Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 57(1); 2023 > Article
-
Review
Infections and immunity: associations with obesity and related metabolic disorders -
Amitabha Ray1
 , Melissa J. L. Bonorden2
, Melissa J. L. Bonorden2 , Rajashree Pandit3
, Rajashree Pandit3 , Katai J. Nkhata4
, Katai J. Nkhata4 , Anupam Bishayee5
, Anupam Bishayee5
-
Journal of Pathology and Translational Medicine 2023;57(1):28-42.
DOI: https://doi.org/10.4132/jptm.2022.11.14
Published online: January 15, 2023
1College of Medical Science, Alderson Broaddus University, Philippi, WV, USA
2Division of Research and Development, Hormel Foods Corporation, Austin, MN, USA
3Division of Medical & Behavioral Health, Pueblo Community College, Pueblo, CO, USA
4WuXi AppTec, St. Paul, MN, USA
5Lake Erie College of Osteopathic Medicine, Bradenton, FL, USA
- Corresponding Author: Amitabha Ray, MD, PhD, College of Medical Science, Alderson Broaddus University, 101 College Hill Drive, Philippi, WV 26416, USA Tel: +1-304-457-6587, Fax: +1-304-457-6308, E-mail: ray.amit213@gmail.com
© 2023The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Abstract
- CORONAVIRUS DISEASE, OTHER VIRAL INFECTIONS, AND OBESITY-RELATED PROBLEMS
- COVID-19–ASSOCIATED OPPORTUNISTIC FUNGAL DISEASES AND DIABETES
- OTHER NON-VIRAL PATHOLOGIES: PRION AND PRION-LIKE DISEASES
- PARASITES IN OBESITY-RELATED PATHOLOGIES
- IMMUNE CELLS IN OBESITY
- INFLUENCE OF OBESITY ON VACCINE EFFECTIVENESS
- POTENTIAL INDICATORS OF OBESITY AND INFLAMMATION
- CONCLUSION
- NOTES
- REFERENCES
Abstract
- About one-fourth of the global population is either overweight or obese, both of which increase the risk of insulin resistance, cardiovascular diseases, and infections. In obesity, both immune cells and adipocytes produce an excess of pro-inflammatory cytokines that may play a significant role in disease progression. In the recent coronavirus disease 2019 (COVID-19) pandemic, important pathological characteristics such as involvement of the renin-angiotensin-aldosterone system, endothelial injury, and pro-inflammatory cytokine release have been shown to be connected with obesity and associated sequelae such as insulin resistance/type 2 diabetes and hypertension. This pathological connection may explain the severity of COVID-19 in patients with metabolic disorders. Many studies have also reported an association between type 2 diabetes and persistent viral infections. Similarly, diabetes favors the growth of various microorganisms including protozoal pathogens as well as opportunistic bacteria and fungi. Furthermore, diabetes is a risk factor for a number of prion-like diseases. There is also an interesting relationship between helminths and type 2 diabetes; helminthiasis may reduce the pro-inflammatory state, but is also associated with type 2 diabetes or even neoplastic processes. Several studies have also documented altered circulating levels of neutrophils, lymphocytes, and monocytes in obesity, which likely modifies vaccine effectiveness. Timely monitoring of inflammatory markers (e.g., C-reactive protein) and energy homeostasis markers (e.g., leptin) could be helpful in preventing many obesity-related diseases.
- During the last two decades, there have been three coronavirus disease outbreaks. The first was the 2002–2004 outbreak of severe acute respiratory syndrome coronavirus (SARS-CoV or SARSCoV-1) that emerged in China. A similar disease, caused by Middle East respiratory syndrome coronavirus (MERS-CoV), was initially detected in Saudi Arabia in 2012. The latest coronavirus pandemic (COVID-19) caused by SARS-CoV-2 began in December 2019 in China. The rapid spread of this infectious disease has been documented in different parts of the world, and about 6.7 million deaths have been recorded so far. SARS-CoV-2 is an enveloped positive-sense single-stranded linear RNA virus; the envelope is coated with envelope (E) and membrane (M) proteins as well as a spike (S) glycoprotein that is responsible for binding to the host target cell receptor angiotensin-converting enzyme 2 (ACE-2). In addition, other cellular components such as extracellular matrix metalloproteinase inducer/CD147, transmembrane serine protease 2, and ADAM metallopeptidase domain 17 are implicated in viral endocytosis [15].
- ACE catalyzes the conversion of angiotensin I to angiotensin II, which is an important step in the regulation of blood pressure via the renin-angiotensin-aldosterone system (RAAS). Moreover, ACE acts on several biomolecules including bradykinin, encephalin, substance P, and amyloid β-peptide (Aβ), as well as in various physiological processes such as renal development, male fertility, hematopoiesis, and immune responses [16,17]. Conversely, ACE-2 is an important homolog of ACE and responsible for the conversion of angiotensin II to angiotensin 1-7, thereby counterbalancing ACE activity [18]. Both ACE and ACE-2 are cell membrane-anchored proteins, expressed in several organs, and functionally antagonistic to each other.
- As mentioned earlier, to enter host cells, SARS-CoV-2 utilizes ACE-2 expressed in various organs, e.g., lung cells (pneumocytes and bronchial epithelium), gastrointestinal epithelium, and endothelial cells. Besides the lung parenchymal injury, there may be generalized endotheliitis (accumulation of lymphocytes, plasma cells and macrophages below the endothelium and in the perivascular spaces) [19]. In COVID-19, endothelial dysfunction is associated with the recruitment of immune cells and can result in many complications such as vasoconstriction, ischemia, inflammation, a pro-coagulant state, edema, and finally organ damage [20]. Moreover, abnormally increased levels of immune reaction and cytokines in different organs may cause a cytokine storm that can lead to additional organ dysfunction.
- Obesity and insulin resistance are strongly connected to the activity of RAAS. Interestingly, expression of ACE-2, the functional receptor for viral entry, has been found to be higher in adipose tissue (and therefore higher in obesity) [21,22]. Furthermore, obesity and its complications such as hypertension, insulin resistance, and type 2 diabetes are associated with a higher risk of COVID-19 disease severity and mortality [22-24]. The impact of obesity and/or diabetes on SARS-CoV-1 infection has not been properly evaluated, although a few studies have investigated MERS-CoV–linked pathologies. One study of 32 MERSCoV infected patients observed that mortality was significantly correlated with both obesity and diabetes [25]. A meta-analysis of 637 MERS-CoV cases revealed that diabetes and hypertension were present in roughly 50% of the patients [26]. Additionally, in the 2009 H1N1 influenza pandemic, obesity was also identified as an important risk factor for a poor prognosis [27,28]. In general, a number of reports have documented an association between type 2 diabetes and chronic viral infections (Table 1) [29-48].
- In addition to the disrupted immune response in obesity, other plausible mechanisms responsible for the poor prognosis of SARS-CoV-2 infection include obesity-associated pre-existing endothelial dysfunction, a reduction in respiratory compliance, dysregulated lipid metabolism, and an overabundance of pro-inflammatory cytokines [20,22,49]. It is worth mentioning that obese individuals have defective/decreased responses to vaccination [49]. As a result, the combined effects of obesity and viral infection may worsen the existing status of cytokines, which normally coordinate the immune system and physiological homeostasis.
CORONAVIRUS DISEASE, OTHER VIRAL INFECTIONS, AND OBESITY-RELATED PROBLEMS
- Generally, the majority of COVID-19 patients are asymptomatic or have minor symptoms. Less than 20% of cases require medical attention. According to the National Institutes of Health (NIH, USA), approximately 65% of individuals with serious illness from SARS-CoV-2 infection also had metabolic disorders such as obesity, type 2 diabetes, hypertension, and heart issues. Disease severity is associated with an uncontrolled immune response involving macrophages, neutrophils, different complement components, and a number of cytokines including TNF-α and interleukin-6 (IL-6). All these factors ultimately lead to a ‘cytokine storm’ and accompanying problems such as acute respiratory distress syndrome (ARDS), widespread vascular inflammation, and disseminated intravascular coagulation.
- Therefore, to prevent the aforementioned abnormal immune reactions, intervention with corticosteroids (like dexamethasone) has been considered [50]. Of note, apart from immune suppression, corticosteroid therapy can aggravate insulin resistance/type 2 diabetes, which is a risk factor for COVID-19 disease severity. Moreover, immune suppression can also allow the growth of opportunistic bacterial and fungal pathogens. Among hospitalized COVID-19 patients, investigators have isolated various bacterial strains such as coagulase-negative staphylococci, Klebsiella pneumoniae, and Pseudomonas aeruginosa, as well as a number of fungal agents including Candida albicans, Aspergillus fumigatus, and Cryptococcus neoformans [51-53]. Although Aspergillus and Candida species are commonly found in severely ill COVID-19 patients, studies have also documented other fungal pathogens. For example, researchers detected Histoplasma capsulatum complement fixation titers in patients with serious SARS-CoV-2 infection [53,54]. Other factors also thought to play a key role in fungal co-infection include hypoxemia/lack of tissue oxygenation, mechanical ventilation, and type 2 diabetes and associated hyperglycemia [55].
- Inappropriately managed diabetes increases the risk of infection of various body organs. Furthermore, diabetes can hinder both innate and adaptive immune mechanisms [56]. In the second wave of the COVID-19 pandemic in India (roughly from March to July 2021), there was a mysterious outbreak of mucormycosis or black fungus infection among patients with SARS-CoV-2 infection, and diabetic patients were more susceptible to mucormycosis [57]. In the normal immune system, all three complement activation pathways (classical, lectin, and alternative) play an important role protecting against fungal pathogens through mechanisms such as opsonization, humoral immune response stimulation, and chemotaxis of immune cells [58,59]. In diabetes, glycation of complement components (functional low levels) may lead to an impaired immune response [55,60]. Consequently, the impaired immune response in severe COVID-19 illness allows fungal infection.
