, Hirsch FR, Yatabe Y, eds. IASLC atlas of ALK and ROS1 testing in lung cancer. 2nd ed. North Fort Myers: Editorial Rx Press, 2016; 41-52.
- 11. Parker BM, Parker JV, Lymperopoulos A, Konda V. A case report: pharmacology and resistance patterns of three generations of ALK inhibitors in metastatic inflammatory myofibroblastic sarcoma. J Oncol Pharm Pract 2019; 25: 1226-30. ArticlePubMedPDF
- 12. Rush NI, Sinnott M. Malignant inflammatory myofibroblastic tumor of the lung with IgG4-positive plasma cells. Ann Clin Pathol 2016; 4: 1069.
- 13. Du X, Shao Y, Qin HF, Tai YH, Gao HJ. ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac Cancer 2018; 9: 423-30. ArticlePubMedPMCPDF
- 14. Kim H, Jang SJ, Chung DH, et al. A comprehensive comparative analysis of the histomorphological features of ALK-rearranged lung adenocarcinoma based on driver oncogene mutations: frequent expression of epithelial-mesenchymal transition markers than other genotype. PLoS One 2013; 8: e76999.ArticlePubMedPMC
- 15. Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 2009; 22: 508-15. ArticlePubMedPDF
- 16. Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 2009; 15: 5216-23. ArticlePubMedPMCPDF
- 17. Yoshida A, Tsuta K, Watanabe S, et al. Frequent ALK rearrangement and TTF-1/p63 co-expression in lung adenocarcinoma with signet-ring cell component. Lung Cancer 2011; 72: 309-15. ArticlePubMed
- 18. Nishino M, Klepeis VE, Yeap BY, et al. Histologic and cytomorphologic features of ALK-rearranged lung adenocarcinomas. Mod Pathol 2012; 25: 1462-72. ArticlePubMedPDF
- 19. Popat S, Gonzalez D, Min T, et al. ALK translocation is associated with ALK immunoreactivity and extensive signet-ring morphology in primary lung adenocarcinoma. Lung Cancer 2012; 75: 300-5. ArticlePubMed
- 20. Cook JR, Dehner LP, Collins MH, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol 2001; 25: 1364-71. PubMed
- 21. Campo E, Gascoyne RD. ALK-positive large B-cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon: IARC Press, 2017; 319-20.
- 22. Busam KJ, Vilain RE, Lum T, et al. Primary and metastatic cutaneous melanomas express ALK through alternative transcriptional initiation. Am J Surg Pathol 2016; 40: 786-95. ArticlePubMedPMC
- 23. Couts KL, Bemis J, Turner JA, et al. ALK inhibitor response in melanomas expressing EML4-ALK fusions and alternate ALK isoforms. Mol Cancer Ther 2018; 17: 222-31. ArticlePubMedPMCPDF
- 24. Cessna MH, Zhou H, Sanger WG, et al. Expression of ALK1 and p80 in inflammatory myofibroblastic tumor and its mesenchymal mimics: a study of 135 cases. Mod Pathol 2002; 15: 931-8. ArticlePubMed
- 25. Gasparini P, Casanova M, Villa R, et al. Anaplastic lymphoma kinase aberrations correlate with metastatic features in pediatric rhabdomyosarcoma. Oncotarget 2016; 7: 58903-14. ArticlePubMedPMC
- 26. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007; 448: 561-6. ArticlePubMedPDF
- 27. Kim H, Chung JH. Overview of clinicopathologic features of ALKrearranged lung adenocarcinoma and current diagnostic testing for ALK rearrangement. Transl Lung Cancer Res 2015; 4: 149-55. PubMedPMC
- 28. Fabre D, Fadel E, Singhal S, et al. Complete resection of pulmonary inflammatory pseudotumors has excellent long-term prognosis. J Thorac Cardiovasc Surg 2009; 137: 435-40. ArticlePubMed
- 29. Sakurai H, Hasegawa T, Watanabe S, Suzuki K, Asamura H, Tsuchiya R. Inflammatory myofibroblastic tumor of the lung. Eur J Cardiothorac Surg 2004; 25: 155-9. ArticlePubMed
