Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(6); 2022 > Article
-
Original Article
A clinicopathologic and immunohistochemical study of primary and secondary breast angiosarcoma -
Evi Abada1
 , Hyejeong Jang2
, Hyejeong Jang2 , Seongho Kim2
, Seongho Kim2 , Rouba Ali-Fehmi1, Sudeshna Bandyopadhyay1
, Rouba Ali-Fehmi1, Sudeshna Bandyopadhyay1 -
Journal of Pathology and Translational Medicine 2022;56(6):342-353.
DOI: https://doi.org/10.4132/jptm.2022.08.31
Published online: October 27, 2022
1Department of Pathology, Wayne State University School of Medicine/Detroit Medical Center, Detroit, MI, USA
2Biostatistics and Bioinformatics Core, Karmanos Cancer Institute, Department of Oncology, Wayne State University School of Medicine, Detroit, MI, USA
- Corresponding Author: Evi Abada, MD, MS, Department of Pathology, Wayne State University School of Medicine/Detroit Medical Center, 3990 John R Street, Detroit, MI 48201, USA Tel: +1-313-577-1102, Fax: +1-313-577-0057, E-mail: gs5839@wayne.edu
© 2022 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- We aimed to study the clinicopathologic and immunohistochemical (IHC) (CD117, c-Myc, and p53) characteristics, and overall survival of primary and secondary breast angiosarcoma (BAS).
-
Methods
- This was a retrospective study of BAS cases diagnosed between 1997 and 2020 at our institution. Hematoxylin and eosin-stained slides were reviewed for tumor morphology, margin status, and lymph node metastasis. CD117, p53, D2-40, CD31, and c-Myc IHC stains were performed on 11 viable tissue blocks. Additional clinical information was obtained from the electronic medical records.
-
Results
- Seventeen patients with BAS were identified. Of these, five (29%) were primary and 12 (71%) were secondary BAS, respectively. The median age at diagnosis for primary BAS was 36 years. The median age at diagnosis for secondary BAS was 67 years. The median time to secondary BAS development following radiotherapy was 6.5 years (range, 2 to 12 years). There was no significant difference between primary and secondary BAS in several histopathologic parameters examined, including histologic grade, necrosis, mitotic count, lymph node metastasis, and positive tumor margins. There was also no difference in CD117, p53, D2-40, CD31, and c-Myc expression by IHC between primary and secondary BAS. During a median followup of 21 months, primary BAS had two (40%) reported deaths and secondary BAS had three (25%) reported deaths. However, this difference in survival between both groups was not statistically significant (hazard ratio, 0.51; 95% confidence interval, 0.09 to 3.28; p = .450).
-
Conclusions
- BAS is a rare and aggressive disease. No histologic, IHC (CD117, c-Myc, and p53), or survival differences were identified between primary and secondary BAS in this study.
- We retrospectively reviewed our database to identify patients diagnosed with primary and secondary BAS between January 1997 and February 2020. Study inclusion criteria included a prior diagnosis of BAS. Cases were categorized as primary BAS if they had no prior history of breast cancer or secondary BAS following post-radiation therapy for a prior breast cancer. Two surgical pathologists with expertise in breast pathology (RAF and SB) and EA reviewed pathology records including histologic slides to confirm the diagnosis of BAS. Additionally, IHC staining for CD117, p53, c-Myc, CD31, and D2-40 was performed on eleven 4-micron thick paraffin-embedded tissue sections. The slides were dried at 62°C and counterstained with Mayer’s hematoxylin and bluing reagent. Appropriate positive and negative controls were employed throughout. The antibody incubation and detection were performed on an autostainer (Ventana Bench Mark UltraView, Universal DAB kit, Ventana Medical Systems, Tucson, AZ, USA). Table 1 summarizes the clones, dilutions, incubation times, and sources for the antibodies used. CD117 immunoreactivity was interpreted as positive with the presence of uniform cytoplasmic (or membranous) staining in the tumor cells. For p53, the immunoreactivity scoring was counted as the percentage of nuclear staining per 10 high-power fields (HPF), in several areas, regardless of the staining intensity [20]. A 20% cutoff value for detection of positive nuclear reactivity was selected for p53 antibody as previously described [21,22]. Strong nuclear staining in greater than 50% of the tumor cells was interpreted as positive for c-Myc and a negative result was represented by faint staining in a small percentage of cells (less than 50%) [23]. CD31 and D2-40 were both interpreted as positive with membranous (and cytoplasmic) staining in the tumor cells. Histologic parameters reviewed include tumor size, tumor grade, mitotic count (number of mitoses per 10 HPF, using a 40× objective and a 10× ocular lens; field size 0.25 mm2), necrosis, lymphovascular invasion, lymph node metastasis, and margin status. Tumors were histologically graded as low, intermediate, or high [24,25]. Low-grade tumors contain open, anastomosing vascular channels that proliferate within dermis, subcutaneous tissue or breast tissue [26]. A single layer of flat endothelial cells which may be hyperchromatic with small nucleoli line these channels, which dissect through the stroma, causing distortion but little destruction of the preexisting lobules and ducts, with the absence of solid/spindle cell foci, blood lakes, and necrosis [26]. Intermediate-grade angiosarcoma differs from low-grade by containing additional cellular foci of papillary formations and/or solid/spindle cell proliferation with slightly increased mitoses [26]. High-grade angiosarcoma contains tumor lined by malignant endothelial cells with prominent cytologic atypia. Endothelial tufting and papillary formations are present, with conspicuous solid and spindle cell areas mostly devoid of vascular formations [26]. In addition, mitoses may be brisk especially in more cellular or solid areas and areas of hemorrhage, known as “blood lakes” and necrosis are also prominent [26]. Additional clinical data, including demography, tumor laterality, time to development of secondary breast angiosarcoma, comorbidities including obesity, diabetes, hypertension, and smoking history, treatment received and OS were obtained from the electronic medical record.
- Patient baseline characteristics were summarized by median (range) and frequency (%) for continuous and categorical variables, respectively. Group comparisons were performed by Fisher exact tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. The distribution of OS was graphically described using Kaplan-Meier curve along with a median and 95% confidence interval (CI). The median follow-up time was estimated using the reverse Kaplan-Meier method. A log-rank test was used to compare Kaplan-Meier curves between groups. When a categorical variable has more than two levels, a global log-rank p-value was calculated using likelihood ratio tests. Due to the small sample size, Cox proportional regression analyses were limited to univariable analyses. Firth Cox regression models were used to reduce bias in maximum likelihood estimation caused by rare events. The proportional hazard assumption was verified based on Schoenfeld residuals, and no violation was found except for positive margins and tumor site that were further confirmed using restricted mean survival time.
MATERIALS AND METHODS
- Seventeen women with a diagnosis of BAS were identified and of these, 12 (71%) were Caucasians and five (29%) were Black/African Americans (AA). Primary BAS was seen in five cases (29%) and secondary BAS was seen in 12 cases (71%), respectively. In terms of race and its association with disease occurrence, secondary BAS was more common in both Caucasian and Black/AA women. However, the impact of race on disease occurrence was not statistically significant (p>.99). The median age at diagnosis for primary BAS was 36 years (range, 23 to 71 years) and the median age at diagnosis for secondary BAS was 67 years (range, 33 to 76 years). However, this difference in age was not statistically significant (p=.246).
- Right-sided tumors were more common (80%) in women with primary BAS, compared with women with secondary BAS in which left-sided tumors were more common (58%). However, this difference in tumor laterality was not statistically significant (p=.294). Multifocal tumors were observed in one case of primary BAS (20%) and three cases of secondary BAS (25%) (p>.99). Skin involvement was present in seven secondary BAS cases (58%); however, there was no involvement of the skin in any of the primary BAS cases (p=.044). Positive margins were seen in two cases (40%) of primary BAS and six cases (50%) of secondary BAS. However, this difference was not statistically significant (p>.99). For the remaining cases with negative tumor margins, the median tumor size for primary BAS was 2 cm (range, 0.5 to 28 cm) and the median tumor size for secondary BAS was 0.95 cm (range, 0.4 to 2.5 cm). However, this difference in tumor size between both groups was not statistically significant (p=.437).