COVID-19–ASSOCIATED OPPORTUNISTIC FUNGAL DISEASES AND DIABETES
- Prions or protease-resistant misfolded proteins are unusual protein aggregates (also termed amyloids/amyloid fibrils) that have a high proportion of β-sheets. The first prion identified was the PrP protein [61]. The gene encoding the normal cellular prion protein (PrPC, misfolded–PrPSc) is located on chromosome 20, and this glycoprotein is commonly present on the cell surface and can serve as a receptor for the Aβ peptide [62]. In addition, PrPC is expressed in different organs, particularly in the nervous and immune systems, and is thought to have numerous physiological functions such as cell surface scaffolding. Nevertheless, the mechanism of misfolding and aggregation into an abnormal prion-like conformation that again influences the misfolding of other associated protein copies in a self-propagating manner is indeed a unique biological process, and we can see this phenomenon even in yeast and fungi.
- Apart from typical prion diseases, which include CreutzfeldtJakob disease (CJD), kuru, Gerstmann-Sträussler-Scheinker disease, and fatal familial insomnia [63], there are other prion-like diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, type 2 diabetes, and amyloidosis. Certain proteins such as Aβ, tau, α-synuclein, and serum amyloid-A, which share some pathological characteristics with prions, have been implicated in these prion-like diseases [64,65]. For example, in Alzheimer’s and Parkinson’s diseases, aggregates of Aβ, tau, and α-synuclein can transmit the disease-pathology to experimental animals [65]. Of note, the fundamental features of Alzheimer’s disease lesions are the formation of Aβ in plaques and tau in tangles — both are β-sheet-rich misfolded variants of normal proteins.
- Currently, about 50 different proteins are thought to form various human disease-related amyloid fibrils [66]. Furthermore, some of these abnormal proteins can cross species barriers and affect humans such as bovine spongiform encephalopathy or madcow disease, which is linked to variant CJD (vCJD) [67]. Interestingly, aggregation of amylin or islet amyloid polypeptide has been found in the pancreatic islets of Langerhans in individuals with type 2 diabetes [66]. In addition, patients with type 2 diabetes have an increased risk of developing Alzheimer’s disease. Of note, a number of pathophysiological associations have been documented between Alzheimer’s disease and metabolic disorders such as type 2 diabetes, obesity, and metabolic syndrome [68]. On the other hand, both Alzheimer’s and prion diseases are neurodegenerative disorders, and there are several neuropathological commonalities and genetic connections between these diseases [69].
- In humans, the most common prion disease is CJD with an incidence of about 1 case per 1 million population per year worldwide [70]. The majority (~85%) of cases of this rare disease occur sporadically, while ~10%–15% cases are due to familial or genetic mutations. The remaining cases (less than 1%) are linked to obvious environmental causes such as contaminated tissue transplant or surgical instruments (i.e., iatrogenic CJD) and contaminated meat consumption (i.e., vCJD). Another prion disease, kuru, once endemic in the Eastern Highlands of Papua New Guinea, disappeared rapidly after the cessation of ritual cannibalism. Clinically, kuru has a prodromal phase and three stages. Interestingly, in the second stage, obesity is a common feature, which could also exist in early disease in association with bulimia [71]. However, unlike dissemination of microbial infections, prion disease is spread through ingestion or inoculation of contaminated materials (aside from sporadic and genetic inheritance). Therefore, different mechanisms by which conventional infectious diseases are spread, e.g., skin/mucosal contact, droplet/aerosol (airborne, coughs or sneezes), body fluids (like urine), fecal-oral route, vector-borne transmission, and fomites, have no role in the spread of prion diseases.
- A number of investigators recorded an alteration of gut microbiota (dysbiosis) in prion disease and other neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease [72-76]. Gut microbiota dysbiosis has also been reported in obesity and metabolic disorders including type 2 diabetes [77-80]. Nonetheless, there is a close similarity between protein misfolding disorders and pathogenesis of prions (infectious/transmissible proteins). For example, misfolded Aβ and tau (of Alzheimer’s disease) spread in a way very similar to misfolded PrP [81-83]. As mentioned above, PrPC functions as a cell surface receptor for Aβ. Fascinatingly, PrPC acts in the propagation of prions as well as to transduce the neurotoxic signals from Aβ oligomers [82]. Experimentally, the transmission of kuru and CJD to various in vivo models has been performed by different laboratories [84-86]. Similarly, the pathologies of Aβ, tau, and α-synuclein could be transmitted in a prion-like manner to in vivo models by injecting the misfolded proteins. In this connection, the results of selected studies have been mentioned briefly in Table 2 [87-92].
- Both in vitro and in vivo studies have identified a number of compounds that have anti-prion activity. Congo red, polyanionic glycans, quinacrine, and compB either inhibit the formation of PrPSc or enhance the degradation of PrPSc. Interestingly, in experimental studies, anle138b (a recently developed drug) has been documented to inhibit the formation of pathological aggregates of α-synuclein (Parkinson’s disease) and tau (Alzheimer’s disease) proteins in addition to inhibiting aggregation of prion protein (PrPSc) [93,94]. A clear understanding of protein misfolding and its association with metabolic disorders at the molecular level may provide insights into their precise pathogenesis and result in the development of prevention strategies. Furthermore, early diagnosis (preferably in the preclinical stage) and prompt intervention are critical when there are few pathological protein aggregates in the brain. Accordingly, identification of appropriate early diagnostic markers is essential.
OTHER NON-VIRAL PATHOLOGIES: PRION AND PRION-LIKE DISEASES
- Parasitic infestations can have a diverse range of consequences/ sequelae. Ryan et al. [95] posited that helminth-related anti-inflammatory mechanisms may be beneficial. Lifestyle-linked chronic diseases usually have a strong connection with inflammation. In this context, hookworm species, particularly Necator americanus, may have a protective effect in inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis as well as in celiac disease. In addition, these authors reported an inverse association between human helminth infection and insulin resistance/type 2 diabetes. In an experimental study on male C57BL/6 wild-type mice, infection with Nippostrongylus brasiliensis (rodent hookworm) significantly decreased various diabetes-associated parameters such as fasting blood glucose and weight gain [96]. Similarly, studies in human subjects have demonstrated that infection with Strongyloides stercoralis (threadworm) can reduce the risk of type 2 diabetes by modulating the expression of different pro-inflammatory cytokines [97-99]. A study from Thailand showed that Opisthorchis viverrini (liver fluke) infection had a protective effect against hyperglycemia and metabolic disease risk [100]. In contrast, many studies have reported that individuals with parasitic diseases are more susceptible to diabetes or that diabetic persons are at higher risk of infection with various parasites, e.g., Ascaris lumbricoides, Echinococcus granulosus, Enterobius vermicularis, Schistosoma mansoni, S. haematobium, Hymenolepis nana, hookworm, and Taenia species, as well as protozoan parasites such as Giardia lamblia, Entamoeba histolytica, and Cryptosporidium species (Table 3) [101-116]. Some of these helminths are also considered to be responsible for cancer development. For example, S. haematobium can induce squamous cell carcinoma of the urinary bladder, and O. viverrini may cause cholangiocarcinoma/bile duct cancer [117]. Unprecedentedly, a report revealed the dissemination of cancer cells from H. nana to different organs of a human host [118].
- Two major types of primary liver cancer, i.e., hepatocellular carcinoma (~75% of all liver cancers) and cholangiocarcinoma (10%–20% of cases), are uniquely linked to a diverse group of risk factors namely viral hepatitis (hepatitis B virus and hepatitis C virus), obesity, type 2 diabetes, alcohol consumption, smoking, and toxic substances including aflatoxins produced by Aspergillus species. Moreover, risk factors for cholangiocarcinoma are inflammatory bowel disease, parasitic infections, and hepatolithiasis. Along with O. viverrini, infection with Clonorchis sinensis (another liver fluke) can cause the development of cholangiocarcinoma, particularly in Southeast Asia [119]. In addition, C. sinensis and A. lumbricoides may promote hepatolithiasis [119,120]. Interestingly, the co-occurrence of O. viverrini infection and diabetes has been shown to be associated with hepatobiliary tract damage and malignant transformation [121,122].
- For helminthic infestations that are predominantly connected with tissue migration, numerous studies have documented the presence of peripheral eosinophilia (or increased number of eosinophils in the peripheral blood) or Loeffler’s syndrome (i.e., accumulation of eosinophils in the lung) [123,124]. Although eosinophils play a significant role in various physiologic processes including innate and adaptive immunity, data on the precise role of human eosinophils in defense against helminths are limited. Data on the specific anti-parasitic role of mast cells and basophils, which behave functionally similar to eosinophils in hypersensitivity/allergic inflammation, are also inadequate [125-127]. By contrast, there is a growing body of evidence that neutrophils play a protective role against several parasitic infections such as E. histolytica, Leishmania, and Plasmodium infections [128-130]. Neutrophils may clear the parasites by a number of mechanisms including phagocytosis, generation of reactive oxygen species (ROS), and formation of neutrophil extracellular traps.
PARASITES IN OBESITY-RELATED PATHOLOGIES
- White blood cells (WBCs or leukocytes) are fundamental components of the immune system. Several studies have reported a quantitative increase in WBCs among obese people [131-133]. A major component of the observed increase in WBC counts is neutrophils. In peripheral blood, more than half of the WBCs (up to about 70%) are neutrophils. It is notable that these cells are present in marginated (recoverable part) and circulating pools in almost equal proportions, while circulating cells have a remarkably short lifespan. Nonetheless, along with increased WBC counts, many investigators have noticed significantly higher levels of neutrophils in obese people [134-136]. Neutrophils from obese subjects have also been found to be functionally more active than neutrophils from lean controls. In obesity, the levels of neutrophil-released superoxides are significantly greater than in normal controls [137]. Apart from an elevated leukocyte count and release of ROS like superoxide (i.e., oxidative burst), the lymphocyte count is also elevated in obesity. In a communitybased study of 116 obese women, investigators found that obesity was connected with an increase in certain lymphocyte subset counts, excepting natural killer (NK) and cytotoxic/suppressor T cells [138]. Multivariate analyses of 322 women who were longitudinally followed from 1999 through 2003 revealed that increasing body weight was independently related to higher WBC, total lymphocyte, CD4, and CD8 counts [139]. Similarly, a study of 119 Saudi female university students showed that both BMI and waist-to-hip ratio (WHR) were significantly correlated with WBC, neutrophil, and CD4 lymphocyte counts [140]. Furthermore, in a cross-sectional, retrospective study of 223 participants (female- 104), BMI was found to have a significant positive relationship with WBC, neutrophil, and lymphocyte counts [141]. These findings indicate that being overweight or obese may impact both innate and adaptive immune responses to numerous pathophysiological phenomena including infections by various pathogens.
