Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(6); 2022 > Article
-
Original Article
Diagnostic distribution and pitfalls of glandular abnormalities in cervical cytology: a 25-year single-center study -
Jung-A Sung1
 , Ilias P. Nikas2
, Ilias P. Nikas2 , Haeryoung Kim1
, Haeryoung Kim1 , Han Suk Ryu1,3
, Han Suk Ryu1,3 , Cheol Lee,1
, Cheol Lee,1
-
Journal of Pathology and Translational Medicine 2022;56(6):354-360.
DOI: https://doi.org/10.4132/jptm.2022.09.05
Published online: November 9, 2022
1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
2School of Medicine, European University Cyprus, Nicosia, Cyprus
3Center for Medical Innovation, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea
- Corresponding Author: Cheol Lee, MD, PhD, Department of Pathology, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-2072-4919, Fax: +82-2-743-5530, E-mail: fe98134@snu.ac.kr
© 2022 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Detection of glandular abnormalities in Papanicolaou (Pap) tests is challenging. This study aimed to review our institute’s experience interpreting such abnormalities, assess cytohistologic concordance, and identify cytomorphologic features associated with malignancy in follow-up histology.
-
Methods
- Patients with cytologically-detected glandular lesions identified in our pathology records from 1995 to 2020 were included in this study.
-
Results
- Of the 683,197 Pap tests performed, 985 (0.144%) exhibited glandular abnormalities, 657 of which had tissue follow-up available. One hundred eighty-eight cases were cytologically interpreted as adenocarcinoma and histologically diagnosed as malignant tumors of various origins. There were 213 cases reported as atypical glandular cells (AGC) and nine cases as adenocarcinoma in cytology, yet they were found to be benign in follow-up histology. In addition, 48 cases diagnosed with AGC and six with adenocarcinoma cytology were found to have cervical squamous lesions in follow-up histology, including four squamous cell carcinomas. Among the cytomorphological features examined, nuclear membrane irregularity, three-dimensional clusters, single-cell pattern, and presence of mitoses were associated with malignant histology in follow-up.
-
Conclusions
- This study showed our institute’s experience detecting glandular abnormalities in cervical cytology over a 25-year period, revealing the difficulty of this task. Nonetheless, the present study indicates that several cytological findings such as membrane irregularity, three-dimensional clusters, single-cell pattern, and evidence of proliferation could help distinguishing malignancy from a benign lesion.
- In this retrospective study, the records of Seoul National University Hospital were searched within the period from January 1995 to December 2020 to identify all reported cervical cytology cases with glandular abnormalities. These cases were prepared either as LBC slides or conventional smears. Among the 683,197 Pap tests over this 25-year period, 985 cases from 923 patients were reported with a glandular abnormality, while the 657 of these with available surgical pathology follow-up were included in the study. Of the 985 Pap tests, 322 (32.7%) were prepared as conventional smears, whereas 663 (67.3%) as LBC. The cytologic interpretations regarding the glandular abnormalities were made by board-certified cytopathologists of our laboratory, based on the following categories of the Bethesda system [5,26]: atypical glandular cells not otherwise specified (AGC-NOS), atypical endocervical cells (AGC-EC), atypical endometrial cells (AGC-EM), atypical glandular cells favor neoplastic (AGC-FN), and ADC. Several Pap tests that were diagnosed as atypical glandular cells of undetermined significance, favor reactive based on the Bethesda system 1991, were considered AGC-NOS lesions in 2014 Bethesda system. Surgical pathology follow-up included any of the following samples: cervical biopsies, endocervical curettages, loop electrosurgical excision procedure or conizations, endometrial curettages, and hysterectomies.
- A few cytomorphologic features of selected Pap test cases exhibiting glandular abnormalities and cytohistologic discrepant findings were also compared between the cases subsequently found to be histologically benign and malignant, to identify cytologic criteria associated with malignancy. These features included architectural characteristics (overlapping; presence of single cells; and presence of three-dimensional clusters), nuclear enlargement, irregular nuclear membranes, increased nuclear-to-cytoplasm (N/C) ratio, nuclear hyperchromasia, prominent nucleoli, and mitotic activity. The Fisher exact test was used to identify the importance of cytomorphologic features in the diagnosis of malignancies. A p-value of <.05 was considered statistically significant.