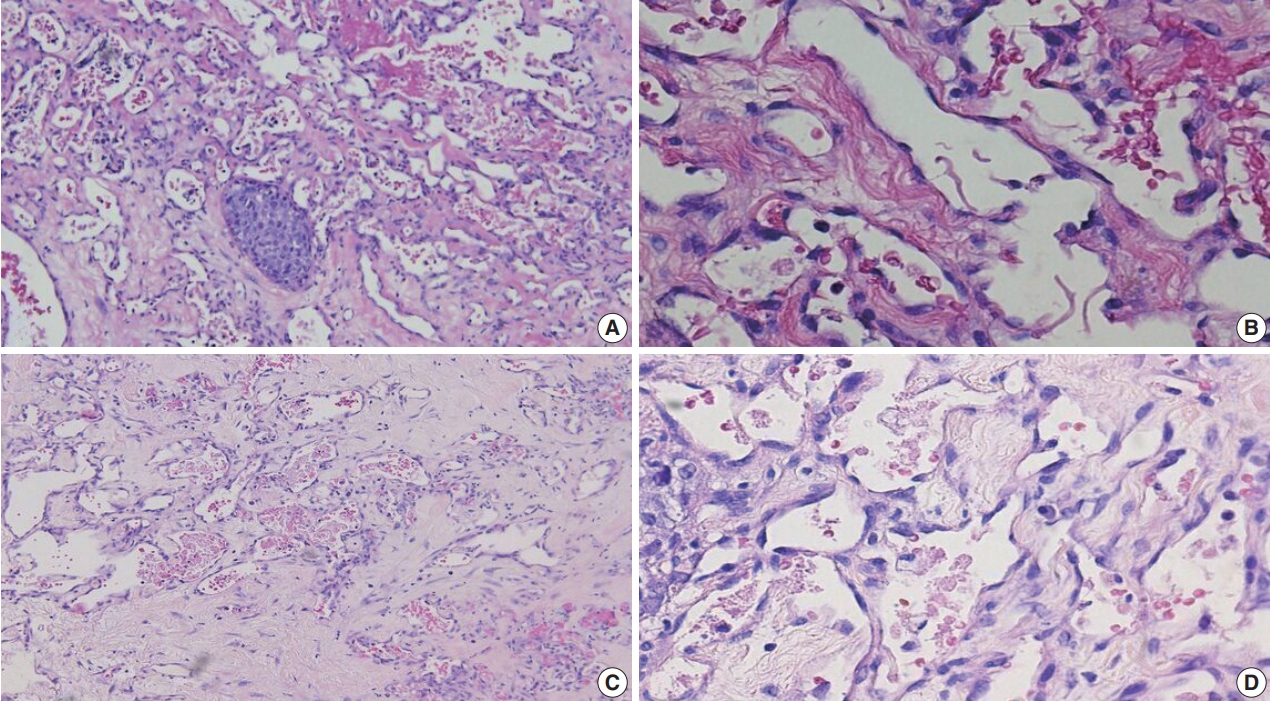
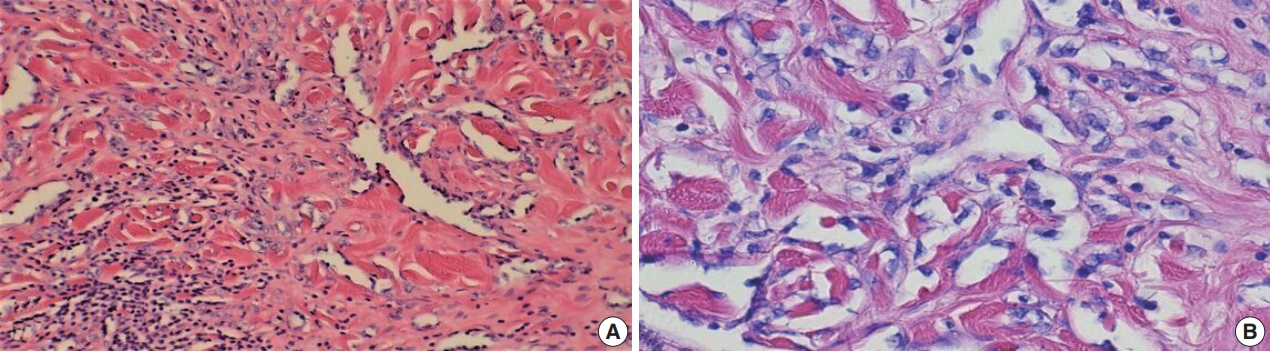
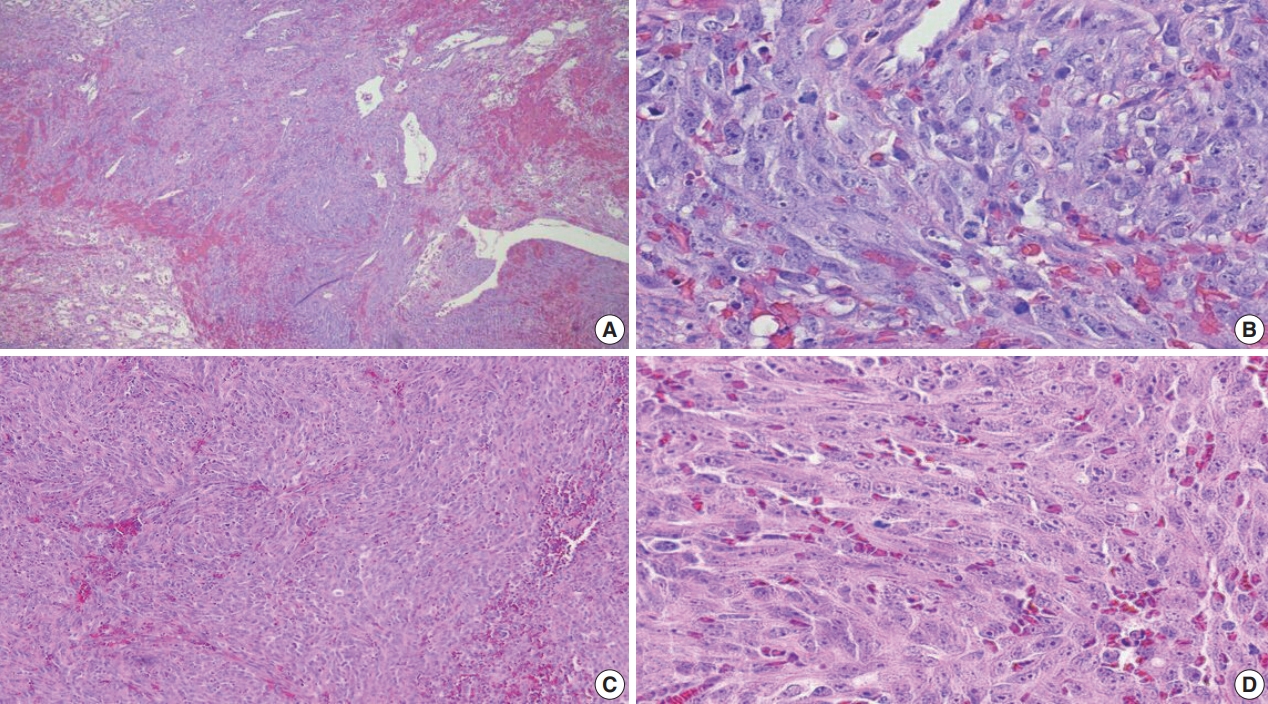
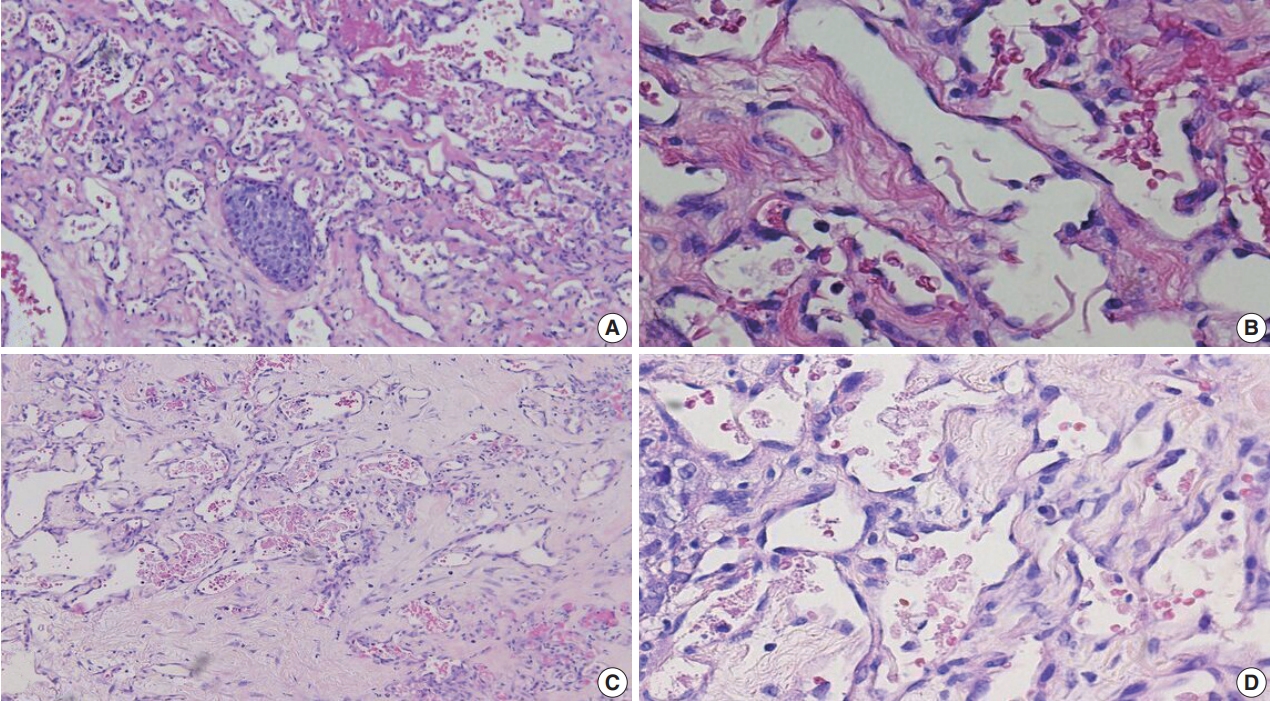
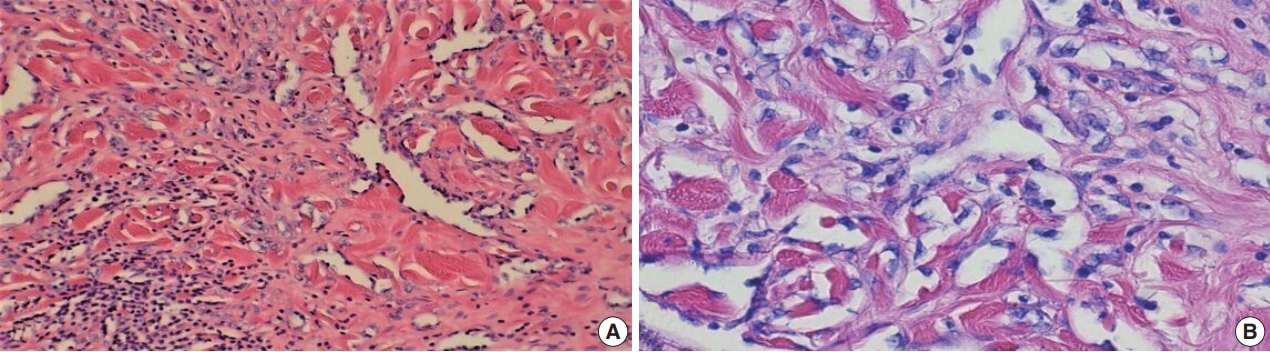
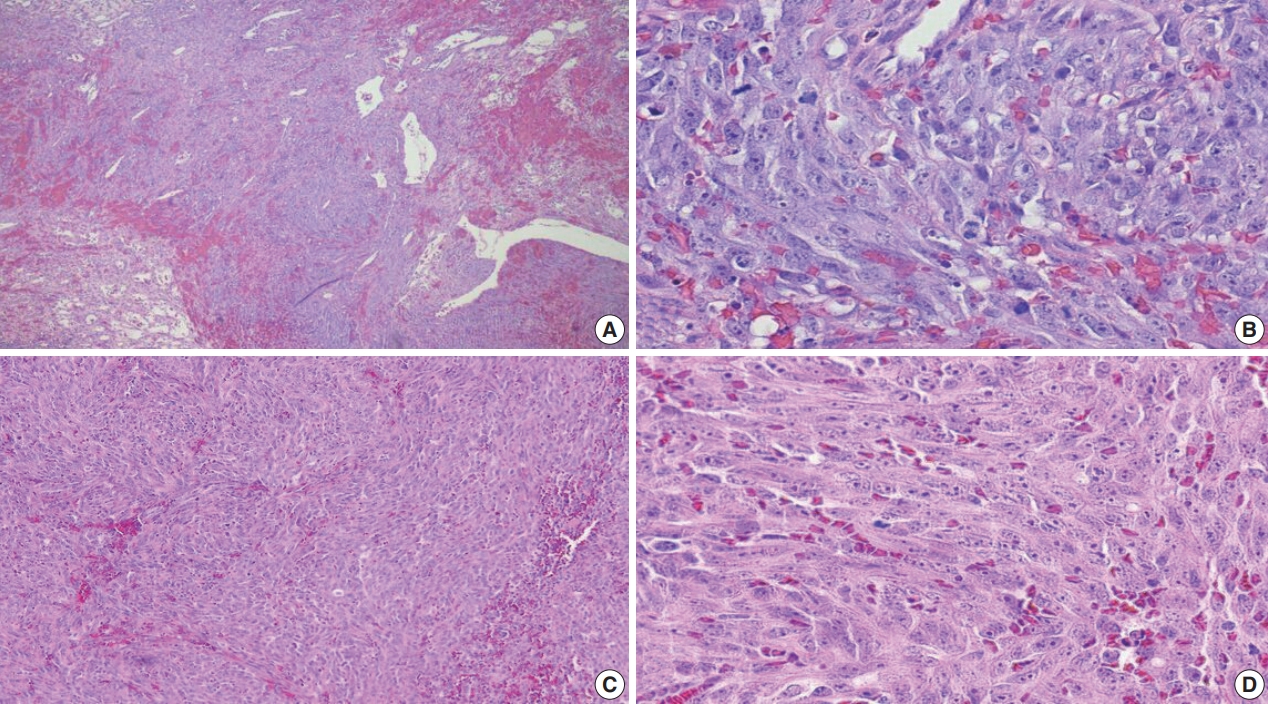
- The tumors were graded into low (Fig. 1A, B: primary BAS; Fig. 1C, D: secondary BAS), intermediate (Fig. 2A, B: primary BAS) and high grade (Fig. 3A, B: primary BAS; Fig. 3C, D: secondary BAS) based on previously defined histologic criteria [24,25]. However, we found no difference in histologic grade between primary and secondary BAS (p=.087). Other histologic parameters examined include presence of tumor necrosis, lymph node metastasis, and mitotic count (categorized into >10/10 HPF and <10/10 HPF). There was no difference between primary and secondary BAS with regard to tumor necrosis (p=.538), lymph node metastasis (p=.191), and mitotic count (p=.593).