- Obesity is associated with chronic low-grade inflammation. A relatively inexpensive method to assess the systemic pro-inflammatory state is to determine the blood neutrophil-to-lymphocyte ratio (NLR) [142]. It is believed that this parameter also indicates the stress situation of our body. A healthy range is between 1 and 2; values more than 3 or less than 0.7 in adults are pathognomonic [142]. In a study that compared NLR between obese individuals with insulin resistance (n=46) and those without (n=51), both the neutrophil count and NLR were found to be significantly higher in the insulin resistance group [143]. It is worth mentioning that insulin resistance or type 2 diabetes, which is a common sequela of obesity, is also associated with elevated total and differential WBC counts [131,132,144]. In a study of 600 subjects (BMI: 27.9±4.7) selected from 474,616 patients who visited Severance Hospital, Seoul, South Korea between January 2008 and March 2017, NLR was significantly associated with intra-abdominal visceral adipose tissue volume [145]. In addition, WBCs and levels of the serum inflammatory marker high-sensitivity C-reactive protein (hs-CRP) were strongly correlated with visceral adipose tissue. In a cross-sectional study in Taiwan, a total of 26,016 subjects with metabolic syndrome were recruited between 2004 and 2013 [146]. Of note, the American Heart Association criteria for metabolic syndrome are central obesity, hypertension, high blood glucose and triglycerides, and lower high-density lipoprotein cholesterol levels in the blood. In this study, obesity and related anthropometric parameters such as WHR were positively associated with NLR and C-reactive protein (CRP) in both sexes. Another study of 1,267 subjects (1,068 female and 199 male) collected from the out-patient clinic of Düzce University Hospital, Turkey during 2012–2013 reported that while WBC, neutrophil, and lymphocyte counts as well as level of hs-CRP exhibited a significant interrelationship with BMI, BMI was not correlated with NLR [147]. NLR may not be a better pro-inflammatory indicator than CRP or hs-CRP. Nonetheless, a different study from Turkey of 306 morbidly obese subjects (BMI ≥40) demonstrated significantly higher NLR levels in these subjects than normal controls [148]. Moreover, the authors concluded that elevated NLR was an independent and strong predictor of type 2 diabetes in morbidly obese individuals. A cross-sectional study from the 2011–2016 National Health and Nutrition Examination Survey (NHANES 2011–2016, a US population database) recorded a positive association between BMI and NLR in healthy adult female participants (n=3,201) [149]. The above studies indicate that being overweight or obese is linked with certain circulating markers that can be used affordably to evaluate systemic inflammatory status.
- Lymphocytes (T-cells, B-cells, NK cells) play a significant role in obesity-linked inflammation [150]. A study from Germany revealed an impaired NK cells phenotype and subset alterations in obesity [151]. Another study of 169 subjects demonstrated an increase in total lymphocytes along with granulocytes and a decrease in the NK cell population among persons with metabolic syndrome and increased visceral adipose tissue [152]. Furthermore, an increase in memory cells was also documented in those subjects with an increased BMI and visceral adipose tissue. Impaired B-cell and T-cell function has been observed in high-fat (HF) diet-induced obese mice [153].
- In HF diet-induced obese C57BL/6J female mice, investigators noted higher circulating monocytes in the HF group than the standard chow diet-fed mice [154]. Likewise, a number of studies involving human subjects have recorded an increased monocyte count in obese individuals [132,138,155,156]. However, other studies found no correlation between BMI and blood monocyte count [140,151]. Monocytes are the largest cells in our blood and normally up to 10% of WBCs are monocytes. Monocytes can be classified into three categories depending on their surface receptors: classical (CD14+), intermediate (CD14+ and low levels of CD16+), and non-classical (CD16+ along with lower levels of CD14+). In a study of 58 obese subjects and 25 metabolically healthy lean controls, numbers of both intermediate and non-classical monocytes were higher in obese subjects than lean controls [157]. Interestingly, these investigators also found that the levels of intermediate monocytes were positively and significantly related with the obese group’s serum triglyceride levels and mean blood pressure. Monocytes can differentiate into macrophages after migration to different tissues of our body— therefore, macrophages are present in the extracellular space. A number of reports have confirmed the accumulation of macrophages in the excess adipose tissue of obese individuals [157-159]. These infiltrated macrophages in adipose tissue create an inflammatory environment due to their production of several pro-inflammatory molecules. Consequently, macrophage infiltration and adipose tissue inflammation are important pathological processes that contribute to systemic inflammation and various complications such as insulin resistance and metabolic syndrome.
IMMUNE CELLS IN OBESITY
- The efficacy and effectiveness of any vaccine varies considerably and no vaccines can provide 100% protection. According to the WHO, vaccine effectiveness is associated with a number of factors including age, gender, ethnicity, and other accompanying health conditions. The efficacy of a vaccine is evaluated by estimating the development of disease among vaccinated people in comparison with a placebo/control group in controlled clinical trials (i.e., ideal conditions). Conversely, vaccine effectiveness refers to how a vaccine actually performs in different populations.