MATERIALS AND METHODS
- Distribution of glandular abnormalities in cervical cytology
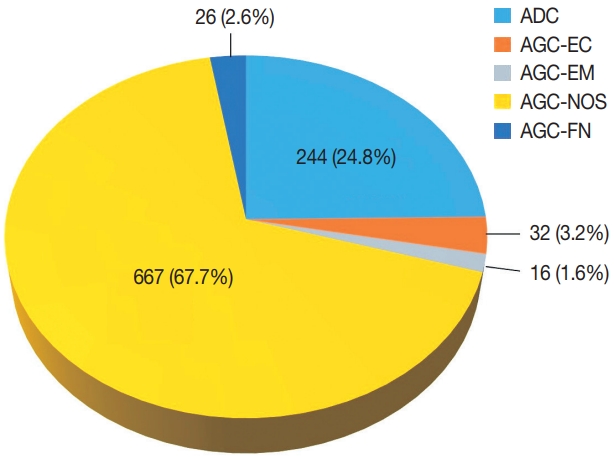
- Among 683,197 Pap tests interpreted from January 1995 to December 2020 in our center, the number of cases with glandular abnormalities was 985 (0.144%), of which 244 (24.8%) were diagnosed as ADC. In the cytological evaluation of the 741 cases with AGC, the distribution of AGC subcategories according to the Bethesda system was as follows: AGC-NOS 667 (67.7%), AGC-EC 32 (3.2%), AGC-EM 16 (1.6%), and AGC-FN 26 (2.6%) (Fig. 1). The mean age of the patients was 49 years (range, 14 to 86 years).
- Assessment of cytohistologic concordance in our cohort
- Of the 985 Pap tests interpreted with glandular abnormalities, histologic follow-up was available for 657 cases (66.7%). Among them, 409 cases (62.3%) were interpreted as AGC-NOS, 206 (31.4%) as ADC, 21 (3.2%) as AGC-FN, 12 (1.8%) as AGC-EC, and nine (1.4%) as AGC-EM. The cytohistologic concordance was summarized by referring to the Quality Improvement program recommended by the Korean Society for Cytopathology [27].
- Table 1 shows the cytohistopathological correlations of the patients according to subclassification of glandular lesions. One hundred eighty-eight cases were cytologically interpreted as ADC and histologically diagnosed as malignant tumors, including the cervix, endometrium, ovary, and other organ origins. Among the cases with AGC Pap test interpretation, 213 were found to be benign in follow-up histology, while most of the others were diagnosed as malignant lesions. In addition, there were 48 cases diagnosed with an AGC and six cases with ADC cytologically, where a cervical squamous