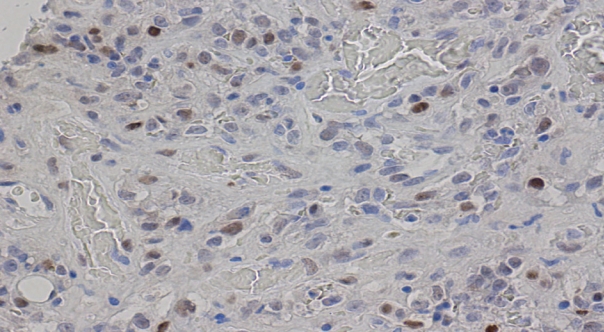
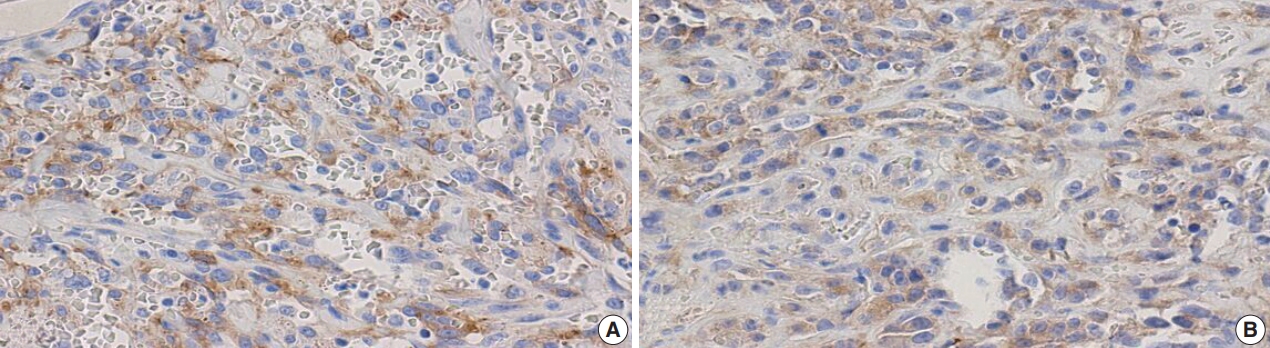
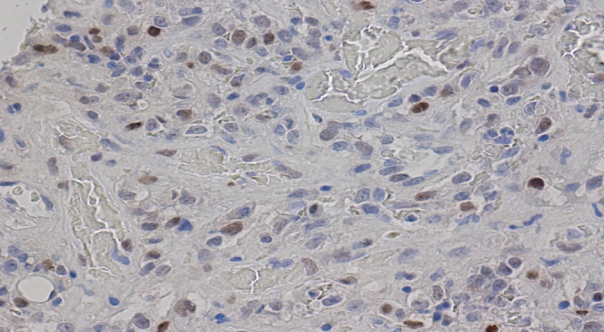
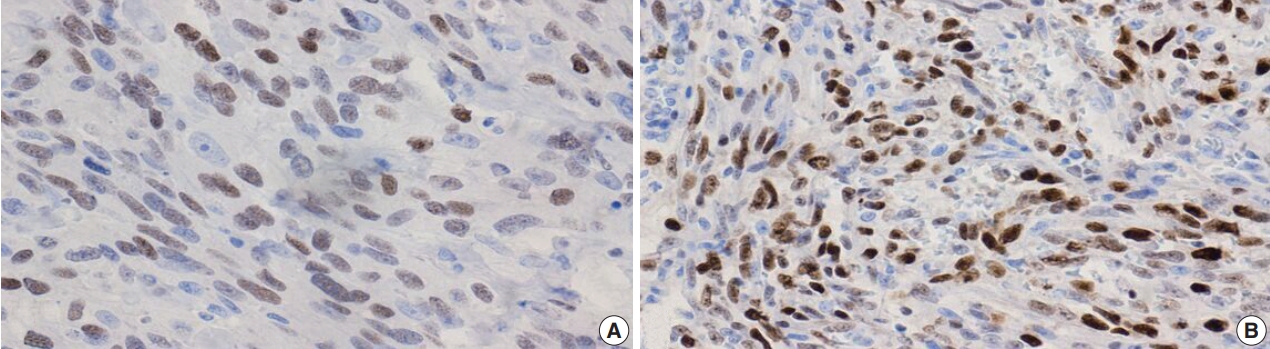
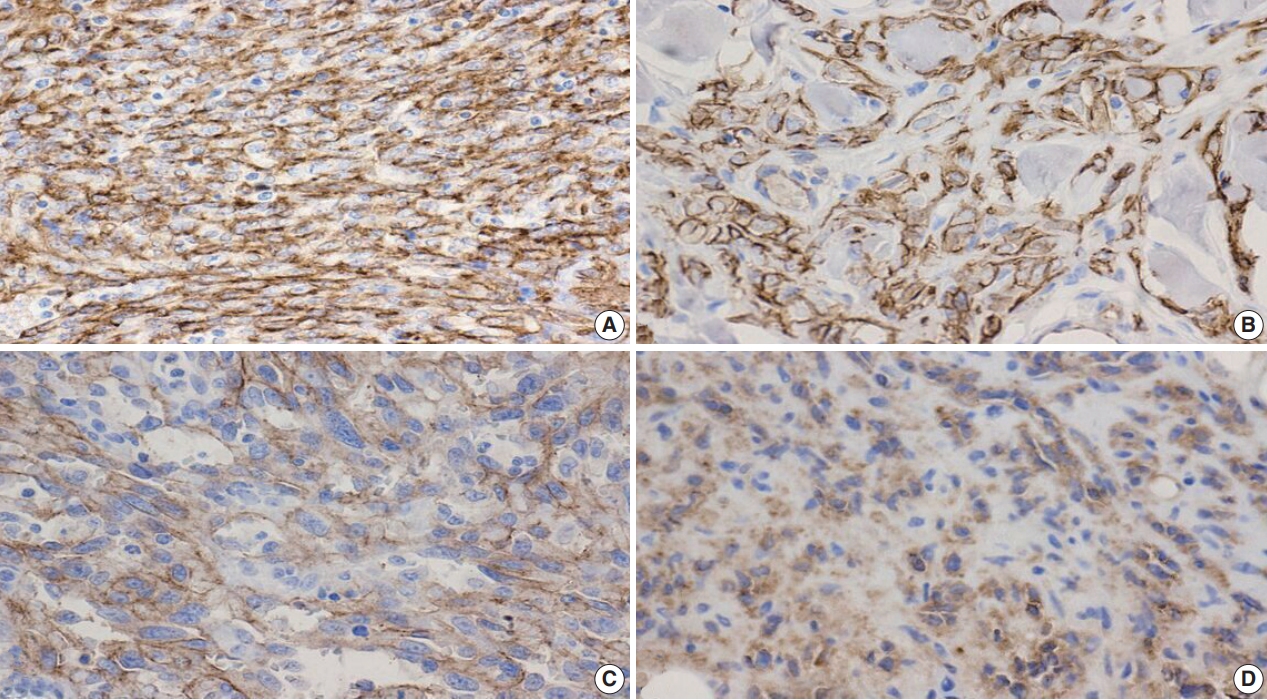
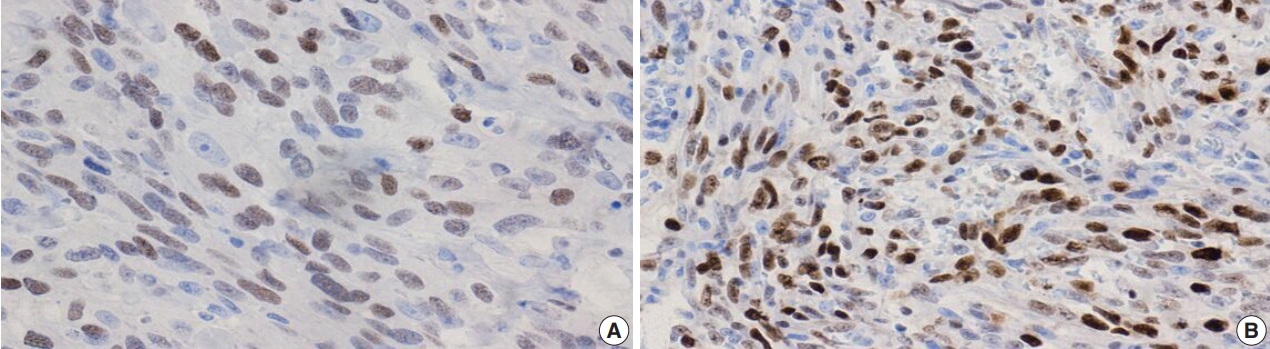
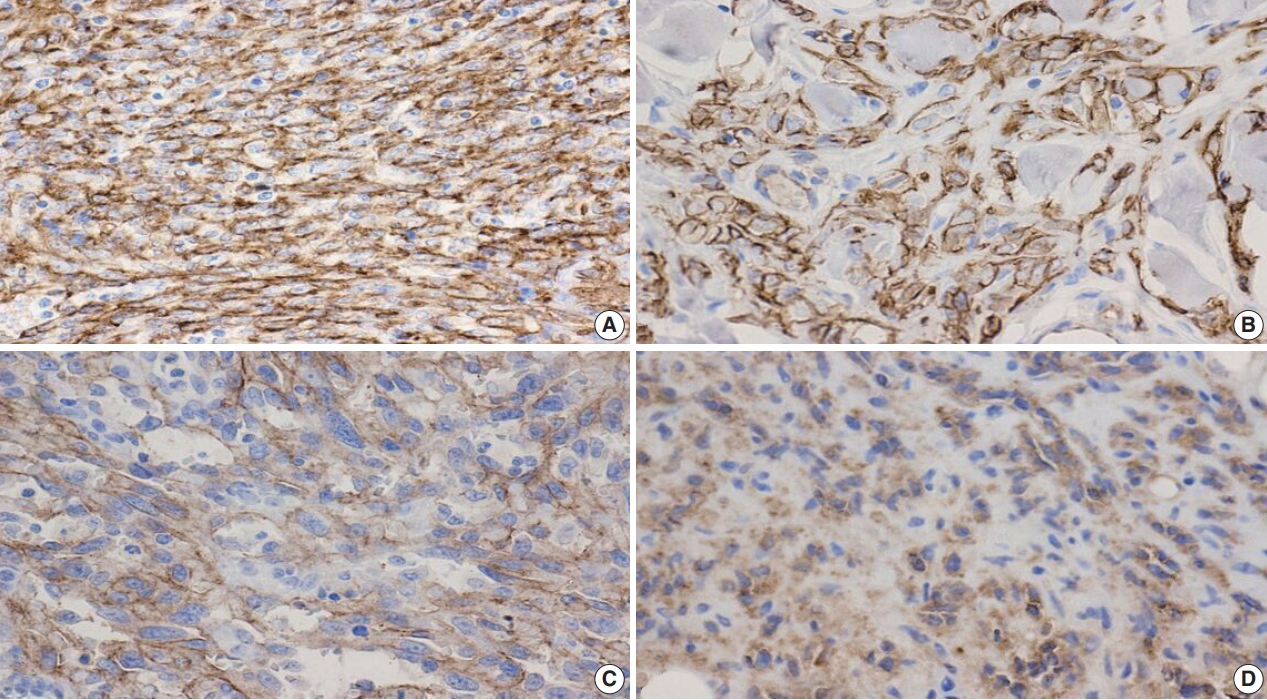
- Additional clinical characteristics evaluated from our patient population include the incidence of obesity (body mass index ≥30), hypertension, smoking, diabetes, and the treatment received for their BAS diagnosis. We observed that patients with secondary BAS appear to have more concurrent clinical comorbidities; however, these findings were not statistically significant when compared with patients with primary BAS (obesity: p=.117; hypertension: p=.102; smoking: p>.99; diabetes: p=.338). In line with published literature, CD117 (Fig. 4A: primary BAS; Fig. 4B: secondary BAS), p53 (Fig. 5: secondary BAS), c-Myc (Fig. 6A: primary BAS; Fig. 6B: secondary BAS), CD31 (Fig. 7A: primary BAS; Fig. 7B: secondary BAS), and D240 (Fig. 7C: primary BAS; Fig. 7D: secondary BAS) IHC stains were performed on 11 (3 primary and 8 secondary BAS) viable tissue blocks. Although there was no significant difference in the expression of CD117 (p>.99), p53 (p=.236), D240 (p>.99), CD31 (p>.99), and c-Myc (p>.99) between primary and secondary BAS, it’s noteworthy that p53 was only expressed in secondary BAS cases, and lacked expression in the primary BAS cases evaluated. Furthermore, c-Myc showed expression in both primary and secondary BAS cases. Table 2 summarizes the IHC pattern of expression in both primary and secondary BAS. All patients with BAS were managed with wide local excision or mastectomy. Two patients with secondary BAS received additional chemotherapy and one patient with primary BAS received additional chemotherapy and radiotherapy. However, there was no difference between patients who were treated with surgery alone and those who received additional treatments (p=.353). Table 3 summarizes the clinicopathologic characteristics, comorbidities, and treatment received by BAS type, respectively.
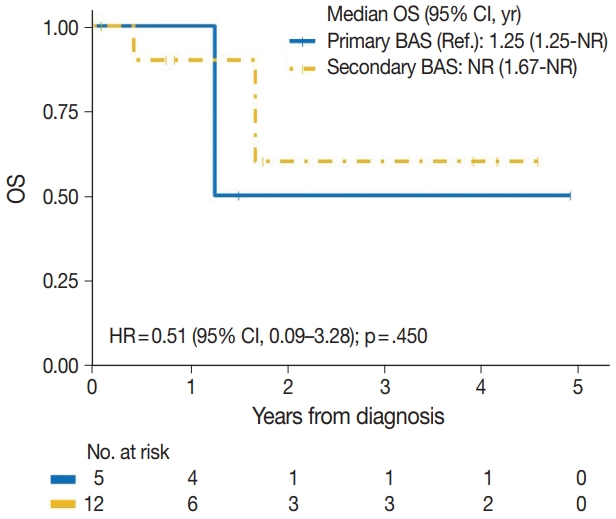
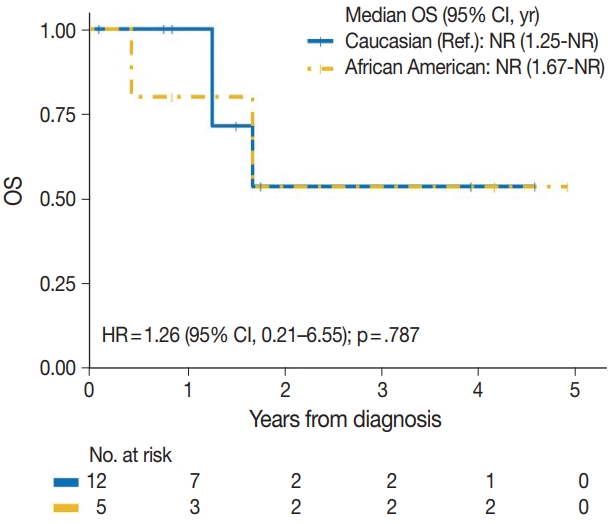
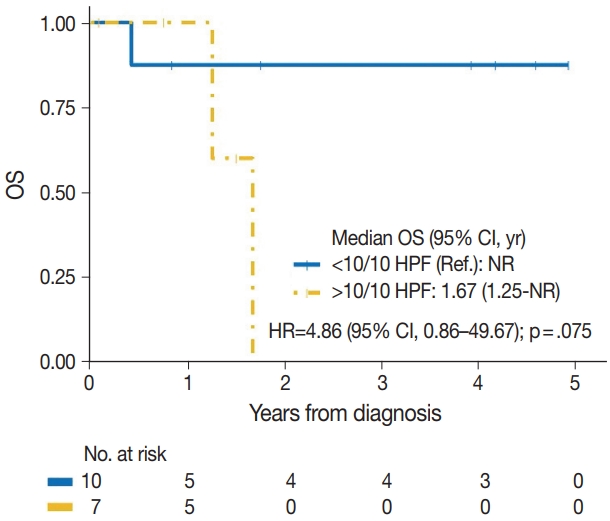
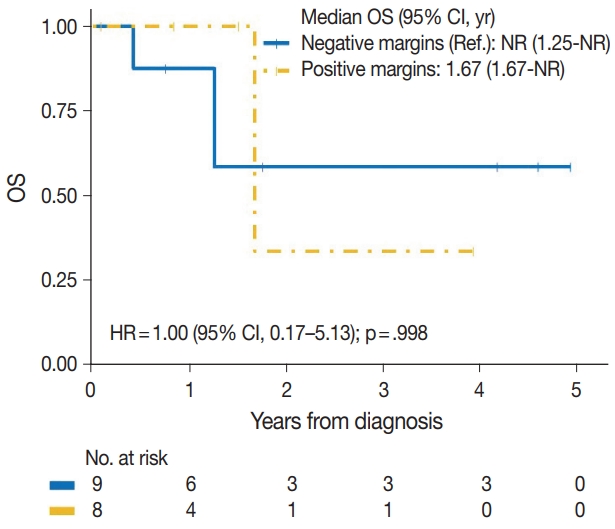
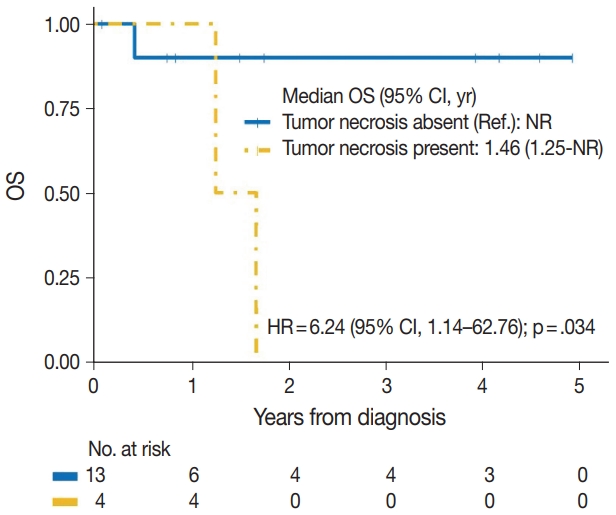
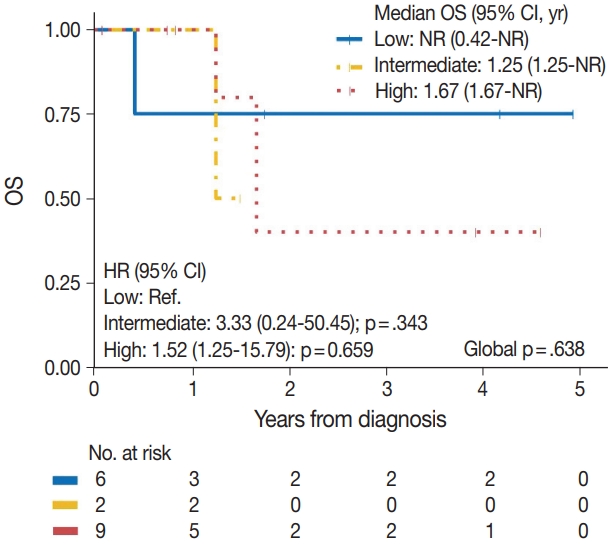
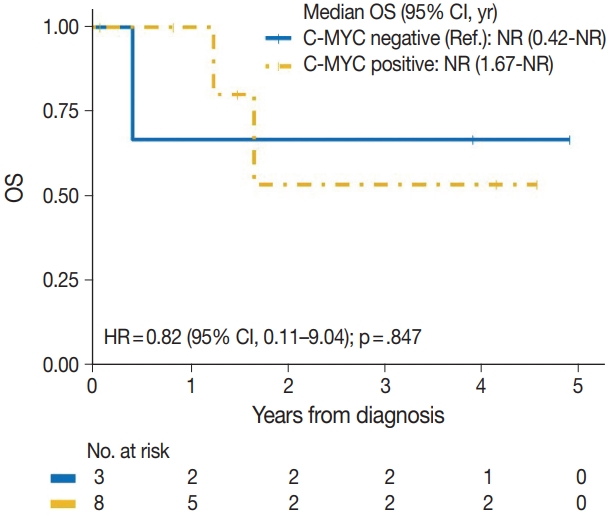
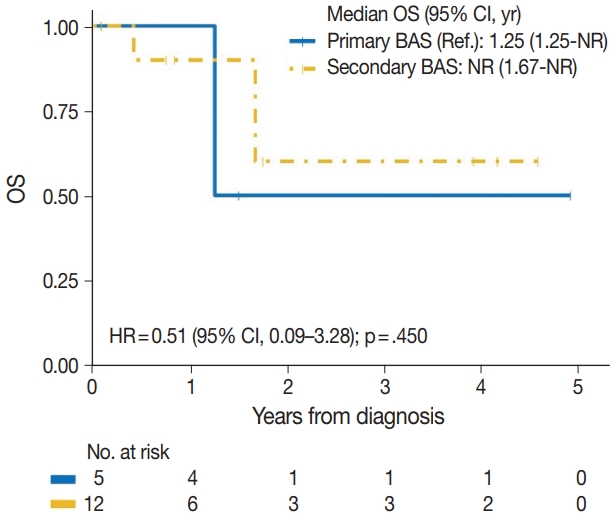
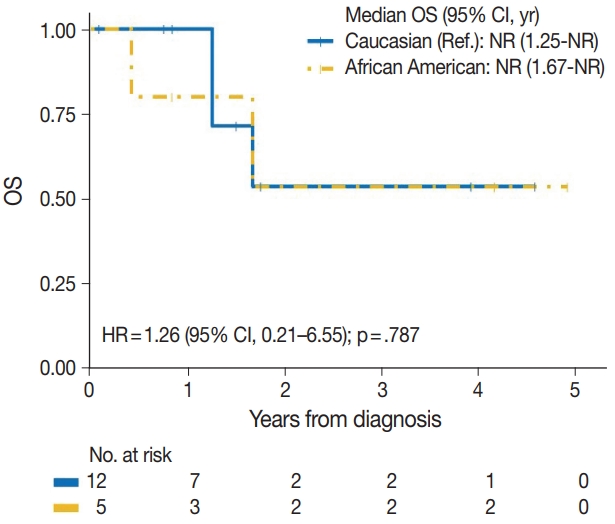
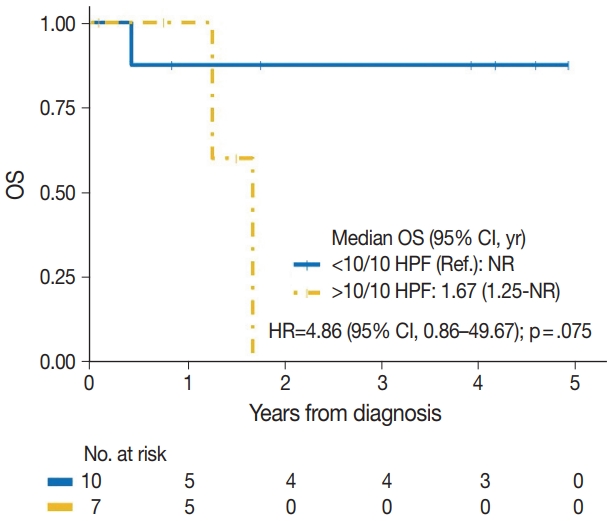