- The basic biological mechanisms underlying vaccine non-responsiveness are not well known. However, there is evidence that both carbohydrate and fat metabolic pathways are involved in responsiveness to vaccines [160]. Furthermore, obesity has been proposed to be associated with inadequate vaccine responsiveness [161]. Apart from well-established health-related problems such as insulin resistance and hypertension, the obesity-related chronic low-grade inflammatory state has adverse effects on the immune system [162]. Obviously, more research is needed to understand the effects of obesity on vaccine effectiveness.
INFLUENCE OF OBESITY ON VACCINE EFFECTIVENESS
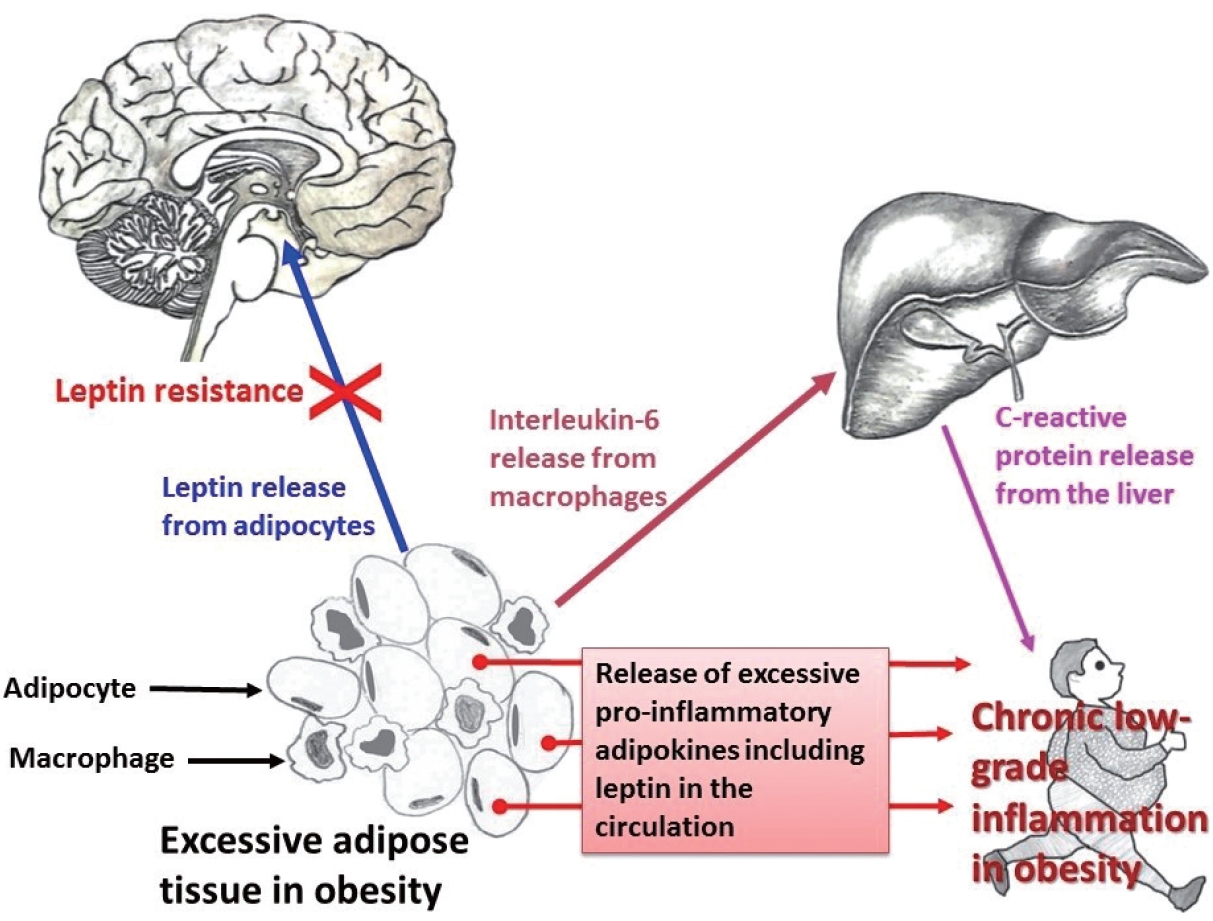
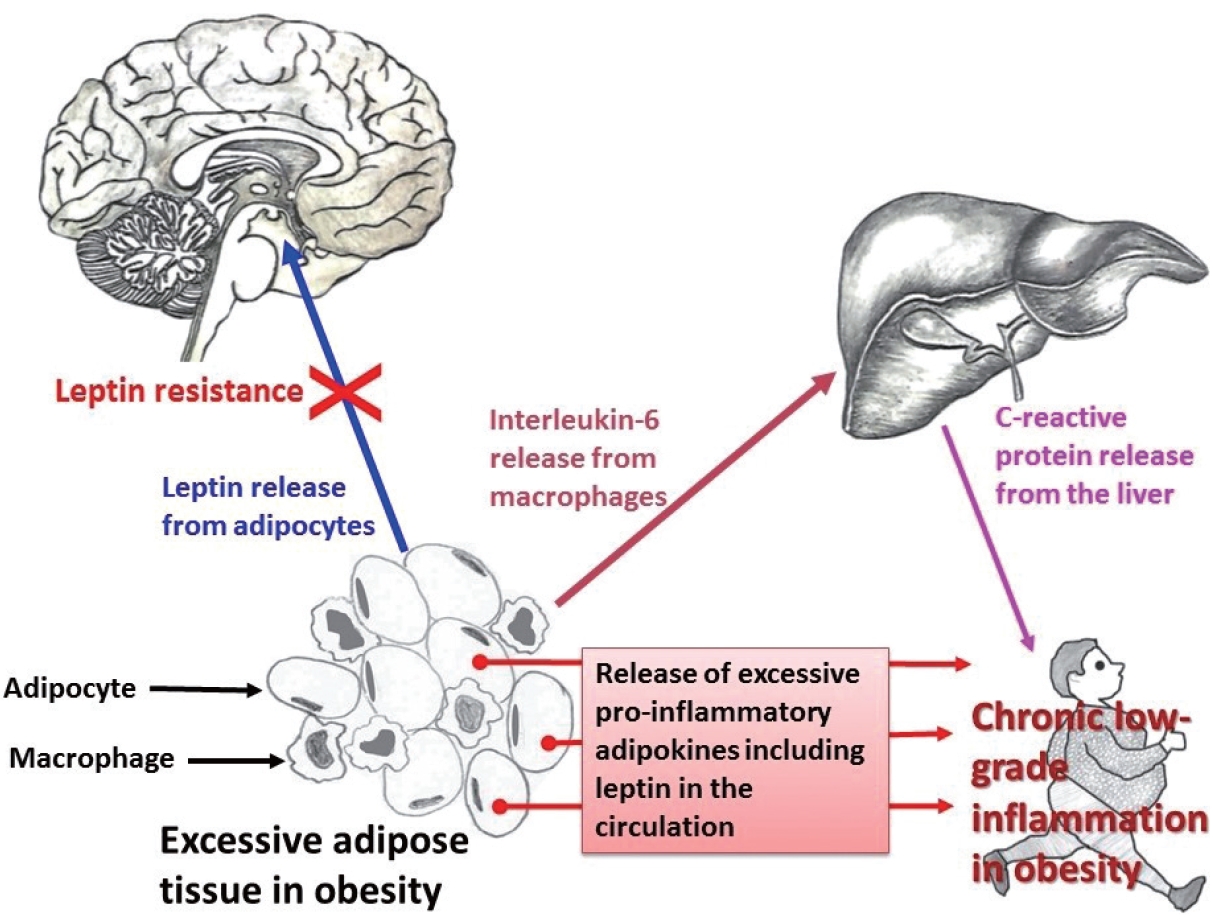
- Obesity and CRP have been demonstrated to be positively correlated [163]. Furthermore, CRP is widely used as a marker of inflammation. Along with its role in inflammation, CRP also functions significantly in host defense against different pathogenic organisms [164]. CRP is present in at least two distinct forms: pentameric and monomeric (mCRP) isoforms, which have diverse activities and functional characteristics. Dissociation of the pentameric group into monomeric forms occurs at sites of inflammation and the monomeric form then may participate in local inflammation. CRP is primarily produced by the liver and its blood levels may increase from 0.8 mg/L (approximate normal value) to more than 500 mg/L in inflammatory conditions [165]. However, CRP is involved in several pathophysiological processes such as activation of the complement system, phagocytosis, promotion of apoptosis, release of nitric oxide (NO), and biosynthesis of various cytokines particularly pro-inflammatory cytokines such as TNF-α, MCP-1, and IL-6 [166]. In addition, it is believed that mCRP can stimulate the process of chemotaxis and recruitment of circulating WBCs to sites of inflammation. Studies have documented associations (both positive and negative) between CRP and various hormone-like cytokines (adipokines) that are released from adipose tissue [167-169]; in particular, with the pro-inflammatory adipokine leptin (Fig. 1).
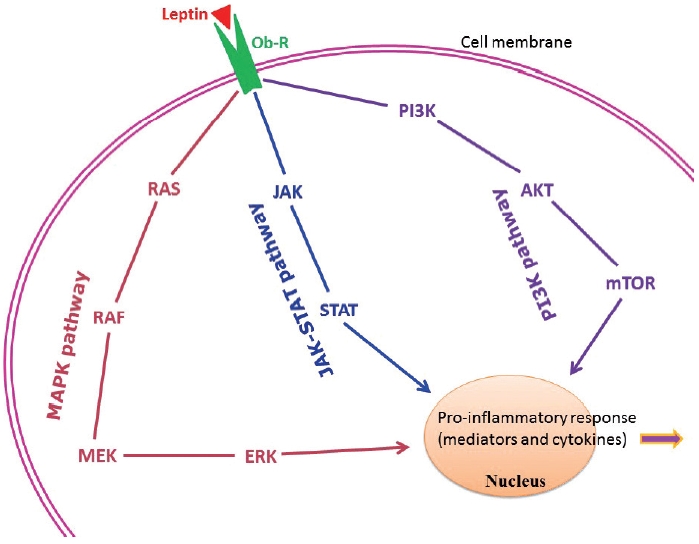
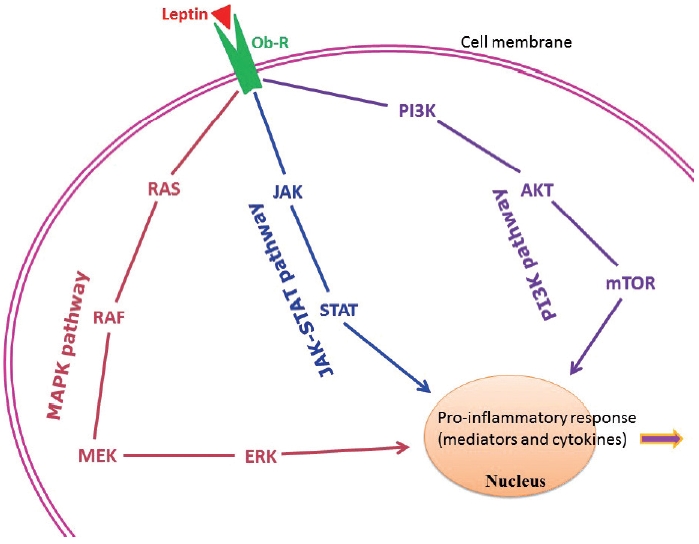
- Adipose tissue behaves like an endocrine organ. As mentioned earlier, several hormone-like cytokines or adipokines are secreted from adipose tissue or fat cells. In general, the majority of these adipokines are pro-inflammatory, for example, leptin, visfatin, and resistin. However, a few anti-inflammatory adipokines such as omentin, apelin, and adiponectin are also secreted. Nevertheless, the majority of published studies have focused mainly on two adipokines– pro-inflammatory leptin and anti-inflammatory adiponectin. These two adipokines are involved in a number of biological mechanisms both under normal health conditions as well as under pathological circumstances. Interestingly, both adipokines are closely linked with our immune system. Leptin is a 16-kD protein produced primarily by adipocytes. Its main function is maintenance of energy homeostasis through regulation of the arcuate nucleus of the hypothalamus. Leptin is associated with both innate and adaptive immune responses [170]. This adipokine has a close connection with inflammatory molecules including IL-6, TNF-α, NO, eicosanoid, and cyclooxygenase (particularly cyclooxygenase 2) [171], as well as intracellular signaling pathways connected with inflammation such as mitogen-activated protein kinase, Janus kinase/signal transducer and activator of transcription, and phosphatidylinositol-3-kinase (Fig. 2). In addition, leptin promotes chemotaxis, phagocytosis, and release of ROS [170,172].
- Higher circulating levels of both leptin and CRP have been demonstrated to be correlated with disease severity and poor prognosis in patients with COVID-19 [173-175]. In a recently published report from Italy, COVID-19 patients with pneumonia had increased circulating levels of leptin and IL-6 and lower adiponectin levels than age- and sex-matched healthy controls [176]. Similar findings were documented in another study from the Netherlands [177]. In contrast, a group of investigators hypothesized that increased blood levels of leptin could be due to patients’ obesity and unrelated to disease pathology [178,179]. A number of mechanisms have been proposed to explain the poor prognostic role of leptin in COVID-19. van der Voort et al. reasoned that SARS-CoV-2 infection, by inducing higher leptin production, might overactivate leptin receptors in pulmonary tissue, ultimately enhancing local inflammation in the lungs [177]. Higher leptin levels could also activate monocytes and thus upregulate the expression of pro-inflammatory cytokines in monocytes, resulting in dysregulation of immune responses, finally leading to ARDS and multiple organ failure [173,180]. As mentioned earlier, leptin receptors are present in all immune cells. Therefore, leptin may affect the functions of these cells. Understanding the precise role of leptin and its interactions with different adipokines (both pro-inflammatory and anti-inflammatory) and other classical hormones such as insulin, insulin-like growth factors, and estrogen, will help elucidate the relationships between obesity-related problems and immune mechanisms.