lesion was detected in their paired histology, including four squamous cell carcinomas. There was also a reported coexistence of glandular and squamous abnormalities in 50 Pap tests (AGC-NOS, 44; AGC-EM, 1; AGC-FN, 1; ADC, 4); these cases were solely categorized based on their glandular component. Notably, nine cases with an ADC cytologic interpretation were histologically diagnosed as benign.
- Cytomorphologic features of AGC associated with malignancy in follow-up histology
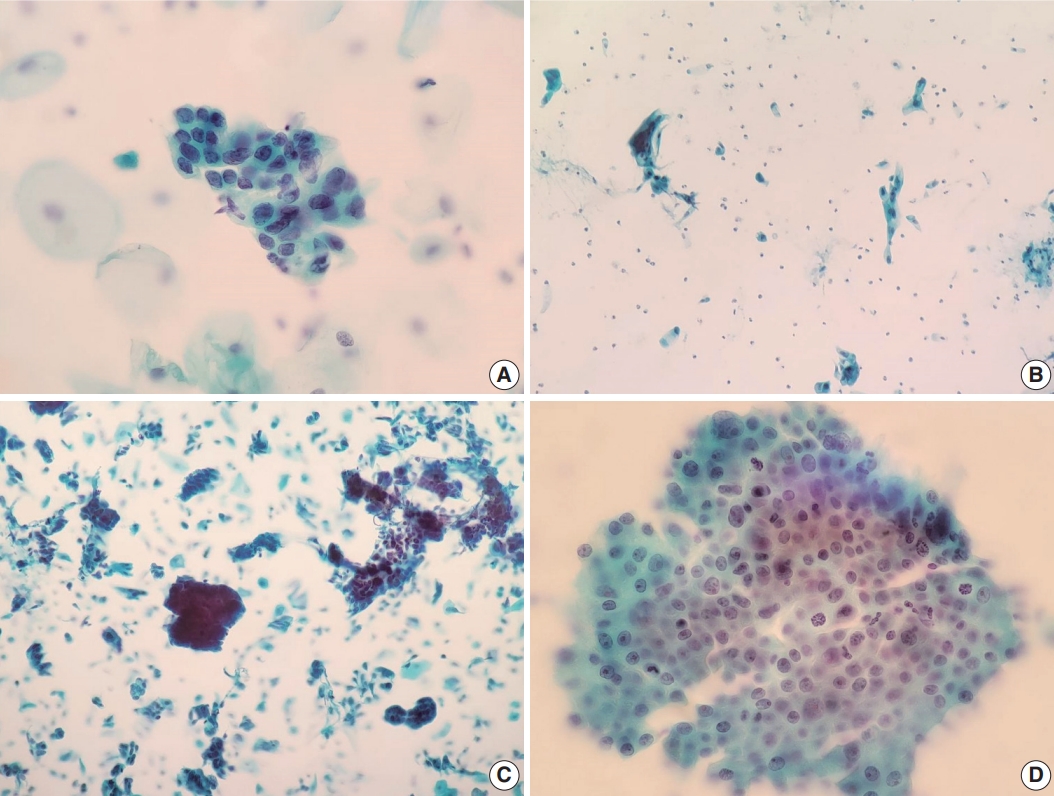
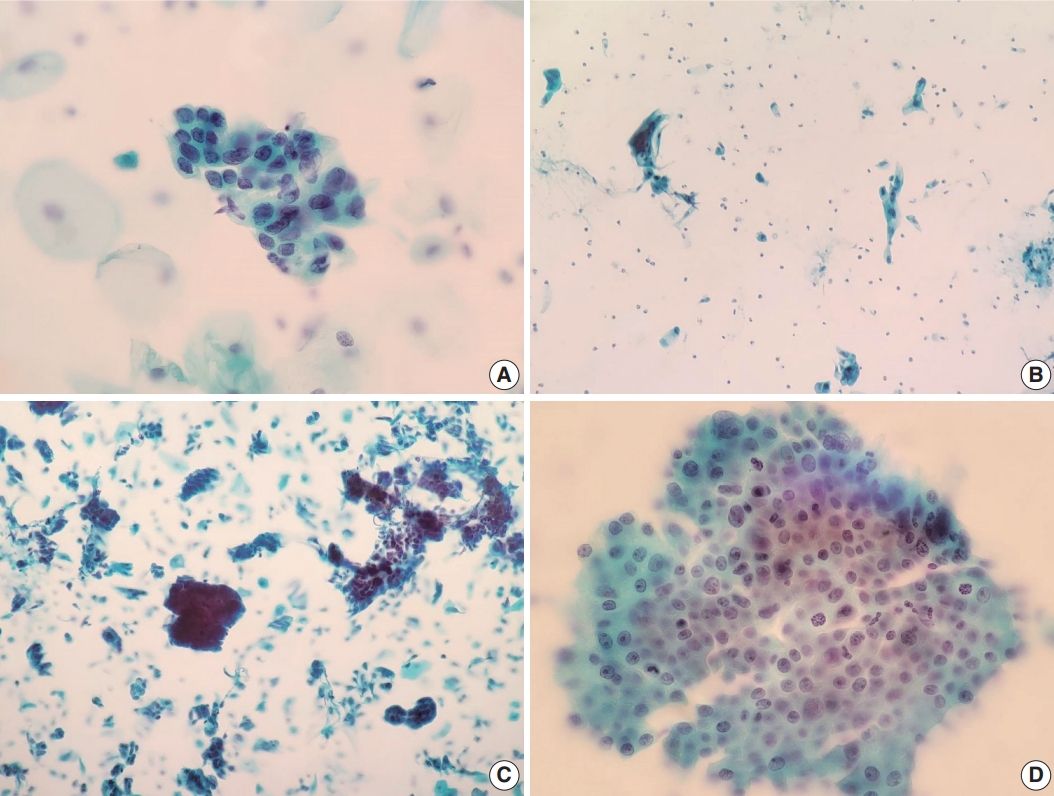
- We also reviewed a few cytomorphologic features in some of our cytology AGC cases exhibiting cytohistologic discordance. Seventy-four AGC cases with available slides in our records were selected. Fifty-one cases with AGC cytology diagnosed as benign, 14 as cervical ADC, and nine as cervical ADC in situ (AIS) in their follow-up histology were included. Histologically, cases diagnosed with diseases other than cervical ADC or AIS were excluded. We examined the abnormal glandular cells in these Pap tests to identify cytomorphologic features associated with subsequent malignant histology. Table 2 shows the comparison of selected features in the 74 eligible cases examined, according to their histologic follow-up. Whereas irregular nuclear membranes, three-dimensional clusters, single-cell pattern, and presence of mitoses were statistically significant (p<.05) for the presence of malignancy, increased N/C ratios, overlapping, nuclear hyperchromasia, and prominent nucleoli were not (p>.05). Fig. 2 shows some cytomorphologic features of the Pap smear samples from some AGC cases, histologically-confirmed as malignant.