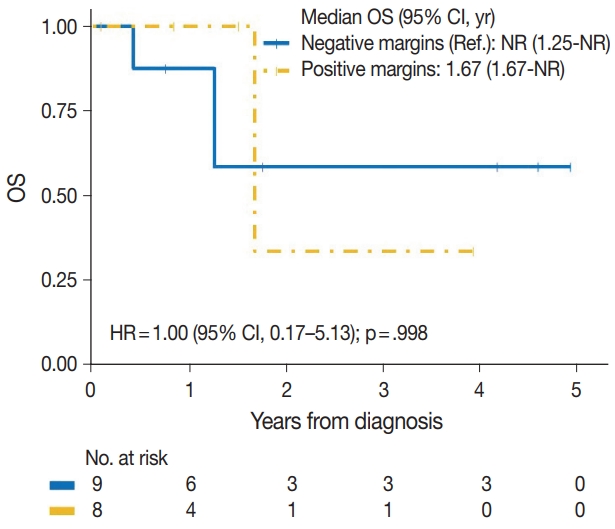
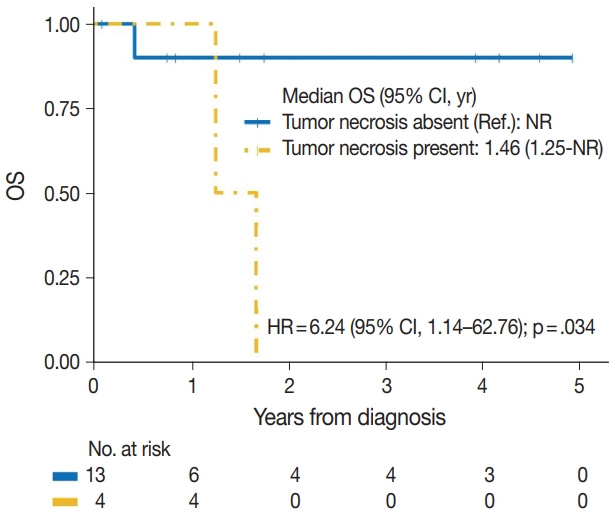
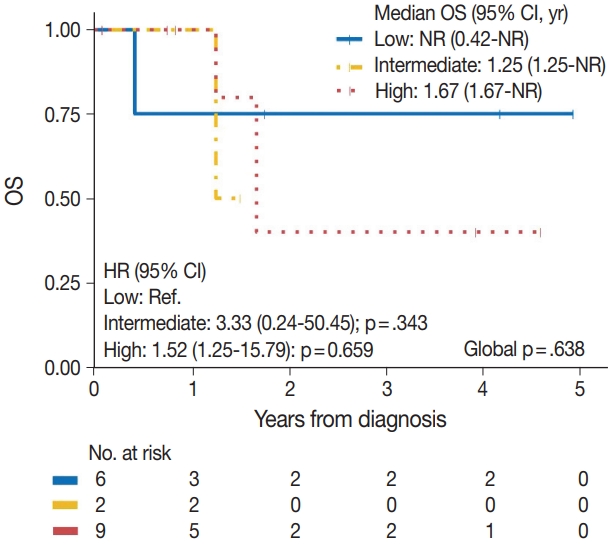
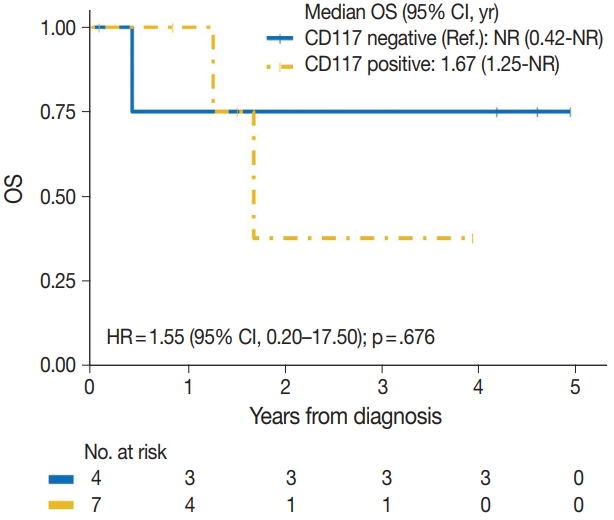
- The median time to development of secondary BAS following radiation therapy was 6.5 years (range, 2 to 12 years). The presence of tumor necrosis had an adverse effect on OS in BAS and this observation was statistically significant (p=.034). However, other histologic variables, including tumor size (p=.307), tumor grade (global p=.638), mitotic count (p=.075), lymph node metastasis (p=.278), and positive margin status (p=.998) had no significant effect on OS in BAS. Factors such as age at diagnosis (p=.845), race (p=.787) and type of BAS primary vs. secondary (p=.450) all had no significant effect on OS. Additionally, comorbid characteristics including obesity (p=.063), hypertension (p=.990), smoking (p=.551), and diabetes (p=.548) also had no significant impact on OS in BAS. There was also no difference in OS between patients who were treated with surgery alone and those who were treated with surgery and chemotherapy or radiotherapy (p=.671). By IHC, CD117 (p=.676), p53 (p=.847), D2-40 (p=.960), and c-Myc (p=.847) all had no significant effect on OS in BAS. The median follow-up period for both primary and secondary BAS was 21 months. The Kaplan-Meier curve of OS suggests that patients with primary BAS may have worse outcomes compared with patients with secondary BAS; however, this finding was not statistically significant (hazard ratio [HR], 0.51; 95% CI, 0.09 to 3.28; p=.450) (Fig. 8). Kaplan-Meier curve also shows no difference in OS between Caucasian and Black/AA women (Fig. 9). Tumors with mitoses >10/10 HPF also appear to have worse OS on the Kaplan-Meier curve; however, this finding was not statistically significant (Fig. 10). Additionally, Kaplan-Meier evaluation of OS, also shows worse OS for tumors with positive margins (Fig. 11), necrosis (Fig. 12), and histologic high grade (Fig. 13). However, these observations were all not statistically significant. By IHC, the Kaplan-Meier evaluation of OS shows that CD117 positive (Fig. 14) and c-Myc positive (Fig. 15) tumors all behave worse than their negative counterparts, respectively; however, these observations were not statistically significant. Table 4 summarizes the univariable Cox proportional hazard regression analyses of risk factors associated with OS. Additional patient and tumor characteristics are presented in the Supplementary Table S1.