POTENTIAL INDICATORS OF OBESITY AND INFLAMMATION
- The recent COVID-19 pandemic has renewed interest in infectious diseases, and the disease pathology itself is a meeting place of both communicable and non-communicable diseases. For this reason, many authors have described the grave situation of 2020 and 2021 as ‘double pandemics’– the pandemic of COVID-19 and the long-continued global problem of obesity [1,181,182]. Apart from bacterial and fungal infections that are a common occurrence in obesity-related health conditions like type 2 diabetes, a number of prion-like diseases have a close link with these metabolic disorders. Regular assessment of a few markers such as CRP and leptin and adjustment of lifestyle factors could help protect against pathogens as well as metabolic diseases.
CONCLUSION
Ethics Statement
Not applicable.
Availability of Data and Material
The datasets generated or analyzed during the current study are available in the [MEDLINE] repository.
Code Availability
Not applicable.
Author Contributions
Conceptualization: AR. Data collection and assembly: RP. Formal analysis: KJN. Project administration: MJLB, RP. Supervision: MJLB, RP. Writing— original draft: AR, MJLB. Figures: KJN. Writing—review & editing: RP, KJN, AB. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
| Study | Patients’ details | Finding |
|---|---|---|
| Ndako et al. (2021); Nigeria [29] | 180 Diabetic patients and 100 non-diabetics controls | Higher risk of HBV infection among type 2 diabetic patients than non-diabetics |
| Iovanescu et al. (2015); Romania [30] | 246 Patients with chronic liver disease (136 chronic viral hepatitis, 110 viral liver cirrhosis) | A significant association between diabetes mellitus and HCV-induced chronic liver disease |
| Cheng et al. (2006); Hong Kong [31] | 2,838 Type 2 diabetes patients | HBV-infected patients had earlier onset of diabetes, higher frequency of retinopathy, and increased risk of end-stage renal disease than non-HBV–infected patients |
| Vírseda Chamorro et al. (2006); Spain [32] | 305 Patients who came for HCV assessment | A relationship between HCV infection and type 2 diabetes |
| Arao et al. (2003); Japan [33] | 866 Patients with chronic viral disease (707 HCV-infected and 159 HBV-infected) | HCV infection was closely associated with diabetes, and cirrhosis was an independent risk factor for diabetes |
| Dworzanski et al. (2019); Poland [34] | 173 Diabetic patients and 50 persons without diabetes | Prevalence of EBV, HPV, and EBV+HPV co-infection was significantly higher in diabetic patients than those without diabetes |
| Karjala et al. (2011); USA [35] | Data from the National Health and Examination and Nutritional Examination Survey (NHANES) 2007–2008 | Obesity was significantly associated with HSV-1 infection |
| Fernandez-Real et al. (2007); Spain [36] | 74 Healthy middle-aged men from the general population | Significant positive relation between HSV-1 titer and fat mass |
| Sun et al. (2003); China [37] | 1,244 Inpatients (408 with dyslipidemia and 836 controls) | Prevalence of HSV-2 seropositivity was significantly higher in patients with dyslipidemia. BMI, diabetes, and hypertension were more common in patients with dyslipidemia than those without |
| Woelfle et al. (2022); Germany [38] | From the German population–based KORA cohort (pre-diabetes, n = 1,257) | HSV-2 and CMV were associated with pre-diabetes incidence |
| Yoo et al. (2019); Korea [39] | 576 Adults with CMV diseases | Type 2 diabetes cases had a higher incidence of CMV diseases |
| Chen et al. (2012); Netherlands [40] | 549 Participants | CMV seropositive subjects were more likely to have type 2 diabetes |
| Roberts and Cech (2005); USA [41] | 113 Hemodialysis patients (83 type 2 diabetes and 30 controls) | A higher seroprevalence of anti-CMV IgG among diabetes patients |
| Chiu et al. (1997); Canada [42] | Endarterectomy specimens from 76 patients with carotid artery stenosis and 20 normal carotid artery and aortic tissue autopsy specimens | CMV was detected in carotid atherosclerotic plaques from 27 cases (35.5%) |
| Reinholdt et al. (2021); Denmark [43] | Male population (n = 2,528,756), nationwide registry-based cohort study | Increased incidence rate of HPV-related anogenital intraepithelial neoplasia and cancer among men with diabetes than non-diabetic men |
| Sobti et al. (2019); UK [44] | 210 Patients with HNSCC | Prevalence of developing HPV-16–positive HNSCC was 3.79 times higher in diabetic patients than in those without diabetes. Moreover, diabetes was a risk factor for a poorer prognosis |
| Slama et al. (2021); USA [45] | 1,584 Men with pre-diabetes (793 with HIV, 791 without HIV), over a median 12-year follow-up | 40% higher risk for the development of diabetes among men with HIV |
| Kubiak et al. (2021); South Africa [46] | 1,369 Persons with HIV | Among adults with HIV, diabetes and pre-diabetes were common |
| Hema et al. (2021); Burkina Faso [47] | 4,259 Patients in a cross-sectional study | Prevalence of diabetes and hypertension was higher among persons with HIV on ART than the general population |
| Jeremiah et al. (2020); Tanzania [48] | 1,947 Adults (336 with HIV on ART, 956 with HIV ART-naïve, 655 without HIV) | Prevalence of diabetes was high, particularly among HIV-infected ART-naïve persons |
| Study | Study design | Finding |
|---|---|---|
| Ayers et al. (2017) [87] | Injection of β-synuclein fibrils in M83 transgenic micea through different peripheral routes, i.e., intramuscular (hind limb muscle), intravenous (tail veins), and intraperitoneal. | Injection of α-synuclein fibrils via these peripheral routes in M83 mice induced a robust α-synuclein pathology in the central nervous system. |
| Betemps et al. (2014) [88] | Transgenic M83 mice were inoculated intracerebrally in the striato-cortical areab with brain homogenates from sick M83 mice. | Disease acceleration following intracerebral inoculation suggests that disease propagation involves a prion-like mechanism. |
| Boluda et al. (2015) [89] | Intracerebral injection of Alzheimer’s disease brain extracts enriched in pathological tau in young mutant P301S tau transgenic mice (PS19)c approximately 6–9 months before they show the onset of mutant tau transgene-induced tau pathology. | At 1-month post-injection, inoculated Alzheimer’s disease-tau in young PS19 mice induced tau pathology predominantly in neuronal perikarya (neuron cell body). With longer post-injection survival periods of up to 6 months, tau pathology spread to different brain regions distant from the inoculated sites. |
| Guo et al. (2016) [90] | 2–3-Month-old C57BL6 and C57BL6/C3H F1 mice were intracerebrally inoculated with different tau fibrils; 15–19-month-old C57BL6 mice were injected with Alzheimer’s disease-tau. | Intracerebral inoculation of tau fibrils purified from Alzheimer’s disease brains, but not synthetic tau fibrils, resulted in the formation of abundant tau inclusions in the brain of non-transgenic mice. |
| Lam et al. (2021) [91] | The posterior cingulate cortexd areas of 1.5-year-old male mouse lemurs (Microcebus murinus) were inoculated with either Alzheimer’s disease or control brain extracts. | After 21 months, amyloid beta (Aβ) and tau pathologies developed in all Alzheimer-inoculated animals (n = 12) while no control brain extract-inoculated animals (n = 6) developed such lesions. |
| Morales et al. (2015) [92] | Brain extracts from 18–20 months old tg2576 micee (having significant amyloid deposits) were serially diluted (10−7 dilution) and intracerebrally injected into 50–55-day-old tg2576 mice. | Administration of misfolded Aβ significantly accelerated amyloid deposition in young mice. |
aThe M83 transgenic mouse model overexpresses A53T mutated human α-synuclein protein, which is connected with buildup of pathognomonic Ser129-phosphorylated α-synuclein in the central nervous system. Abnormal accumulation of misfolded α-synuclein is linked to synucleinopathies including Parkinson’s disease;
bStriato-cortical area: The corpus striatum (subcortical basal ganglia) and the adjacent cerebral cortex in the forebrain region;
cPS19 transgenic mouse expresses the P301S mutant form of human microtubule-associated protein tau. This hyper-phosphorylated and insoluble protein accumulates in the brain;
dPosterior cingulate cortex: Situated at the posterior part of the cingulate gyrus in the medial part of the inferior parietal lobe, above the posterior end of the corpus callosum;
eThe Tg2576 mouse model overexpresses a mutant form of amyloid precursor protein (APP695SWE, found in early-onset familial Alzheimer’s disease), which has the double mutation- APPK670M/671L. The most common neurodegenerative diseases: Alzheimer’s disease and Parkinson’s disease.