RESULTS
- Although the efficacy of cervical cytology diagnosing squamous cell carcinoma and its precursors is well-established, detecting glandular abnormalities is a challenge due to their rarity, pathologists’ lack of experience with their cytomorphologic criteria, and poor interobserver reproducibility [10-12]. The aim of the present single-center study was to investigate the diagnostic distribution and assess the cytohistologic concordance of glandular abnormalities in cervical cytology, also to identify selected cytomorphologic findings associated with malignancy in follow-up surgical pathology.
- According to our findings, glandular abnormalities accounted for 0.144% of all cervical cytology cases in our center from 1995 to 2020, showing their rarity and the diagnostic challenge interpreting them. This finding is consistent with what is reported in the literature [15,28,29]. In respect of the distribution of glandular lesions, none was diagnosed as AIS in cytology. Of the 16 cases that were histologically-confirmed to be cervical AIS, 13 were interpreted as AGC-NOS and three as ADC in cytology. This indicates that identifying AIS in cytology is a rather challenging task. Previous studies have identified the pitfalls of the AIS cytologic diagnosis, also its suboptimal accuracy and low levels of interobserver agreement [30-32]. Some authors have also reported that many AIS lesions could be interpreted as AGC, AGC-FN, ADC, or squamous intraepithelial lesions in cervical cytology [32,33].
- Assessing the cytohistologic correlation of the glandular abnormalities detected in our cervical cytology cohort, we found that most Pap test cases reported to have glandular abnormalities cytologically actually had some significant lesions in their subsequent histopathology. One hundred eighty-eight out of 657 cases (28.6%) were turned out to be completely concordant, all of which were diagnosed as malignant tumors in follow-up histology (including cervical 92, endometrial, and ovarian 73). Also, there were 121 cases reported as AGC in cervical cytology, which turned out to be malignant tumors of various origins. On the other hand, 213 AGC Pap tests were histologically benign. Zhao et al. [25] analyzed 662 patients with an AGC cytologic interpretation and available tissue biopsy material and found that AGC cytology revealed cancer in 15.3% of the cases during histologic follow-up, most likely located in the cervix (8.3%), the endometrium (6.3%), and the ovaries (0.6%), respectively. In another study by Pradhan et al. [34], the histologic diagnoses of 3,709 AGC cases consisted of: negative (70.5%), cervical intraepithelial neoplasia/low grade squamous intraepithelial lesion (LSIL) and high grade squamous intraepithelial lesion (HSIL) (20.7%), endometrial ADC (5.5%), endocervical AIS and ADC (1.9%), and metastatic carcinomas (0.5%).
- We also reviewed 74 AGC Pap tests to identify any cytomorphologic feature differences between the cases confirmed as benign and malignant during follow-up surgical pathology. In this examination, irregular nuclear membranes, three-dimensional clusters, single-cell pattern, and presence of mitoses were found to be significantly associated with malignancy in subsequent histology. On the contrary, no significant association was identified regarding increased N/C ratio, nuclear hyperchromasia, prominent nucleoli, or overlapping. These particular findings are largely concordant with what has so far been reported the literature. Raab et al. [35] showed that the presence of single dysplastic cells, nuclear membrane irregularities, and reduced amount of cytoplasm indicated cancer or a cancer precursor in histology. In another study by Yucel Polat et al. [29], the presence of feathering, papillary pattern, polarity loss, 3D clusters, irregular nuclear membranes, and prominent nucleoli were found to be significant; in contrast, the formation of rosettes, overlapping, increased N/C ratio, and nuclear hyperchromasia were not significantly associated with cancer in follow-up histology. Torres et al. [36] showed that finding cells exhibiting high N/C ratio and dyskeratosis indicated intraepithelial neoplasia or cancer, whereas glandular lesions were composed of cells with reduced amount of cytoplasm, nuclear membrane irregularity, and macronuleoli. Lastly, Reynolds et al. [37] reported that the presence of single dysplastic cells, 3D clusters, intracytoplasmic neutrophils, increased N/C ratio and larger nuclei, nuclear border irregularity, reniform-shaped nuclei, polarity loss, overlapping, and macronucleoli were significantly associated with a clinically significant lesion (HSIL or cancer) in surgical pathology follow-up.
- Whenever possible, it is necessary to identify the cytomorphologic differences between squamous and glandular abnormalities in Pap tests, in addition to the cytologic features observed in benign glandular lesions that could mimic malignancy, to reduce potential misinterpretations. AGC is often found to be a benign lesion or a squamous intraepithelial lesion (e.g., a HSIL with endocervical gland involvement), rather than a glandular abnormality, in follow-up histology [34,38]. HSIL with endocervical gland involvement could indeed be misinterpreted as a glandular abnormality in cervical cytology. However, the loss of polarity within the hyperchromatic crowded groups, the flattening of the cells at the periphery of such groups, and the identification of single squamous dyskaryotic cells could help identify such lesions as squamous rather than glandular [26,38].