RESULTS
- Our study is unique because we describe a single academic medical center’s experience with primary and secondary BAS spanning over 2 decades. Our findings emphasize the rarity of these tumors and presents information that describes the similarities and differences between primary and secondary BAS, including clinicopathologic and IHC characteristics, which only very few studies have hitherto described. The rarity of this disease indeed precludes any prospective study and poses significant challenges in its diagnosis, treatment, and research [4]. Results from our patient population shows that secondary BAS occurs at a higher frequency than primary BAS and this finding is in agreement with other studies [1,2,4]. The higher incidence of secondary BAS may be explained by the fact that more women with breast cancer are seeking breast-conserving surgeries with adjuvant radiotherapy, which may put them at risk of developing secondary BAS. Although we found no significant difference in age distribution between both groups of patients, it is remarkable to note that patients with primary BAS are much younger (median age, 36 years) than patients with secondary BAS (median age, 67 years). This observation is important, as breast cancer is known to be more common in older women. Therefore, for younger patients with BAS, this diagnosis may be missed, especially if it is misclassified as other benign skin pathologies. Our findings are similar to what has been previously described, with primary BAS occurring in women ages 30–50 years [27] and, secondary BAS occurring in older women (median age, 67 to 71 years) following a median of 10.5 years after radiotherapy for breast cancer [27-30]. In our study, however, patients with secondary BAS appeared to develop the disease at shorter latency times with a median time of 6.5 years. This finding may be explained by the small sample size of our patient population, which is not unexpected due to the rarity of this disease. In addition, it is unclear whether the finding of more concurrent comorbidities in patients with secondary BAS in our study had any role to play in terms of disease latency, and thus leaves room for further research. While our study showed that BAS was more common in Caucasian women compared with Black/AA women and is similar to what has been reported in another study [1], it is important to emphasize that incidence by race had no significant impact on the characteristics of BAS of the women in this study.