| Study | Subject | Important finding |
|---|---|---|
| Udoh et al. (2020); Nigeria [105] | Cross-sectional study of 208 diabetic patients | Diabetic patients were reservoirs of asymptomatic Plasmodium falciparum. |
| Wyss et al. (2017); Sweden [106] | Retrospective observational study on 937 adults with malaria | Comorbidities, specifically obesity and diabetes, were risk factors for severe malaria in adults diagnosed with Plasmodium falciparum. |
| Danquah et al. (2010); Ghana [107] | Case-control study of 946 diabetic patients and 520 controls | Patients with type 2 diabetes had a 46% increased risk for infection with Plasmodium falciparum. |
| Vizzoni et al. (2018); Brazil [108] | Cross-sectional study of 619 patients with Chagas disease | Elderly patients had a high frequency of hypertension and other comorbidities such as diabetes and dyslipidemia. |
| dos Santos et al. (1999); Brazil [109] | Cross-sectional study of female patients with Chagas disease (n = 362) and controls (n = 285) | Diabetes/hyperglycemia was more prevalent in patients with the cardiac form of Chagas disease than in controls, or patients with gastrointestinal problems or the asymptomatic form of the disease. |
| Soltani et al. (2021); Iran [110] | Case-control study of 105 diabetic patients and 150 controls | Chronic Toxoplasma gondii infection was significantly associated with diabetes. |
| Li et al. (2018); China [111] | Case-control study of 1,200 diabetic patients (type 1, 2, and gestational) and 1,200 matched controls | Diabetic patients had a significantly higher Toxoplasma gondii seroprevalence than controls. |
| Reeves et al. (2013); Germany [112] | 999 Randomly selected adults | Obese persons had significantly higher Toxoplasma gondii seropositivity than non-obese individuals. |
| Machado et al. (2018); Brazil [113] | Descriptive study of 156 diabetic individuals | Frequencies of Giardia lamblia were higher in individuals with type 2 diabetes than those without. |
| Sisu et al. (2021); Ghana [114] | Cross-sectional study of 152 diabetes patients | Diabetes patients appeared susceptible to infections with Giardia lamblia, Entamoeba hystolytica, and Cryptosporidium parvum. |
| Akinbo et al. (2013); Nigeria [115] | 150 Diabetic patients and 30 controls | Diabetes was significantly associated with intestinal parasitic infections (like Entamoeba histolytica). |
| Alemu et al. (2018); Ethiopia [116] | Cross-sectional study of 215 diabetic patients | Intestinal parasites were found more frequently in diabetic patients compared to data from other similar studies. Cryptosporidium parvum was the parasite found with the highest frequency. |
- 1. Gammone MA, D’Orazio N. COVID-19 and obesity: overlapping of two pandemics. Obes Facts 2021; 14: 579-85. ArticlePubMedPMCPDF
- 2. Sattar N, Valabhji J. Obesity as a risk factor for severe COVID-19: summary of the best evidence and implications for health care. Curr Obes Rep 2021; 10: 282-9. ArticlePubMedPMCPDF
- 3. Dayaramani C, De Leon J, Reiss AB. Cardiovascular disease complicating COVID-19 in the elderly. Medicina (Kaunas) 2021; 57: 833.ArticlePubMedPMC
- 4. Ghilotti F, Bellocco R, Ye W, Adami HO, Trolle Lagerros Y. Obesity and risk of infections: results from men and women in the Swedish National March Cohort. Int J Epidemiol 2019; 48: 1783-94. ArticlePubMedPDF
- 5. Dhurandhar NV, Bailey D, Thomas D. Interaction of obesity and infections. Obes Rev 2015; 16: 1017-29. ArticlePubMed
- 6. Frydrych LM, Bian G, O'Lone DE, Ward PA, Delano MJ. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J Leukoc Biol 2018; 104: 525-34. ArticlePubMedPDF
- 7. Chakraborty S, Bhattacharyya R, Banerjee D. Infections: a possible risk factor for type 2 diabetes. Adv Clin Chem 2017; 80: 227-51. PubMed
- 8. de Heredia FP, Gomez-Martinez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc 2012; 71: 332-8. ArticlePubMed
- 9. Cinkajzlova A, Mraz M, Haluzik M. Adipose tissue immune cells in obesity, type 2 diabetes mellitus and cardiovascular diseases. J Endocrinol 2021; 252: R1-22. ArticlePubMed
- 10. Iskander K, Farhour R, Ficek M, Ray A. Obesity-related complications: few biochemical phenomena with reference to tumorigenesis. Malays J Pathol 2013; 35: 1-15. PubMed
- 11. Ray A. Cancer and comorbidity: the role of leptin in breast cancer and associated pathologies. World J Clin Cases 2018; 6: 483-92. ArticlePubMedPMC
- 12. Taylor EB. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin Sci (Lond) 2021; 135: 731-52. ArticlePubMedPMCPDF
- 13. Fernandez-Riejos P, Najib S, Santos-Alvarez J, et al. Role of leptin in the activation of immune cells. Mediators Inflamm 2010; 2010: 568343.PubMedPMC
- 14. Conus S, Bruno A, Simon HU. Leptin is an eosinophil survival factor. J Allergy Clin Immunol 2005; 116: 1228-34. ArticlePubMed
- 15. Ahmetaj-Shala B, Vaja R, Atanur SS, George PM, Kirkby NS, Mitchell JA. Cardiorenal tissues express SARS-CoV-2 entry genes and basigin (BSG/CD147) increases with age in endothelial cells. JACC Basic Transl Sci 2020; 5: 1111-23. ArticlePubMedPMC
- 16. Lubbe L, Cozier GE, Oosthuizen D, Acharya KR, Sturrock ED. ACE2 and ACE: structure-based insights into mechanism, regulation and receptor recognition by SARS-CoV. Clin Sci (Lond) 2020; 134: 2851-71. ArticlePubMedPMCPDF
- 17. Wong MK. Angiotensin converting enzymes. In: Takei Y, Ando H, Tsutsui K, eds. Handbook of hormones. Oxford: Elsevier Academic Press, 2016; 263-e29D-4.
- 18. Kuba K, Imai Y, Penninger JM. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ J 2013; 77: 301-8. ArticlePubMed
- 19. Varga Z. Endotheliitis in COVID-19. Pathologe 2020; 41(Suppl 2):99-102. ArticlePubMedPMCPDF
- 20. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395: 1417-8. ArticlePubMedPMC
- 21. Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes Med 2020; 19: 100283.ArticlePubMedPMC
- 22. Al-Sabah S, Al-Haddad M, Al-Youha S, Jamal M, Almazeedi S. COVID-19: impact of obesity and diabetes on disease severity. Clin Obes 2020; 10: e12414.PubMedPMC
- 23. Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ 2020; 371: m3862.ArticlePubMed
- 24. Albini A, Di Guardo G, Noonan DM, Lombardo M. The SARSCoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blockerbased cardiovascular therapies. Intern Emerg Med 2020; 15: 759-66. ArticlePubMedPMCPDF
- 25. Halim AA, Alsayed B, Embarak S, Yaseen T, Dabbous S. Clinical characteristics and outcome of ICU admitted MERS corona virus infected patients. Egypt J Chest Dis Tuberc 2016; 65: 81-7. ArticlePubMed
- 26. Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis 2016; 49: 129-33. ArticlePubMedPMC
- 27. Honce R, Schultz-Cherry S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol 2019; 10: 1071.ArticlePubMedPMC
- 28. Bhattacharya I, Ghayor C, Perez Dominguez A, Weber FE. From influenza virus to novel corona virus (SARS-CoV-2): the contribution of obesity. Front Endocrinol (Lausanne) 2020; 11: 556962.ArticlePubMedPMC
- 29. Ndako JA, Nwankiti OO, Olorundare JO, et al. Studies on the serological markers for hepatitis B virus infection among type 2 diabetic patients. J Clin Lab Anal 2021; 35: e23464.ArticlePubMedPMCPDF
- 30. Iovanescu VF, Streba CT, Ionescu M, et al. Diabetes mellitus and renal involvement in chronic viral liver disease. J Med Life 2015; 8: 483-7. PubMedPMC
- 31. Cheng AY, Kong AP, Wong VW, et al. Chronic hepatitis B viral infection independently predicts renal outcome in type 2 diabetic patients. Diabetologia 2006; 49: 1777-84. ArticlePubMedPDF
- 32. Virseda Chamorro I, Virseda Chamorro M, Prieto Carbajo RI, Jaqueti Aroca J. Hepatitis C as a risk factor of diabetes mellitus type 2. Rev Clin Esp 2006; 206: 167-71. PubMed
- 33. Arao M, Murase K, Kusakabe A, et al. Prevalence of diabetes mellitus in Japanese patients infected chronically with hepatitis C virus. J Gastroenterol 2003; 38: 355-60. ArticlePubMedPDF
- 34. Dworzanski J, Drop B, Kliszczewska E, Strycharz-Dudziak M, Polz-Dacewicz M. Prevalence of Epstein-Barr virus, human papillomavirus, cytomegalovirus and herpes simplex virus type 1 in patients with diabetes mellitus type 2 in south-eastern Poland. PLoS One 2019; 14: e0222607.ArticlePubMedPMC
- 35. Karjala Z, Neal D, Rohrer J. Association between HSV1 seropositivity and obesity: data from the National Health and Nutritional Examination Survey, 2007-2008. PLoS One 2011; 6: e19092.ArticlePubMedPMC
- 36. Fernandez-Real JM, Ferri MJ, Vendrell J, Ricart W. Burden of infection and fat mass in healthy middle-aged men. Obesity (Silver Spring) 2007; 15: 245-52. ArticlePubMed
- 37. Sun YH, Pei WD, Wu YJ, Wang GG. Association of herpes simplex virus type2 infection with dyslipidemia in Chinese. Zhonghua Yi Xue Za Zhi 2003; 83: 1774-7. PubMed