- Some cases in our cohort included histologically benign cases interpreted as ADCs in cytology. Interestingly, among the cases interpreted as AGCs or ADCs in cytology, there were a few cases that were neither ADC nor squamous cell carcinoma in histology, including a smooth muscle tumor of uncertain malignant potential, an ovarian diffuse large B-cell lymphoma, an extramammary Paget disease of vulva, a small cell carcinoma, and a poorly differentiated carcinoma of cervix. The pathologists’ lack of experience and/or the presence of ambiguous cytomorphological criteria may be responsible for their misinterpretation.
- A limitation of this study is that a few of our cases cytologically containing both glandular and squamous abnormalities (e.g., ACG-NOS and atypical squamous cells-cannot exclude HSIL (ASC-H) were solely classified based on their glandular component in this study, before assessing our cases’ distribution and cytology-histology correlation. According to the literature, glandular and squamous lesions in the cervix often coexist [39,40]. In our study, 50 of the 657 cases with histologic follow-up diagnosis were found to have both glandular and squamous abnormalities in their cytology reports. Forty-four of 409 AGC-NOS cases exhibited such coexistence, whereas 19 of them were finally diagnosed as cervical squamous neoplasias (LSIL, HSIL, or invasive squamous cell carcinoma) during surgical pathology follow-up, without any glandular lesions present. As previously mentioned, we evaluated only the glandular component for the cytohistologic correlation assessment of such cases. For example, a case interpreted as AGC-NOS and ASC-H in cytology followed by an LSIL diagnosis in histology, was regarded as a lesion with AGC-NOS only, ignoring its cytologic interpretation about squamous component. This way of classification, however, can be controversial, as it may not carry a major clinical impact when AGC patients end up having a squamous lesion.
- In conclusion, this study showed the current status of glandular abnormalities detected by cervical cytology in a single center over a 25-year-period. They revealed a few cytomorphologic features, such as nuclear membrane irregularity, three-dimensional clusters, single-cell pattern and presence of mitoses, associated with malignancy in follow-up histology. As detecting glandular lesions is rare in Pap tests and pathologists are often unfamiliar with them, we hope this study will add some value to the relevant literature.
DISCUSSION
Ethics Statement
All procedures performed in the current study were approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. H-2112-004-1277) in accordance with the 1964 Helsinki declaration and its later amendments. The committee waived the demand to obtain informed consent.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author contributions
Conceptualization: CL, HSR. Data curation: JAS. Investigation: JAS. Methodology: JAS, CL, HSR. Supervision: CL. Visualization: JAS. Writing— original draft: JAS. Writing—review & editing: CL, IPN, HK. Approval of final manuscript: all authors.
Conflicts of Interest
HK, a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
No funding to declare.
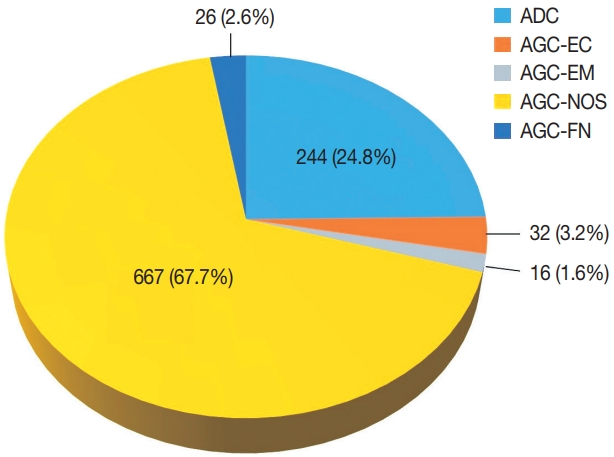
Pap, Papanicolaou; AGC-NOS, atypical glandular cells not otherwise specified; AGC-EC, atypical endocervical cells; AGC-EM, atypical endometrial cells; AGC-FN, atypical glandular cells favor neoplastic; ADC, adenocarcinoma; EM, endometrial; LSIL, low grade squamous intraepithelial lesion; HSIL, high grade squamous intraepithelial lesion; TIFD, tissue insufficient for diagnosis.