- 30. Childress MA, Himmelberg SM, Chen H, Deng W, Davies MA, Lovly CM. ALK fusion partners impact response to ALK inhibition: differential effects on sensitivity, cellular phenotypes, and biochemical properties. Mol Cancer Res 2018; 16: 1724-36. ArticlePubMedPMCPDF
- 31. Fordham AM, Xie J, Gifford AJ, et al. CD30 and ALK combination therapy has high therapeutic potency in RANBP2-ALK-rearranged epithelioid inflammatory myofibroblastic sarcoma. Br J Cancer 2020; 123: 1101-13. ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

- Inflammatory Myofibroblastic Tumor: An Updated Review
Joon Hyuk Choi
Cancers.2025; 17(8): 1327. CrossRef - Epithelioid Inflammatory Myofibroblastic Sarcoma: Case Series With a First Report of CLTC::ALK Fusion in an Aggressive Disease
Daisy Maharjan, Carina Dehner, Ali Alani, Robert Bell, Sheila Segura
Genes, Chromosomes and Cancer.2025;[Epub] CrossRef - ALK rearranged malignant mesenchymal neoplasms of thorax: therapeutically targetable ‘ALKomas’ beyond the spectrum of non-small cell lung carcinomas and thoracic inflammatory myofibroblastic tumors
Shreya Sadhu, Adarsh Barwad, Asit Ranjan Mridha, Prabhat Singh Malik, Aruna Nambirajan, Deepali Jain
Virchows Archiv.2025; 487(5): 1003. CrossRef - Mediastinal epithelioid inflammatory myofibroblastic sarcoma with the EML4‐ALK fusion: A case report and literature review
Tingyu Pan, Xinyu Sun, Xiao Wu, Futing Tang, Xianmei Zhou, Qian Wang, Shi Chen
Respirology Case Reports.2024;[Epub] CrossRef - Primary epithelioid inflammatory myofibroblastic sarcoma of the brain with EML4::ALK fusion mimicking intra-axial glioma: a case report and brief literature review
Eric Eunshik Kim, Chul-Kee Park, Koung Mi Kang, Yoonjin Kwak, Sung-Hye Park, Jae-Kyung Won
Journal of Pathology and Translational Medicine.2024; 58(3): 141. CrossRef - Epithelioid Inflammatory Myofibroblastic Sarcoma: A Report of a Rare Case
Varun Ronanki, Vaddatti Tejeswini, Inuganti Venkata Renuka, Shaik Raheema, Bakkamanthala S K Kanth
Cureus.2024;[Epub] CrossRef - Thoracic epithelioid inflammatory myofibroblastic sarcoma: a rare and aggressive disease with case report and literature review
Linke Yang, Pei Li, Runze Liu, Baomin Feng, Huiqing Mao, Xiaoyong Tang, Guangjian Yang
Discover Oncology.2024;[Epub] CrossRef - Epithelioid inflammatory myofibroblastic sarcoma with exceptionally long response to lorlatinib—a case report
Rafał Becht, Kajetan Kiełbowski, Justyna Żychowska, Wojciech Poncyljusz, Aleksandra Łanocha, Katarzyna Kozak, Ewa Gabrysz-Trybek, Paweł Domagała
Therapeutic Advances in Medical Oncology.2024;[Epub] CrossRef - Rare giant epithelioid inflammatory myofibroblastic sarcoma of the abdominal cavity in a child: a case report and review of the literature
Jinzhou Li, Haixing Su, Sheng Zhang, Xianyun Chen, Chongzhi Hou, Tao Cheng
Frontiers in Oncology.2024;[Epub] CrossRef - Case report: Epithelioid inflammatory myofibroblastic sarcoma treated with an ALK TKI ensartinib
Mengmeng Li, Ruyue Xing, Jiuyan Huang, Chao Shi, Chunhua Wei, Huijuan Wang
Frontiers in Oncology.2023;[Epub] CrossRef - Epithelioid Inflammatory Myofibroblastic Sarcoma With Poor Response to Crizotinib: A Case Report
Soheila Aminimoghaddam, Roghayeh Pourali
Clinical Medicine Insights: Case Reports.2023;[Epub] CrossRef - Epithelioid inflammatory myofibroblastic sarcoma: a case report and brief literature review
Weidong Dou, Yu Guan, Tao Liu, Hang Zheng, Shuo Feng, Yingchao Wu, Xin Wang, Zhanbing Liu
Frontiers in Oncology.2023;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1.
Fig. 2.