- 38. Woelfle T, Linkohr B, Waterboer T, et al. Health impact of seven herpesviruses on (pre)diabetes incidence and HbA(1c): results from the KORA cohort. Diabetologia 2022; 65: 1328-38. ArticlePubMedPMCPDF
- 39. Yoo SG, Han KD, Lee KH, La Y, Kwon DE, Han SH. Impact of cytomegalovirus disease on new-onset type 2 diabetes mellitus: population-based matched case-control cohort study. Diabetes Metab J 2019; 43: 815-29. ArticlePubMedPMCPDF
- 40. Chen S, de Craen AJ, Raz Y, et al. Cytomegalovirus seropositivity is associated with glucose regulation in the oldest old: results from the Leiden 85-plus study. Immun Ageing 2012; 9: 18.ArticlePubMedPMCPDF
- 41. Roberts BW, Cech I. Association of type 2 diabetes mellitus and seroprevalence for cytomegalovirus. South Med J 2005; 98: 686-92. ArticlePubMed
- 42. Chiu B, Viira E, Tucker W, Fong IW. Chlamydia pneumoniae, cytomegalovirus, and herpes simplex virus in atherosclerosis of the carotid artery. Circulation 1997; 96: 2144-8. ArticlePubMed
- 43. Reinholdt K, Thomsen LT, Munk C, et al. Incidence of HPV-related anogenital intraepithelial neoplasia and cancer in men with diabetes compared with the general population. Epidemiology 2021; 32: 705-11. ArticlePubMed
- 44. Sobti A, Sharif-Askari FS, Khan S, et al. Logistic regression prediction model identify type 2 diabetes mellitus as a prognostic factor for human papillomavirus-16 associated head and neck squamous cell carcinoma. PLoS One 2019; 14: e0217000.ArticlePubMedPMC
- 45. Slama L, Barrett BW, Abraham AG, et al. Risk for incident diabetes is greater in prediabetic men with HIV than without HIV. AIDS 2021; 35: 1605-14. ArticlePubMed
- 46. Kubiak RW, Kratz M, Motala AA, et al. Clinic-based diabetes screening at the time of HIV testing and associations with poor clinical outcomes in South Africa: a cohort study. BMC Infect Dis 2021; 21: 789.ArticlePubMedPMCPDF
- 47. Hema A, Poda A, Tougouma JB, et al. Diabetes mellitus and high blood pressure over risk in HIV-infected people followed at Souro Sanou University Hospital Day Hospital, Bobo-Dioulasso 2018. Rev Epidemiol Sante Publique 2021; 69: 72-7. PubMed
- 48. Jeremiah K, Filteau S, Faurholt-Jepsen D, et al. Diabetes prevalence by HbA1c and oral glucose tolerance test among HIV-infected and uninfected Tanzanian adults. PLoS One 2020; 15: e0230723.ArticlePubMedPMC
- 49. Albashir AA. The potential impacts of obesity on COVID-19. Clin Med (Lond) 2020; 20: e109-13. ArticlePubMedPMC
- 50. Singh AK, Majumdar S, Singh R, Misra A. Role of corticosteroid in the management of COVID-19: a systemic review and a Clinician’s perspective. Diabetes Metab Syndr 2020; 14: 971-8. ArticlePubMedPMC
- 51. Hughes S, Troise O, Donaldson H, Mughal N, Moore LS. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect 2020; 26: 1395-9. ArticlePubMedPMC
- 52. Rawson TM, Wilson RC, Holmes A. Understanding the role of bacterial and fungal infection in COVID-19. Clin Microbiol Infect 2021; 27: 9-11. ArticlePubMed
- 53. Cafardi J, Haas D, Lamarre T, Feinberg J. Opportunistic fungal infection associated with COVID-19. Open Forum Infect Dis 2021; 8: ofab016.ArticlePubMedPMCPDF
- 54. Taylor M, Ghodasara A, Ismail A, Gauhar U, El-Kersh K. Disseminated histoplasmosis in an immunocompetent patient after COVID-19 pneumonia. Cureus 2021; 13: e17269.ArticlePubMedPMC
- 55. Amin A, Vartanian A, Poladian N, et al. Root causes of fungal coinfections in COVID-19 infected patients. Infect Dis Rep 2021; 13: 1018-35. ArticlePubMedPMC
- 56. Erener S. Diabetes, infection risk and COVID-19. Mol Metab 2020; 39: 101044.ArticlePubMedPMC
- 57. Choudhary NK, Jain AK, Soni R, Gahlot N. Mucormycosis: a deadly black fungus infection among COVID-19 patients in India. Clin Epidemiol Glob Health 2021; 12: 100900.ArticlePubMedPMC
- 58. Kuchi Bhotla H, Balasubramanian B, Meyyazhagan A, et al. Opportunistic mycoses in COVID-19 patients/survivors: epidemic inside a pandemic. J Infect Public Health 2021; 14: 1720-6. ArticlePubMedPMC
- 59. Speth C, Rambach G, Wurzner R, Lass-Florl C. Complement and fungal pathogens: an update. Mycoses 2008; 51: 477-96. ArticlePubMed
- 60. Soni S, Namdeo Pudake R, Jain U, Chauhan N. A systematic review on SARS-CoV-2-associated fungal coinfections. J Med Virol 2022; 94: 99-109. ArticlePubMedPDF
- 61. Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 1982; 216: 136-44. ArticlePubMed
- 62. Linden R. The biological function of the prion protein: a cell surface scaffold of signaling modules. Front Mol Neurosci 2017; 10: 77.ArticlePubMedPMC
- 63. Sikorska B, Liberski PP. Human prion diseases: from Kuru to variant Creutzfeldt-Jakob disease. Subcell Biochem 2012; 65: 457-96. ArticlePubMed
- 64. Aguilar-Calvo P, Garcia C, Espinosa JC, Andreoletti O, Torres JM. Prion and prion-like diseases in animals. Virus Res 2015; 207: 82-93. ArticlePubMed
- 65. Duyckaerts C, Clavaguera F, Potier MC. The prion-like propagation hypothesis in Alzheimer’s and Parkinson’s disease. Curr Opin Neurol 2019; 32: 266-71. ArticlePubMed
- 66. Iadanza MG, Jackson MP, Hewitt EW, Ranson NA, Radford SE. A new era for understanding amyloid structures and disease. Nat Rev Mol Cell Biol 2018; 19: 755-73. ArticlePubMedPDF
- 67. Lee J, Kim SY, Hwang KJ, Ju YR, Woo HJ. Prion diseases as transmissible zoonotic diseases. Osong Public Health Res Perspect 2013; 4: 57-66. ArticlePubMedPMC
- 68. Tumminia A, Vinciguerra F, Parisi M, Frittitta L. Type 2 diabetes mellitus and Alzheimer’s disease: role of insulin signalling and therapeutic implications. Int J Mol Sci 2018; 19: 3306.ArticlePubMedPMC
- 69. Kellett KA, Hooper NM. Prion protein and Alzheimer disease. Prion 2009; 3: 190-4. ArticlePubMedPMC
- 70. Sitammagari KK, Masood W, et al. Creutzfeldt Jakob disease [Internet]. Treasure Island: StatPearls Publishing 2022 [cited 2022 Mar 9]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507860/.
- 71. Collinge J, Whitfield J, McKintosh E, et al. A clinical study of kuru patients with long incubation periods at the end of the epidemic in Papua New Guinea. Philos Trans R Soc Lond B Biol Sci 2008; 363: 3725-39. ArticlePubMedPMCPDF
- 72. Guo Y, Xu Y, Lin X, et al. Creutzfeldt-Jakob disease: alterations of gut microbiota. Front Neurol 2022; 13: 832599.ArticlePubMedPMC
- 73. Yang D, Zhao D, Shah SZA, et al. Implications of gut microbiota dysbiosis and metabolic changes in prion disease. Neurobiol Dis 2020; 135: 104704.ArticlePubMed
- 74. Fang P, Kazmi SA, Jameson KG, Hsiao EY. The microbiome as a modifier of neurodegenerative disease risk. Cell Host Microbe 2020; 28: 201-22. ArticlePubMedPMC
- 75. Liu S, Gao J, Zhu M, Liu K, Zhang HL. Gut microbiota and dysbiosis in Alzheimer’s disease: implications for pathogenesis and treatment. Mol Neurobiol 2020; 57: 5026-43. ArticlePubMedPMCPDF
- 76. Nishiwaki H, Ito M, Ishida T, et al. Meta-analysis of gut dysbiosis in Parkinson’s disease. Mov Disord 2020; 35: 1626-35. PubMedPDF
- 77. Gasmi Benahmed A, Gasmi A, Dosa A, et al. Association between the gut and oral microbiome with obesity. Anaerobe 2021; 70: 102248.ArticlePubMed
- 78. Thomas MS, Blesso CN, Calle MC, Chun OK, Puglisi M, Fernandez ML. Dietary influences on gut microbiota with a focus on metabolic syndrome. Metab Syndr Relat Disord 2022; 20: 429-39. ArticlePubMed
- 79. Gradisteanu Pircalabioru G, Liaw J, Gundogdu O, et al. Effects of the lipid profile, type 2 diabetes and medication on the metabolic syndrome-associated gut microbiome. Int J Mol Sci 2022; 23: 7509.ArticlePubMedPMC
- 80. Bielka W, Przezak A, Pawlik A. The role of the gut microbiota in the pathogenesis of diabetes. Int J Mol Sci 2022; 23: 480.ArticlePubMedPMC
- 81. Gomez-Gutierrez R, Morales R. The prion-like phenomenon in Alzheimer’s disease: evidence of pathology transmission in humans. PLoS Pathog 2020; 16: e1009004.ArticlePubMedPMC
- 82. Zhou J, Liu B. Alzheimer’s disease and prion protein. Intractable Rare Dis Res 2013; 2: 35-44. ArticlePubMedPMC
- 83. Soto C. Transmissible proteins: expanding the prion heresy. Cell 2012; 149: 968-77. ArticlePubMedPMC
- 84. Liberski PP, Gajos A, Sikorska B, Lindenbaum S. Kuru, the first human prion disease. Viruses 2019; 11: 232.ArticlePubMedPMC
- 85. Brandner S, Jaunmuktane Z. Prion disease: experimental models and reality. Acta Neuropathol 2017; 133: 197-222. ArticlePubMedPMCPDF
- 86. Manuelidis L, Chakrabarty T, Miyazawa K, Nduom NA, Emmerling K. The kuru infectious agent is a unique geographic isolate distinct from Creutzfeldt-Jakob disease and scrapie agents. Proc Natl Acad Sci U S A 2009; 106: 13529-34. ArticlePubMedPMC