| Cytomorphological feature |
Histologic diagnosis |
p-valuea | |
|---|---|---|---|
| Benign (n = 51) | ADC/AIS (n = 23) | ||
| Architectural features | |||
| Overlapping | .058 | ||
| Present | 30 | 18 | |
| Absent | 21 | 5 | |
| Single-cell pattern | < .001 | ||
| Present | 1 | 8 | |
| Absent | 50 | 15 | |
| Three-dimensional clusters | < .001 | ||
| Present | 0 | 8 | |
| Absent | 51 | 15 | |
| Nuclear features | |||
| Increased N/C ratio | .434 | ||
| Present | 50 | 22 | |
| Absent | 1 | 1 | |
| Hyperchromasia | .233 | ||
| Present | 39 | 18 | |
| Absent | 12 | 5 | |
| Membrane irregularity | .012 | ||
| Present | 18 | 15 | |
| Absent | 33 | 8 | |
| Prominent nucleoli | .124 | ||
| Present | 16 | 10 | |
| Absent | 35 | 13 | |
| Mitoses | .009 | ||
| 0/HPF | 49 | 17 | |
| > 1/HPF | 2 | 6 | |
- 1. Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2013; 2: 35.ArticlePubMedPMCPDF
- 2. Arbyn M, Rebolj M, De Kok IM, et al. The challenges of organising cervical screening programmes in the 15 old member states of the European Union. Eur J Cancer 2009; 45: 2671-8. ArticlePubMed
- 3. Hong S, Won YJ, Park YR, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat 2020; 52: 335-50. ArticlePubMedPMCPDF
- 4. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209-49. ArticlePubMedPDF
- 5. Nayar R, Wilbur DC. The Pap test and Bethesda 2014. “The reports of my demise have been greatly exaggerated.” (after a quotation from Mark Twain). Acta Cytol 2015; 59: 121-32. PubMed
- 6. Kang M, Ha SY, Cho HY, et al. Comparison of papanicolaou smear and human papillomavirus (HPV) test as cervical screening tools: can we rely on HPV test alone as a screening method? An 11-year retrospective experience at a single institution. J Pathol Transl Med 2020; 54: 112-8. ArticlePubMedPMCPDF
- 7. Polman NJ, Snijders PJ, Kenter GG, Berkhof J, Meijer C. HPV-based cervical screening: rationale, expectations and future perspectives of the new Dutch screening programme. Prev Med 2019; 119: 108-17. ArticlePubMed
- 8. Naucler P, Ryd W, Tornberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 2009; 101: 88-99. ArticlePubMed
- 9. Wentzensen N, Schiffman M, Palmer T, Arbyn M. Triage of HPV positive women in cervical cancer screening. J Clin Virol 2016; 76 Suppl 1: S49-S55. ArticlePubMedPMC
- 10. Moriarty AT, Wilbur D. Those gland problems in cervical cytology: faith or fact? Observations from the Bethesda 2001 terminology conference. Diagn Cytopathol 2003; 28: 171-4. ArticlePubMed
- 11. Bansal B, Gupta P, Gupta N, Rajwanshi A, Suri V. Detecting uterine glandular lesions: role of cervical cytology. Cytojournal 2016; 13: 3.ArticlePubMedPMC
- 12. Lin M, Narkcham S, Jones A, et al. False-negative Papanicolaou tests in women with biopsy-proven invasive endocervical adenocarcinoma/adenocarcinoma in situ: a retrospective analysis with assessment of interobserver agreement. J Am Soc Cytopathol 2022; 11: 3-12. ArticlePubMed
- 13. Solomon D, Frable WJ, Vooijs GP, et al. ASCUS and AGUS criteria. International Academy of Cytology Task Force summary. Diagnostic cytology towards the 21st century: an international expert conference and tutorial. Acta Cytol 1998; 42: 16-24. PubMed
- 14. Wood MD, Horst JA, Bibbo M. Weeding atypical glandular cell look-alikes from the true atypical lesions in liquid-based Pap tests: a review. Diagn Cytopathol 2007; 35: 12-7. ArticlePubMed
- 15. Toyoda S, Kawaguchi R, Kobayashi H. Clinicopathological characteristics of atypical glandular cells determined by cervical cytology in Japan: survey of gynecologic oncology data from the Obstetrical Gynecological Society of Kinki District, Japan. Acta Cytol 2019; 63: 361-70. ArticlePubMedPDF
- 16. Galic V, Herzog TJ, Lewin SN, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol 2012; 125: 287-91. ArticlePubMed
- 17. Gallardo-Alvarado L, Cantu-de Leon D, Ramirez-Morales R, et al. Tumor histology is an independent prognostic factor in locally advanced cervical carcinoma: a retrospective study. BMC Cancer 2022; 22: 401.ArticlePubMedPMCPDF
- 18. Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States: a 24-year population-based study. Gynecol Oncol 2000; 78: 97-105. ArticlePubMed
- 19. Vizcaino AP, Moreno V, Bosch FX, et al. International trends in incidence of cervical cancer: II. Squamous-cell carcinoma. Int J Cancer 2000; 86: 429-35. ArticlePubMed
- 20. Wang SS, Sherman ME, Hildesheim A, Lacey JV Jr, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976-2000. Cancer 2004; 100: 1035-44. ArticlePubMed
- 21. Bray F, Loos AH, McCarron P, et al. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev 2005; 14: 677-86. ArticlePubMedPDF
- 22. Takeuchi S. Biology and treatment of cervical adenocarcinoma. Chin J Cancer Res 2016; 28: 254-62. ArticlePubMedPMC
- 23. Gien LT, Beauchemin MC, Thomas G. Adenocarcinoma: a unique cervical cancer. Gynecol Oncol 2010; 116: 140-6. ArticlePubMed
- 24. Katanyoo K, Sanguanrungsirikul S, Manusirivithaya S. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma in locally advanced cervical cancer. Gynecol Oncol 2012; 125: 292-6. ArticlePubMed
- 25. Zhao C, Florea A, Onisko A, Austin RM. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol 2009; 114: 383-9. ArticlePubMed
- 26. Nayar R, Wilbur DC. The Bethesda System for Reporting Cervical Cytology: definitions, criteria, and explanatory notes. Cham: Springer, 2015.