- We studied the histologic similarities and differences between primary and secondary BAS. Following our review, we found no significant difference between histologic parameters examined in primary and secondary BAS. In other words, regardless of whether a patient develops BAS de novo or following radiotherapy for a previous breast cancer diagnosis, the histologic phenotypes are ultimately the same. Similar to what we observed in our study, no difference in tumor characteristics was found between primary and secondary BAS in another study [1]. Of note is that differentiating low-grade angiosarcoma from atypical post-radiation vascular lesions (AVLs) may be difficult because they both represent the low-grade end of the morphologic spectrum of radiationassociated vascular lesions [26]. Nonetheless, AVLs are typically smaller, more superficial, non-infiltrative, and fairly well circumscribed [31].
- The immunostains performed as part of this study were selected based on previous research on angiosarcoma in other regions of the body and the breast, which have shown inconsistent findings. Slightly more than half (28 of 50 [56%]) of the angiosarcomas in one series showed CD117 positivity in the neoplastic endothelial cells by IHC, including post-radiation angiosarcoma [14]. CD117 expression by IHC was also seen in 25% of angiosarcoma in another series [32] and showed weak staining by IHC in two of 14 primary BAS cases in another series [33]. Additionally, multiple studies have shown conflicting p53 immunoreactivity by IHC with one study reporting that p53 was expressed in primary angiosarcoma but not in secondary sarcoma [19], and another study reporting no difference in p53 expression between primary and secondary sarcomas [20]. Furthermore, MYC amplification and c-Myc overexpression were detected almost exclusively in secondary angiosarcoma, compared with primary angiosarcoma [34]. In contrast, one study reported weak c-Myc staining by IHC in two of nine cases of primary BAS in their series [33]. Following our review, we found no significant difference in expression of CD117 (p>.99), p53 (p=.236), D240 (p>.99), CD31 (p> .99), and c-Myc (p>.99) between primary and secondary BAS. Our findings may be explained by the limited number of cases in this study but leaves room for future research. It’s however, noteworthy that p53 was only expressed in the secondary BAS cases in our cohort, and was not expressed in the primary BAS cases evaluated. In addition, c-Myc showed expression in both primary and secondary BAS cases in our series.
- Our study also compared comorbidities (hypertension, obesity, diabetes mellitus, and smoking history) and the treatment received between patients with primary and secondary BAS. Following our review, we found that patients with secondary BAS had more concurrent comorbid conditions compared with patients with primary BAS. However, these findings were not statistically significant. While these comorbid observations in patients with secondary BAS may be explained by the fact that they are older and thus more prone to chronic diseases, these findings may nonetheless have prognostic implications and thus leave room for future research.
- The role of histologic characteristics in the prognostication and outcome of BAS has not been fully validated. Our experience shows that the presence of tumor necrosis is associated with worse OS in BAS and this observation was statistically significant (p=.034). We, however found that other histologic parameters, including tumor grade, mitotic count, lymph node metastasis, and positive tumor margins had no significant effect on OS in BAS patients. Additionally, the expression of CD117 (p=.676), p53 (p= .847), D2-40 (p=.960), and c-Myc (p=.847) all had no impact on OS in BAS patients. While these findings are inconclusive due to the limited number of patients in this study, additional studies are needed to characterize the utility of these markers in BAS.
- During a median follow-up of 21 months, primary BAS with two (40%) reported deaths appears to have a worse OS compared with secondary BAS with three (25%) reported deaths. However, this difference in survival between primary and secondary BAS was not statistically significant (HR, 0.51; 95% CI, 0.09 to 3.28; p=.450). Our findings are consistent with what has been reported in similar studies [1,35]. However, Yin et al. [4] found a nominal increased death risk in secondary BAS due to advanced clinicopathologic features.
- With no consensus management guidelines for BAS, treatment includes a combination of wide local excision or mastectomy, with or without chemotherapy and/or radiotherapy. In this study, all patients with BAS were managed with wide local excision or mastectomy. Two patients with secondary BAS received additional chemotherapy and one patient with primary BAS received additional chemotherapy and radiotherapy. However, there was no difference between patients who were treated with surgery alone and those who received additional treatments (p=.35). One study reported no survival benefit in patients treated with routine radiation therapy in primary and secondary BAS, respectively [36]. Chemotherapy has been reported to be beneficial in high-grade and metastatic settings [36]. Therefore, we suggest that the management of patients with BAS should be optimally selected, based on their clinicopathologic characteristics.
- Our study is not devoid of limitations. We recognize that findings from this study are limited and may not be representative of the general population due to the small sample size and its retrospective nature. We also acknowledge that our findings of no statistically significant differences between primary and secondary BAS, are not conclusive due to the limited number of cases in our cohort. However, this is not unusual because BAS remains a rare disease with catastrophic outcomes, which limits the ability to conduct a prospective study and include a larger study population. Additionally, the median follow-up of less than 5 years is obviously inadequate to establish prognosis in this rare disease. However, our follow-up data is similar to the experiences of other studies [26,27,35,37,38] and this is understandable due to the rarity and adverse outcomes of this disease. Despite these limitations, our study highlights our experience from a tertiary healthcare center and provides additional information that is particularly relevant to the natural history of this very rare disease. In addition, the study team has dedicated surgical pathologists with expertise in breast pathology (SB and RAF) whose review of available study materials were invaluable in the conduct of this study.
- In summary, BAS is a rare and aggressive disease. On the basis of our results, only the presence of tumor necrosis was associated with worse OS in BAS. However, no histologic, immunohistochemical (CD117, p53, and c-Myc) or survival differences were identified between primary and secondary BAS in this study.
DISCUSSION
Supplementary Information
Ethics Statement
All procedures performed in the current study were approved by the Wayne State University IRB and/or national research ethics committee (063419M1X; 08/13/2019) in accordance with the 1964 Helsinki declaration and its later amendments. Formal written informed consent was not required with a waiver by the appropriate IRB and/or national research ethics committee.
Availability of Data and Material
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
Author contributions
Conceptualization: EA, RAF. Data curation: EA, HJ, SK. Formal analysis: HJ, SK. Investigation: EA, RAF, SB. Methodology: EA, RAF, SB, SK. Project administration: EA, RAF. Resources: EA, RAF. Supervision: RAF. Visualization: EA, RAF, SB. Writing—original draft preparation: EA. Writing—review and editing: EA, HJ, SK, RAF, SB.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
The Biostatistics and Bioinformatics Core is supported in part by NIH Center grant P30 CA022453 to the Karmanos Cancer Institute at Wayne State University.


| Variable | Total (n = 11) | Primary BAS (n = 3) | Secondary BAS (n = 8) | p-valuea |
|---|---|---|---|---|
| CD117 | > .99 | |||
| Positive | 7 (63.6) | 2 (66.7) | 5 (62.5) | |
| Negative | 4 (36.4) | 1 (33.3) | 3 (37.5) | |
| p53 | .236 | |||
| Positive | 4 (36.4) | 0 | 4 (50.0) | |
| Negative | 7 (63.6) | 3 (100) | 4 (50.0) | |
| D-240 | > .99 | |||
| Positive | 7 (63.6) | 2 (66.7) | 5 (62.5) | |
| Negative | 4 (36.4) | 1 (33.3) | 3 (37.5) | |
| CD31 | > .99 | |||
| Positive | 11 (100) | 3 (100) | 8 (100) | |
| Negative | 0 | 0 | 0 | |
| C-MYC | > .99 | |||
| Positive | 8 (72.7) | 2 (66.7) | 6 (75.0) | |
| Negative | 3 (27.3) | 1 (33.3) | 2 (25.0) |
| Variable | Total (n = 17) | Primary BAS (n = 5) | Secondary BAS (n = 12) | p-valuea |
|---|---|---|---|---|
| Age at diagnosis (yr) | 66 (23–76) | 36 (23–71) | 67 (33–76) | .246 |
| Race | > .99 | |||
| Caucasian | 12 (70.6) | 4 (80.0) | 8 (66.7) | |
| African American | 5 (29.4) | 1 (20.0) | 4 (33.3) | |
| Tumor size (cm) | 1.1 (0.4–28) | 2 (0.5–28) | 0.95 (0.4–2.5) | .437 |
| Missing | 8 | 2 | 6 | |
| Tumor grade | .087 | |||
| Low | 6 (35.3) | 2 (40.0) | 4 (33.3) | |
| Intermediate | 2 (11.8) | 2 (40.0) | 0 | |
| High | 9 (52.9) | 1 (20.0) | 8 (66.7) | |
| Tumor necrosis | .538 | |||
| Yes | 4 (23.5) | 2 (40.0) | 2 (16.7) | |
| No | 13 (76.5) | 3 (60.0) | 10 (83.3) | |
| Mitotic count | .593 | |||
| > 10/10 HPF | 7 (41.2) | 3 (60.0) | 4 (33.3) | |
| < 10/10 HPF | 10 (58.8) | 2 (40.0) | 8 (66.7) | |
| Lymph node metastasis | .191 | |||
| Yes | 3 (17.6) | 2 (40.0) | 1 (8.3) | |
| No | 14 (82.4) | 3 (60.0) | 11 (91.7) | |
| Positive margins | > .99 | |||
| Yes | 8 (47.1) | 2 (40.0) | 6 (50.0) | |
| No | 9 (52.9) | 3 (60.0) | 6 (50.0) | |
| Tumor site | .294 | |||
| Right | 9 (52.9) | 4 (80.0) | 5 (41.7) | |
| Left | 8 (47.1) | 1 (20.0) | 7 (58.3) | |
| Multifocal tumors | > .99 | |||
| Yes | 4 (23.5) | 1 (20.0) | 3 (25.0) | |
| No | 13 (76.5) | 4 (80.0) | 9 (75.0) | |
| Skin involved | .044 | |||
| Yes | 7 (41.2) | 0 | 7 (58.3) | |
| No | 10 (58.8) | 5 (100) | 5 (41.7) | |
| Obesity | .117 | |||
| Yes | 12 (70.6) | 2 (40.0) | 10 (83.3) | |
| No | 5 (29.4) | 3 (60.0) | 2 (16.7) | |
| Hypertension | .102 | |||
| Yes | 6 (35.3) | 0 | 6 (50.0) | |
| No | 11 (64.7) | 5 (100) | 6 (50.0) | |