Fig. 3.
| No. | Study | Age (yr)/Sex | Anatomic site | Size (cm) | Clinical Presentation | Metastasis at presentation | Immunoprofile | Molecular confirmation (method) | Primary treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Fu et al. [7] | 21/M | Left lower lobe lung | 10 | Weight loss, fatigue | None | Desmin+, ALK (c), CD30– | ALK rearrangement (FISH) | Lobectomy | Bone metastases (pelvis, vertebrae) and underwent laminectomy; death 4 mo after resection |
| 2 | Sarmiento et al. [8] | 71/M | Pleura based, left lower lobe | 12.5 | Dyspnoea, pleural effusion | None | NA | ALK rearrangement (FISH) | Lobectomy and adjuvant crizotinib | Progressed on crizotinib after 2 mo; near complete response to second line ALK inhibitor; remission after 1 yr at last follow-up |
| 3 | Kozu et al. [9] | 57/M | Pleural cavity/chest wall | NA | Pleural effusion and dyspnoea | N/A | Desmin+, cytokeratin+, ALK+ (c), CD30– | RANBP2-ALK fusion (PCR) | Crizotinib | NA |
| 4 | Present case | 25/M | Lung | 7 | Cough, loss of weight, fever, dyspnoea | Liver (solitary) | Desmin+, focal cytokeratin+, ALK+ (c), CD30– | ALK rearrangement (FISH) | Crizotinib | Complete resolution of liver metastases and stable pulmonary disease for 6 mo; bony metastases developed at 10 mo; lobectomy done and stable metastatic disease on crizotinib for 3 mo |
| Right lower lobe |
| Entity | Incidence as lung mass | Epithelial markers | Mesenchymal markers | Other markers | ALK protein expression | Commonest ALK fusions |
|---|---|---|---|---|---|---|
| IMT [2] | Common | Pan CK+/– | SMA+, Desmin+ | CD30– | Cytoplasmic (50%–60%) | TPM3-ALK, TPM4-ALK |
| ALCL [21] | Uncommon | EMA+ | SMA+, Desmin– | CD30+ | Nuclear and cytoplasmic | NPM-ALK |
| ALK+LBL [21] | Rare | EMA+ | – | CD138+, CD38+, Mum1/IRF1+, IgA+, Bob1+ | Cytoplasmic, nuclear and nucleolar | CLTC-ALK |
| ALK-SEC31A | ||||||
| ALK-rearranged NSCLC | Common | CK+ | – | Cytoplasmic | EML4-ALK [14] | |
| Melanoma | Extremely rare | CK– | – | S-100+, Melan A+, HMB45+, SOX10+ | ALKATI (seen in cutaneous melanoma) [22] | EML4-ALK [23] |
| Epithelioid mesothelioma [2] | Uncommon | CK5/6+/–, EMA+/– | Desmin– | Calretinin+/–, WT+, D2-40+ | – | – |
| Rhabdomyosarcoma | Rare | CK/EMA– | Desmin+, Actin+ | MyoD1+, Myogenin+ CD30– | Cytoplasmic | NPM-ALK [24], EML4-ALK [25] |
| Epithelioid leiomyosarcoma [1] | Rare | EMA+ | SMA+, Desmin+, h-caldesmon+ | CD34+/–CK+/–, EMA+/– | – | – |
| EIMS [2] | Rare | CK or EMA–/+ | Desmin+, SMA+ | CD30+ | Nuclear membrane or cytoplasmic with perinuclear accentuation | ALK-RRBP1 [3-5], ALK-RANBP2 |
| Pleomorphic carcinoma [2] | < 1% | NSCC component CK+, TTF-1 +, EMA+ | Vimentin+ | Surfactant protein A+, p53+ | Rare | – |
ALK, anaplastic lymphoma kinase; (c), cytoplasmic; FISH, fluorescent in situ hybridization; NA, not available; RANBP2, Ran specific binding protein 2; PCR, polymerase chain reaction.
ALK, anaplastic lymphoma kinase; ALK ATI, anaplastic lymphoma kinase with alternative transcription initiation; IMT, inflammatory myofibroblastic tumor; CK/PanCK, cytokeratin; SMA, smooth muscle actin; TPM, tropomyosin; ALCL, anaplastic large cell lymphoma; EMA, epithelial membrane antigen; NPM, nucleophosmin; Mum1/IRF1, multiple myeloma 1/interferon regulatory factor 4 protein; CLTC, clathrin heavy chain; NSCLC, non–small cell lung carcinoma; EML4, echinoderm microtubule-associated protein-like 4; HMB45, human melanoma black 45; EIMS, epithelioid Inflammatory myofibroblastic sarcoma;

 E-submission
E-submission