- 27. Oh EJ, Jung CK, Kim DH, et al. Current cytology practices in Korea: a nationwide survey by the Korean Society for Cytopathology. J Pathol Transl Med 2017; 51: 579-87. ArticlePubMedPMCPDF
- 28. Ajit D, Gavas S, Joseph S, Rekhi B, Deodhar K, Kane S. Identification of atypical glandular cells in pap smears: is it a hit and miss scenario? Acta Cytol 2013; 57: 45-53. ArticlePubMedPDF
- 29. Yucel Polat A, Tepeoglu M, Tunca MZ, Ayva ES, Ozen O. Atypical glandular cells in Papanicolaou test: which is more important in the detection of malignancy, architectural or nuclear features? Cytopathology 2021; 32: 344-52. ArticlePubMedPDF
- 30. Niu S, Molberg K, Thibodeaux J, et al. Challenges in the Pap diagnosis of endocervical adenocarcinoma in situ. J Am Soc Cytopathol 2019; 8: 141-8. ArticlePubMed
- 31. Chaump M, Pirog EC, Panico VJ, AB DM, Holcomb K, Hoda R. Detection of in situ and invasive endocervical adenocarcinoma on ThinPrep Pap test: morphologic analysis of false negative cases. Cytojournal 2016; 13: 28.ArticlePubMedPMC
- 32. Umezawa T, Umemori M, Horiguchi A, et al. Cytological variations and typical diagnostic features of endocervical adenocarcinoma in situ: a retrospective study of 74 cases. Cytojournal 2015; 12: 8.ArticlePubMedPMC
- 33. Li S, Tian D, Li Y. Cytological diagnoses of adenocarcinoma in situ of the cervix: common misdiagnoses. Acta Cytol 2015; 59: 91-6. ArticlePubMedPDF
- 34. Pradhan D, Li Z, Ocque R, Patadji S, Zhao C. Clinical significance of atypical glandular cells in Pap tests: an analysis of more than 3000 cases at a large academic women’s center. Cancer Cytopathol 2016; 124: 589-95. ArticlePubMed
- 35. Raab SS, Isacson C, Layfield LJ, Lenel JC, Slagel DD, Thomas PA. Atypical glandular cells of undetermined significance. Cytologic criteria to separate clinically significant from benign lesions. Am J Clin Pathol 1995; 104: 574-82. ArticlePubMed
- 36. Torres JC, Derchain SF, Gontijo RC, et al. Atypical glandular cells: criteria to discriminate benign from neoplastic lesions and squamous from glandular neoplasia. Cytopathology 2005; 16: 295-302. ArticlePubMed
- 37. Reynolds JP, Salih ZT, Smith AL, Dairi M, Kigen OJ, Nassar A. Cytologic parameters predicting neoplasia in Papanicolaou smears with atypical glandular cells and histologic follow-up: a single-institution experience. J Am Soc Cytopathol 2018; 7: 7-15. ArticlePubMed
- 38. Selvaggi SM. Cytologic features of high-grade squamous intraepithelial lesions involving endocervical glands on ThinPrep cytology. Diagn Cytopathol 2002; 26: 181-5. ArticlePubMed
- 39. Kumar N, Bongiovanni M, Molliet MJ, Pelte MF, Egger JF, Pache JC. Diverse glandular pathologies coexist with high-grade squamous intraepithelial lesion in cyto-histological review of atypical glandular cells on ThinPrep specimens. Cytopathology 2009; 20: 351-8. ArticlePubMed
- 40. Colgan TJ, Lickrish GM. The topography and invasive potential of cervical adenocarcinoma in situ, with and without associated squamous dysplasia. Gynecol Oncol 1990; 36: 246-9. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Expertise in Gynecological Pathology Impacts Diagnosis of Atypical Glandular Cell Category in Cervical Cytology
Havva Gökce Terzioglu, Alessa Aragao, Julieta E. Barroeta
Journal of Lower Genital Tract Disease.2025; 29(4): 297. CrossRef - Analysis of atypical glandular cells in ThinPrep Pap smear and follow-up histopathology
Tengfei Wang, Yinan Hua, Lina Liu, Bing Leng
Baylor University Medical Center Proceedings.2024; 37(3): 403. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


Fig. 1.