| Smoking | > .99 | |||
| Yes | 4 (23.5) | 1 (20.0) | 3 (25.0) | |
| No | 13 (76.5) | 4 (80.0) | 9 (75.0) | |
| Diabetes | .338 | |||
| Yes | 7 (41.2) | 1 (20.0) | 6 (50.0) | |
| No | 10 (58.8) | 4 (80.0) | 6 (50.0) | |
| Treatment received | .353 | |||
| Surgery only | 14 (82.4) | 4 (80.0) | 10 (83.3) | |
| Surgery + chemotherapy | 2 (11.8) | 0 | 2 (16.7) | |
| Surgery + radiation + chemotherapy | 1 (5.9) | 1 (20.0) | 0 |
| Variable | Event/No. | HR (95% CI) | p-value |
|---|---|---|---|
| Age at diagnosis | 5/17 | 1.01 (0.96–1.07) | .845 |
| Race | |||
| Caucasian | 3/12 | Reference | |
| African American | 2/5 | 1.26 (0.21–6.55) | .787 |
| BAS type | |||
| Primary BAS | 2/5 | Reference | |
| Secondary BAS | 3/12 | 0.51 (0.09–3.28) | .450 |
| Tumor size (cm) | 3/9 | 1.04 (0.95–1.13) | .307 |
| Tumor grade | .638a | ||
| Low | 1/6 | Reference | |
| Intermediate | 1/2 | 3.33 (0.24–50.45) | .343 |
| High | 3/9 | 1.52 (0.25–15.79) | .659 |
| Tumor necrosis | |||
| No | 1/13 | Reference | |
| Yes | 4/4 | 6.24 (1.14–62.76) | .034 |
| Mitotic count | |||
| < 10/10 HPF | 1/10 | Reference | |
| > 10/10 HPF | 4/7 | 4.86 (0.86–49.67) | .075 |
| Lymph node metastasis | |||
| No | 3/14 | Reference | |
| Yes | 2/3 | 2.61 (0.42–14.00) | .278 |
| Positive margins | |||
| No | 3/9 | Reference | |
| Yes | 2/8 | 1.00 (0.17–5.13) | .998 |
| Tumor site | |||
| Left | 2/8 | Reference | |
| Right | 3/9 | 1.35 (0.26–8.26) | .719 |
| Multifocal tumors | |||
| No | 5/13 | Reference | |
| Yes | 0/4 | 0.37 (0.003–3.32) | .439 |
| Skin involved | |||
| No | 3/10 | Reference | |
| Yes | 2/7 | 0.78 (0.13–4.14) | .773 |
| Obesity | |||
| No | 3/5 | Reference | |
| Yes | 2/12 | 0.20 (0.03–1.10) | .063 |
| Hypertension | |||
| No | 4/11 | Reference | |
| Yes | 1/6 | 0.99 (0.10–5.40) | .990 |
| Smoking | |||
| No | 5/13 | Reference | |
| Yes | 0/4 | 0.45 (0.003–4.04) | .551 |
| Diabetes | |||
| No | 4/10 | Reference | |
| Yes | 1/7 | 0.58 (0.06–3.17) | .548 |
| Treatment received | |||
| Surgery only | 4/14 | Reference | |
| Surgery + chemotherapy/radiotherapy | 1/3 | 1.52 (0.15–8.29) | .671 |
| CD117 | |||
| Negative | 1/4 | Reference | |
| Positive | 2/7 | 1.55 (0.20–17.50) | .676 |
| p53 | |||
| Negative | 2/7 | Reference | |
| Positive | 1/4 | 1.23 (0.11–9.37) | .847 |
| D2-40 | |||
| Negative | 1/4 | Reference | |
| Positive | 2/7 | 0.95 (0.12–10.49) | .960 |
| c-Myc | |||
| Negative | 1/3 | Reference | |
| Positive | 2/8 | 0.82 (0.11–9.04) | .847 |
- 1. Abdou Y, Elkhanany A, Attwood K, Ji W, Takabe K, Opyrchal M. Primary and secondary breast angiosarcoma: single center report and a meta-analysis. Breast Cancer Res Treat 2019; 178: 523-33. ArticlePubMedPMCPDF
- 2. Brar R, West R, Witten D, Raman B, Jacobs C, Ganjoo K. Breast angiosarcoma: case series and expression of vascular endothelial growth factor. Case Rep Oncol 2009; 2: 242-50. ArticlePubMedPMC
- 3. Kunkiel M, Maczkiewicz M, Jagiello-Gruszfeld A, Nowecki Z. Primary angiosarcoma of the breast-series of 11 consecutive cases: a single-centre experience. Curr Oncol 2018; 25: e50-3. ArticlePubMedPMCPDF
- 4. Yin M, Wang W, Drabick JJ, Harold HA. Prognosis and treatment of non-metastatic primary and secondary breast angiosarcoma: a comparative study. BMC Cancer 2017; 17: 295.ArticlePubMedPMCPDF
- 5. Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol 2013; 20: 1267-74. ArticlePubMedPMCPDF
- 6. Arora TK, Terracina KP, Soong J, Idowu MO, Takabe K. Primary and secondary angiosarcoma of the breast. Gland Surg 2014; 3: 28-34. PubMedPMC
- 7. Moore A, Hendon A, Hester M, Samayoa L. Secondary angiosarcoma of the breast: can imaging findings aid in the diagnosis? Breast J 2008; 14: 293-8. ArticlePubMed
- 8. Kaur RP, Vasudeva K, Kumar R, Munshi A. Role of p53 gene in breast cancer: focus on mutation spectrum and therapeutic strategies. Curr Pharm Des 2018; 24: 3566-75. ArticlePubMedPDF
- 9. Croce CM, Thierfelder W, Erikson J, et al. Transcriptional activation of an unrearranged and untranslocated c-myc oncogene by translocation of a C lambda locus in Burkitt. Proc Natl Acad Sci U S A 1983; 80: 6922-6. ArticlePubMedPMC
- 10. Mastronikolis N, Ragos V, Kyrodimos E, et al. Mechanisms of Cmyc oncogenic activity in head and neck squamous cell carcinoma. J BUON 2019; 24: 2242-4. PubMed
- 11. Georgakopoulos G, Tsiambas E, Korkolopoulos P, et al. c-MYC and h-TERT co-expression in colon adenocarcinoma: a tissue microarray digitized image analysis. J BUON 2013; 18: 124-30. PubMed
- 12. La Rosa S, Bernasconi B, Vanoli A, et al. c-MYC amplification and c-myc protein expression in pancreatic acinar cell carcinomas. New insights into the molecular signature of these rare cancers. Virchows Arch 2018; 473: 435-41. ArticlePubMedPDF
- 13. Tsiambas E, Stamatelopoulos A, Baltayiannis N, et al. Evaluation of combined telomerase and c-myc expression in non-small cell lung carcinomas using tissue microarrays and computerized image analysis. J BUON 2005; 10: 533-9. PubMed
- 14. Miettinen M, Sarlomo-Rikala M, Lasota J. KIT expression in angiosarcomas and fetal endothelial cells: lack of mutations of exon 11 and exon 17 of C-kit. Mod Pathol 2000; 13: 536-41. ArticlePubMedPDF
- 15. Billings SD, McKenney JK, Folpe AL, Hardacre MC, Weiss SW. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol 2004; 28: 781-8. ArticlePubMed
- 16. de Bree E, van Coevorden F, Peterse JL, Russell NS, Rutgers EJ. Bilateral angiosarcoma of the breast after conservative treatment of bilateral invasive carcinoma: genetic predisposition? Eur J Surg Oncol 2002; 28: 392-5. ArticlePubMed
- 17. Komdeur R, Hoekstra HJ, Molenaar WM, et al. Clinicopathologic assessment of postradiation sarcomas: KIT as a potential treatment target. Clin Cancer Res 2003; 9: 2926-32. PubMed
- 18. Lae M, Lebel A, Hamel-Viard F, et al. Can c-myc amplification reliably discriminate postradiation from primary angiosarcoma of the breast? Cancer Radiother 2015; 19: 168-74. ArticlePubMed
- 19. Hung J, Hiniker SM, Lucas DR, et al. Sporadic versus radiation-associated angiosarcoma: a comparative clinicopathologic and molecular analysis of 48 cases. Sarcoma 2013; 2013: 798403.ArticlePubMedPMCPDF
- 20. Italiano A, Chen CL, Thomas R, et al. Alterations of the p53 and PIK3CA/AKT/mTOR pathways in angiosarcomas: a pattern distinct from other sarcomas with complex genomics. Cancer 2012; 118: 5878-87. ArticlePubMedPMC
- 21. Cordon-Cardo C, Reuter VE. Alterations of tumor suppressor genes in bladder cancer. Semin Diagn Pathol 1997; 14: 123-32. PubMed
- 22. Mallofre C, Castillo M, Morente V, Sole M. Immunohistochemical expression of CK20, p53, and Ki-67 as objective markers of urothelial dysplasia. Mod Pathol 2003; 16: 187-91. ArticlePubMed
- 23. Nwanze J, Siddiqui MT, Stevens KA, Saxe D, Cohen C. MYC immunohistochemistry Predicts MYC Rearrangements by FISH. Front Oncol 2017; 7: 209.ArticlePubMedPMC
- 24. Donnell RM, Rosen PP, Lieberman PH, et al. Angiosarcoma and other vascular tumors of the breast. Am J Surg Pathol 1981; 5: 629-42. ArticlePubMed
- 25. Rosen PP, Kimmel M, Ernsberger D. Mammary angiosarcoma: the prognostic significance of tumor differentiation. Cancer 1988; 62: 2145-51. ArticlePubMed
- 26. Wang XY, Jakowski J, Tawfik OW, Thomas PA, Fan F. Angiosarcoma of the breast: a clinicopathologic analysis of cases from the last 10 years. Ann Diagn Pathol 2009; 13: 147-50. ArticlePubMed
- 27. Hui A, Henderson M, Speakman D, Skandarajah A. Angiosarcoma of the breast: a difficult surgical challenge. Breast 2012; 21: 584-9. ArticlePubMed
- 28. Seinen JM, Styring E, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol 2012; 19: 2700-6. ArticlePubMedPMC
- 29. Jallali N, James S, Searle A, Ghattaura A, Hayes A, Harris P. Surgical management of radiation-induced angiosarcoma after breast conservation therapy. Am J Surg 2012; 203: 156-61. ArticlePubMed
- 30. Morgan EA, Kozono DE, Wang Q, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol 2012; 19: 3801-8. ArticlePubMedPDF
- 31. Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol 2005; 29: 983-96. PubMed
- 32. Hornick JL, Fletcher CD. Immunohistochemical staining for KIT (CD117) in soft tissue sarcomas is very limited in distribution. Am J Clin Pathol 2002; 117: 188-93. ArticlePubMed
- 33. Shet T, Malaviya A, Nadkarni M, et al. Primary angiosarcoma of the breast: observations in Asian Indian women. J Surg Oncol 2006; 94: 368-74. ArticlePubMed
- 34. Requena C, Rubio L, Lavernia J, et al. Immunohistochemical and fluorescence in situ hybridization analysis of MYC in a series of 17 cutaneous angiosarcomas: a single-center study. Am J Dermatopathol 2018; 40: 349-54. ArticlePubMed
- 35. Vorburger SA, Xing Y, Hunt KK, et al. Angiosarcoma of the breast. Cancer 2005; 104: 2682-8. ArticlePubMed
- 36. Sher T, Hennessy BT, Valero V, et al. Primary angiosarcomas of the breast. Cancer 2007; 110: 173-8. ArticlePubMedPMC
- 37. Marchal C, Weber B, de Lafontan B, et al. Nine breast angiosarcomas after conservative treatment for breast carcinoma: a survey from French comprehensive Cancer Centers. Int J Radiat Oncol Biol Phys 1999; 44: 113-9. ArticlePubMed
- 38. Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX study. J Clin Oncol 2008; 26: 5269-74. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Angiosarcoma: a systematic review of biomarkers in diagnosis, prognosis, and therapeutic strategies
Huyen Thuc Tran Luong, Sofie Vercammen, Ario de Marco, Hilde de Rooster, Antonio Cosma
Frontiers in Oncology.2025;[Epub] CrossRef - Etiology, pathogenesis, and management of angiosarcoma associated with implants and foreign body: Clinical cases and research updates
Ramy Samargandi
Medicine.2024; 103(18): e37932. CrossRef - Ovarian angiosarcoma: A systematic review of literature and survival analysis
Shafi Rehman, Arya Harikrishna, Amisha Silwal, B.R. Sumie, Safdar Mohamed, Nisha Kolhe, Meghana Maddi, Linh Huynh, Jesus Gutierrez, Yoshita Rao Annepu, Ameer Mustafa Farrukh
Annals of Diagnostic Pathology.2024; 73: 152331. CrossRef - Neoadjuvant chemotherapy for radiation associated angiosarcoma (RAAS) of the breast: A retrospective single center study
Stijn J.C. van der Burg, Sophie J.M. Reijers, Anke Kuijpers, Lotte Heimans, Astrid N. Scholten, Rick L.M. Haas, Hester van Boven, Willemijn M. Kolff, Marie-Jeanne T.F.D. Vrancken Peeters, Martijn Kerst, Beatrijs A. Seinstra, Neeltje Steeghs, Winette T.A.
The Breast.2024; 78: 103825. CrossRef - Lymph node involvement in secondary breast angiosarcoma – a case presentation
Adriana Irina Ciuvică, Tiberiu Augustin Georgescu , Andrei Dennis Voichiţoiu , Angela Arsene , Luchian Marinescu , George Ionuţ Bucur , Livia Iordache , Nahedd Saba
Romanian Journal of Morphology and Embryology.2024; 65(3): 523. CrossRef - Primary ovarian angiosarcoma: Two case reports and review of literature
Ying Zhou, Yi-Wen Sun, Xiao-Yang Liu, Dan-Hua Shen
World Journal of Clinical Cases.2023; 11(21): 5122. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure















Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
Fig. 8.
Fig. 9.
Fig. 10.
Fig. 11.
Fig. 12.
Fig. 13.
Fig. 14.
Fig. 15.
| Antibody | Clone | Dilution | Vendor | Retrieval (HIER) (min) | Antibody incubation (min) |
|---|---|---|---|---|---|
| c-kit (CD117) | Rabbit polyclonal | 1:250 | Dako | CC1: 36 | 60 |
| p53 | DO-7 mouse monoclonal | RTU | Ventana Medical Systems | CC1: 36 | 32 |
| c-Myc | Y69 (rabbit) | RTU | Ventana Medical Systems | CC1: 64 | 32 |
| CD31 | JC70 (mouse) | RTU | Cell Marque | CC1: 36 | 40 |
| D2-40 (podoplanin) | D2-40 (mouse) | RTU | Cell Marque | CC1: 36 | 32 |
| Variable | Total (n = 11) | Primary BAS (n = 3) | Secondary BAS (n = 8) | p-value |
|---|---|---|---|---|
| CD117 | > .99 | |||
| Positive | 7 (63.6) | 2 (66.7) | 5 (62.5) | |
| Negative | 4 (36.4) | 1 (33.3) | 3 (37.5) | |
| p53 | .236 | |||
| Positive | 4 (36.4) | 0 | 4 (50.0) | |
| Negative | 7 (63.6) | 3 (100) | 4 (50.0) | |
| D-240 | > .99 | |||
| Positive | 7 (63.6) | 2 (66.7) | 5 (62.5) | |
| Negative | 4 (36.4) | 1 (33.3) | 3 (37.5) | |
| CD31 | > .99 | |||
| Positive | 11 (100) | 3 (100) | 8 (100) | |
| Negative | 0 | 0 | 0 | |
| C-MYC | > .99 | |||
| Positive | 8 (72.7) | 2 (66.7) | 6 (75.0) | |
| Negative | 3 (27.3) | 1 (33.3) | 2 (25.0) |
| Variable | Total (n = 17) | Primary BAS (n = 5) | Secondary BAS (n = 12) | p-value |
|---|---|---|---|---|
| Age at diagnosis (yr) | 66 (23–76) | 36 (23–71) | 67 (33–76) | .246 |
| Race | > .99 | |||
| Caucasian | 12 (70.6) | 4 (80.0) | 8 (66.7) | |
| African American | 5 (29.4) | 1 (20.0) | 4 (33.3) | |
| Tumor size (cm) | 1.1 (0.4–28) | 2 (0.5–28) | 0.95 (0.4–2.5) | .437 |
| Missing | 8 | 2 | 6 | |
| Tumor grade | .087 | |||
| Low | 6 (35.3) | 2 (40.0) | 4 (33.3) | |
| Intermediate | 2 (11.8) | 2 (40.0) | 0 | |
| High | 9 (52.9) | 1 (20.0) | 8 (66.7) | |
| Tumor necrosis | .538 | |||
| Yes | 4 (23.5) | 2 (40.0) | 2 (16.7) | |
| No | 13 (76.5) | 3 (60.0) | 10 (83.3) | |
| Mitotic count | .593 | |||
| > 10/10 HPF | 7 (41.2) | 3 (60.0) | 4 (33.3) | |
| < 10/10 HPF | 10 (58.8) | 2 (40.0) | 8 (66.7) | |
| Lymph node metastasis | .191 | |||
| Yes | 3 (17.6) | 2 (40.0) | 1 (8.3) | |
| No | 14 (82.4) | 3 (60.0) | 11 (91.7) | |
| Positive margins | > .99 | |||
| Yes | 8 (47.1) | 2 (40.0) | 6 (50.0) | |
| No | 9 (52.9) | 3 (60.0) | 6 (50.0) | |
| Tumor site | .294 | |||
| Right | 9 (52.9) | 4 (80.0) | 5 (41.7) | |
| Left | 8 (47.1) | 1 (20.0) | 7 (58.3) | |
| Multifocal tumors | > .99 | |||
| Yes | 4 (23.5) | 1 (20.0) | 3 (25.0) | |
| No | 13 (76.5) | 4 (80.0) | 9 (75.0) | |
| Skin involved | .044 | |||
| Yes | 7 (41.2) | 0 | 7 (58.3) | |
| No | 10 (58.8) | 5 (100) | 5 (41.7) | |
| Obesity | .117 | |||
| Yes | 12 (70.6) | 2 (40.0) | 10 (83.3) | |
| No | 5 (29.4) | 3 (60.0) | 2 (16.7) | |
| Hypertension | .102 | |||
| Yes | 6 (35.3) | 0 | 6 (50.0) | |
| No | 11 (64.7) | 5 (100) | 6 (50.0) | |
| Smoking | > .99 | |||
| Yes | 4 (23.5) | 1 (20.0) | 3 (25.0) | |
| No | 13 (76.5) | 4 (80.0) | 9 (75.0) | |
| Diabetes | .338 | |||
| Yes | 7 (41.2) | 1 (20.0) | 6 (50.0) | |
| No | 10 (58.8) | 4 (80.0) | 6 (50.0) | |
| Treatment received | .353 | |||
| Surgery only | 14 (82.4) | 4 (80.0) | 10 (83.3) | |
| Surgery + chemotherapy | 2 (11.8) | 0 | 2 (16.7) | |
| Surgery + radiation + chemotherapy | 1 (5.9) | 1 (20.0) | 0 |
| Variable | Event/No. | HR (95% CI) | p-value |
|---|---|---|---|
| Age at diagnosis | 5/17 | 1.01 (0.96–1.07) | .845 |
| Race | |||
| Caucasian | 3/12 | Reference | |
| African American | 2/5 | 1.26 (0.21–6.55) | .787 |
| BAS type | |||
| Primary BAS | 2/5 | Reference | |
| Secondary BAS | 3/12 | 0.51 (0.09–3.28) | .450 |
| Tumor size (cm) | 3/9 | 1.04 (0.95–1.13) | .307 |
| Tumor grade | .638 |
||
| Low | 1/6 | Reference | |
| Intermediate | 1/2 | 3.33 (0.24–50.45) | .343 |
| High | 3/9 | 1.52 (0.25–15.79) | .659 |
| Tumor necrosis | |||
| No | 1/13 | Reference | |
| Yes | 4/4 | 6.24 (1.14–62.76) | .034 |
| Mitotic count | |||
| < 10/10 HPF | 1/10 | Reference | |
| > 10/10 HPF | 4/7 | 4.86 (0.86–49.67) | .075 |
| Lymph node metastasis | |||
| No | 3/14 | Reference | |
| Yes | 2/3 | 2.61 (0.42–14.00) | .278 |
| Positive margins | |||
| No | 3/9 | Reference | |
| Yes | 2/8 | 1.00 (0.17–5.13) | .998 |
| Tumor site | |||
| Left | 2/8 | Reference | |
| Right | 3/9 | 1.35 (0.26–8.26) | .719 |
| Multifocal tumors | |||
| No | 5/13 | Reference | |
| Yes | 0/4 | 0.37 (0.003–3.32) | .439 |
| Skin involved | |||
| No | 3/10 | Reference | |
| Yes | 2/7 | 0.78 (0.13–4.14) | .773 |
| Obesity | |||
| No | 3/5 | Reference | |
| Yes | 2/12 | 0.20 (0.03–1.10) | .063 |
| Hypertension | |||
| No | 4/11 | Reference | |
| Yes | 1/6 | 0.99 (0.10–5.40) | .990 |
| Smoking | |||
| No | 5/13 | Reference | |
| Yes | 0/4 | 0.45 (0.003–4.04) | .551 |
| Diabetes | |||
| No | 4/10 | Reference | |
| Yes | 1/7 | 0.58 (0.06–3.17) | .548 |
| Treatment received | |||
| Surgery only | 4/14 | Reference | |
| Surgery + chemotherapy/radiotherapy | 1/3 | 1.52 (0.15–8.29) | .671 |
| CD117 | |||
| Negative | 1/4 | Reference | |
| Positive | 2/7 | 1.55 (0.20–17.50) | .676 |
| p53 | |||
| Negative | 2/7 | Reference | |
| Positive | 1/4 | 1.23 (0.11–9.37) | .847 |
| D2-40 | |||
| Negative | 1/4 | Reference | |
| Positive | 2/7 | 0.95 (0.12–10.49) | .960 |
| c-Myc | |||
| Negative | 1/3 | Reference | |
| Positive | 2/8 | 0.82 (0.11–9.04) | .847 |
HIER, heat-induced epitope retrieval; RTU, ready to use reagent. CC1 is Ventana Medical Systems retrieval solution, RTU, at pH 8.0.
BAS, breast angiosarcoma. Fisher exact test.
Values are presented as median (range) or number (%). BAS, breast angiosarcoma; HPF, high-power field. Fisher exact test or Wilcox rank-sum test as appropriate.
Event/n, the number of events and patients; HR, hazard ratio; CI, confidence interval; BAS, breast angiosarcoma; HPF, high-power field. Global p-value calculated by likelihood ratio test.

 E-submission
E-submission