Fig. 2.
| Histopathologic result | Pap test result |
||||
|---|---|---|---|---|---|
| AGC-NOS | AGC-EC | AGC-EM | AGC-FN | ADC | |
| Benign | 197 | 9 | 3 | 4 | 9 |
| Endometrial lesion | |||||
| Endometrioid adenocarcinoma | 68 | 1 | 5 | 6 | 34 |
| Serous adenocarcinoma | 7 | - | - | - | 15 |
| Clear cell carcinoma | 4 | - | - | - | 3 |
| Adenosquamous carcinoma | 2 | ||||
| Carcinosarcoma | 5 | - | - | - | 3 |
| EM other malignant | 3 | - | - | - | - |
| EM hyperplasia | 5 | - | 1 | - | - |
| Cervical squamous lesion | |||||
| LSIL | 21 | - | - | - | 2 |
| HSIL | 22 | 1 | - | - | 4 |
| Squamous cell carcinoma | 4 | - | - | - | - |
| Cervical glandular lesion | |||||
| Adenocarcinoma in situ | 13 | - | - | - | 3 |
| Adenocarcinoma | 33 | - | - | 7 | 83 |
| Adenosquamous carcinoma | - | - | - | 1 | 6 |
| Cervical other malignant | 2 | - | - | - | 3 |
| Ovarian lesion | |||||
| Serous adenocarcinoma | 9 | - | - | - | 12 |
| Mucinous adenocarcinoma | - | - | - | - | 1 |
| Clear cell carcinoma | - | - | - | - | 2 |
| Endometrioid adenocarcinoma | 1 | - | - | - | - |
| Ovary other malignant | 1 | 1 | - | - | 1 |
| Vaginal lesion | |||||
| Poorly differentiated carcinoma | 1 | - | - | - | 1 |
| Metastatic tumor | 5 | - | - | 2 | 22 |
| Other | 2 | - | - | 1 | - |
| TIFD, unknown | 6 | - | - | - | - |
| Total | 409 | 12 | 9 | 21 | 206 |
| Cytomorphological feature | Histologic diagnosis |
p-value |
|
|---|---|---|---|
| Benign (n = 51) | ADC/AIS (n = 23) | ||
| Architectural features | |||
| Overlapping | .058 | ||
| Present | 30 | 18 | |
| Absent | 21 | 5 | |
| Single-cell pattern | < .001 | ||
| Present | 1 | 8 | |
| Absent | 50 | 15 | |
| Three-dimensional clusters | < .001 | ||
| Present | 0 | 8 | |
| Absent | 51 | 15 | |
| Nuclear features | |||
| Increased N/C ratio | .434 | ||
| Present | 50 | 22 | |
| Absent | 1 | 1 | |
| Hyperchromasia | .233 | ||
| Present | 39 | 18 | |
| Absent | 12 | 5 | |
| Membrane irregularity | .012 | ||
| Present | 18 | 15 | |
| Absent | 33 | 8 | |
| Prominent nucleoli | .124 | ||
| Present | 16 | 10 | |
| Absent | 35 | 13 | |
| Mitoses | .009 | ||
| 0/HPF | 49 | 17 | |
| > 1/HPF | 2 | 6 | |
Pap, Papanicolaou; AGC-NOS, atypical glandular cells not otherwise specified; AGC-EC, atypical endocervical cells; AGC-EM, atypical endometrial cells; AGC-FN, atypical glandular cells favor neoplastic; ADC, adenocarcinoma; EM, endometrial; LSIL, low grade squamous intraepithelial lesion; HSIL, high grade squamous intraepithelial lesion; TIFD, tissue insufficient for diagnosis.
Pap, Papanicolaou; ADC, adenocarcinoma; AIS, adenocarcinoma in situ; N/C, nuclear-to-cytoplasm; HPF, high-power field. The Fisher exact test was used. p-value of < .05 was considered statistically significant.

 E-submission
E-submission