- 87. Ayers JI, Brooks MM, Rutherford NJ, et al. Robust central nervous system pathology in transgenic mice following peripheral injection of alpha-synuclein fibrils. J Virol 2017; 91: e02095-16. PubMedPMC
- 88. Betemps D, Verchere J, Brot S, et al. Alpha-synuclein spreading in M83 mice brain revealed by detection of pathological alpha-synuclein by enhanced ELISA. Acta Neuropathol Commun 2014; 2: 29.PubMedPMC
- 89. Boluda S, Iba M, Zhang B, Raible KM, Lee VM, Trojanowski JQ. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol 2015; 129: 221-37. ArticlePubMedPDF
- 90. Guo JL, Narasimhan S, Changolkar L, et al. Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J Exp Med 2016; 213: 2635-54. ArticlePubMedPMCPDF
- 91. Lam S, Petit F, Herard AS, et al. Transmission of amyloid-beta and tau pathologies is associated with cognitive impairments in a primate. Acta Neuropathol Commun 2021; 9: 165.ArticlePubMedPMCPDF
- 92. Morales R, Bravo-Alegria J, Duran-Aniotz C, Soto C. Titration of biologically active amyloid-beta seeds in a transgenic mouse model of Alzheimer’s disease. Sci Rep 2015; 5: 9349.PubMedPMC
- 93. Wagner J, Ryazanov S, Leonov A, et al. Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease. Acta Neuropathol 2013; 125: 795-813. PubMedPMCPDF
- 94. Wagner J, Krauss S, Shi S, et al. Reducing tau aggregates with anle138b delays disease progression in a mouse model of tauopathies. Acta Neuropathol 2015; 130: 619-31. ArticlePubMedPMCPDF
- 95. Ryan SM, Eichenberger RM, Ruscher R, Giacomin PR, Loukas A. Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities. PLoS Pathog 2020; 16: e1008508.ArticlePubMedPMC
- 96. Khudhair Z, Alhallaf R, Eichenberger RM, et al. Gastrointestinal helminth infection improves insulin sensitivity, decreases systemic inflammation, and alters the composition of gut microbiota in distinct mouse models of type 2 diabetes. Front Endocrinol (Lausanne) 2020; 11: 606530.ArticlePubMed
- 97. Hays R, Esterman A, Giacomin P, Loukas A, McDermott R. Does Strongyloides stercoralis infection protect against type 2 diabetes in humans? Evidence from Australian Aboriginal adults. Diabetes Res Clin Pract 2015; 107: 355-61. ArticlePubMed
- 98. Rajamanickam A, Munisankar S, Bhootra Y, et al. Metabolic consequences of concomitant Strongyloides stercoralis infection in patients with type 2 diabetes mellitus. Clin Infect Dis 2019; 69: 697-704. ArticlePubMed
- 99. Rajamanickam A, Munisankar S, Dolla C, et al. Helminth infection modulates systemic pro-inflammatory cytokines and chemokines implicated in type 2 diabetes mellitus pathogenesis. PLoS Negl Trop Dis 2020; 14: e0008101.ArticlePubMedPMC
- 100. Muthukumar R, Suttiprapa S, Mairiang E, et al. Effects of Opisthorchis viverrini infection on glucose and lipid profiles in human hosts: a cross-sectional and prospective follow-up study from Thailand. Parasitol Int 2020; 75: 102000.ArticlePubMed
- 101. Htun NS, Odermatt P, Paboriboune P, et al. Association between helminth infections and diabetes mellitus in adults from the Lao People’s Democratic Republic: a cross-sectional study. Infect Dis Poverty 2018; 7: 105.ArticlePubMedPMCPDF
- 102. Moudgil V, Rana R, Tripathi PK, Farooq U, Sehgal R, Khan MA. Coprevalence of parasitic infections and diabetes in Sub-Himalayan region of Northern India. Int J Health Sci (Qassim) 2019; 13: 19-24.
- 103. Ambachew S, Assefa M, Tegegne Y, Zeleke AJ. The prevalence of intestinal parasites and their associated factors among diabetes mellitus patients at the University of Gondar Referral Hospital, northwest Ethiopia. J Parasitol Res 2020; 2020: 8855965.ArticlePubMedPMCPDF
- 104. Almugadam BS, Ibrahim MK, Liu Y, et al. Association of urogenital and intestinal parasitic infections with type 2 diabetes individuals: a comparative study. BMC Infect Dis 2021; 21: 20.ArticlePubMedPMCPDF
- 105. Udoh BE, Iwalokun BA, Etukumana E, Amoo J. Asymptomatic falciparum malaria and its effects on type 2 diabetes mellitus patients in Lagos, Nigeria. Saudi J Med Med Sci 2020; 8: 32-40. ArticlePubMed
- 106. Wyss K, Wangdahl A, Vesterlund M, et al. Obesity and diabetes as risk factors for severe Plasmodium falciparum malaria: results from a Swedish nationwide study. Clin Infect Dis 2017; 65: 949-58. ArticlePubMedPMC
- 107. Danquah I, Bedu-Addo G, Mockenhaupt FP. Type 2 diabetes mellitus and increased risk for malaria infection. Emerg Infect Dis 2010; 16: 1601-4. ArticlePubMedPMC
- 108. Vizzoni AG, Varela MC, Sangenis LH, Hasslocher-Moreno AM, do Brasil P, Saraiva RM. Ageing with Chagas disease: an overview of an urban Brazilian cohort in Rio de Janeiro. Parasit Vectors 2018; 11: 354.ArticlePubMedPMCPDF
- 109. dos Santos VM, da Cunha SF, Teixeira Vde P, et al. Frequency of diabetes mellitus and hyperglycemia in chagasic and non-chagasic women. Rev Soc Bras Med Trop 1999; 32: 489-96. PubMed
- 110. Soltani S, Tavakoli S, Sabaghan M, Kahvaz MS, Pashmforosh M, Foroutan M. The probable association between chronic Toxoplasma gondii infection and type 1 and type 2 diabetes mellitus: a casecontrol study. Interdiscip Perspect Infect Dis 2021; 2021: 2508780.ArticlePubMedPMCPDF
- 111. Li YX, Xin H, Zhang XY, et al. Toxoplasma gondii infection in diabetes mellitus patients in China: seroprevalence, risk factors, and case-control studies. Biomed Res Int 2018; 2018: 4723739.ArticlePubMedPMCPDF
- 112. Reeves GM, Mazaheri S, Snitker S, et al. A Positive association between T. gondii seropositivity and obesity. Front Public Health 2013; 1: 73.ArticlePubMedPMC
- 113. Machado ER, Matos NO, Rezende SM, et al. Host-parasite interactions in individuals with type 1 and 2 diabetes result in higher frequency of Ascaris lumbricoides and Giardia lamblia in type 2 diabetic individuals. J Diabetes Res 2018; 2018: 4238435.ArticlePubMedPMCPDF
- 114. Sisu A, Abugri J, Ephraim RK, et al. Intestinal parasite infections in diabetes mellitus patients: a cross-sectional study of the Bolgatanga municipality, Ghana. Sci Afr 2021; 11: e00680.Article
- 115. Akinbo FO, Olujobi SO, Omoregie R, Egbe C. Intestinal parasitic infections among diabetes mellitus patients. Biomark Genom Med 2013; 5: 44-7. Article
- 116. Alemu G, Jemal A, Zerdo Z. Intestinal parasitosis and associated factors among diabetic patients attending Arba Minch Hospital, Southern Ethiopia. BMC Res Notes 2018; 11: 689.ArticlePubMedPMCPDF
- 117. van Tong H, Brindley PJ, Meyer CG, Velavan TP. Parasite infection, carcinogenesis and human malignancy. EBioMedicine 2017; 15: 12-23. ArticlePubMed
- 118. Muehlenbachs A, Bhatnagar J, Agudelo CA, et al. Malignant transformation of Hymenolepis nana in a human host. N Engl J Med 2015; 373: 1845-52. ArticlePubMed
- 119. Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011; 54: 173-84. ArticlePubMed
- 120. Huang MH, Chen CH, Yen CM, et al. Relation of hepatolithiasis to helminthic infestation. J Gastroenterol Hepatol 2005; 20: 141-6. ArticlePubMed
- 121. Chaidee A, Onsurathum S, Intuyod K, et al. Co-occurrence of opisthorchiasis and diabetes exacerbates morbidity of the hepatobiliary tract disease. PLoS Negl Trop Dis 2018; 12: e0006611.ArticlePubMedPMC
- 122. Thinkhamrop K, Khuntikeo N, Laohasiriwong W, Chupanit P, Kelly M, Suwannatrai AT. Association of comorbidity between Opisthorchis viverrini infection and diabetes mellitus in the development of cholangiocarcinoma among a high-risk population, northeastern Thailand. PLoS Negl Trop Dis 2021; 15: e0009741.ArticlePubMedPMC
- 123. Klion AD, Ackerman SJ, Bochner BS. Contributions of eosinophils to human health and disease. Annu Rev Pathol 2020; 15: 179-209. ArticlePubMedPMC
- 124. Shibuya T. Eosinophilic response in parasitic diseases. Nihon Rinsho 1993; 51: 825-31. PubMed
- 125. Linnemann LC, Reitz M, Feyerabend TB, Breloer M, Hartmann W. Limited role of mast cells during infection with the parasitic nematode Litomosoides sigmodontis. PLoS Negl Trop Dis 2020; 14: e0008534.ArticlePubMedPMC
- 126. Lu F, Huang S. The roles of mast cells in parasitic protozoan infections. Front Immunol 2017; 8: 363.ArticlePubMedPMC
- 127. Mitre E, Nutman TB. Lack of basophilia in human parasitic infections. Am J Trop Med Hyg 2003; 69: 87-91. ArticlePubMed
- 128. Rosales C. Neutrophils vs. amoebas: immunity against the protozoan parasite Entamoeba histolytica. J Leukoc Biol 2021; 110: 1241-52. ArticlePubMedPDF
- 129. Passelli K, Billion O, Tacchini-Cottier F. The impact of neutrophil recruitment to the skin on the pathology induced by Leishmania infection. Front Immunol 2021; 12: 649348.ArticlePubMedPMC
- 130. Aitken EH, Alemu A, Rogerson SJ. Neutrophils and malaria. Front Immunol 2018; 9: 3005.ArticlePubMedPMC
- 131. Marzullo P, Minocci A, Giarda P, et al. Lymphocytes and immunoglobulin patterns across the threshold of severe obesity. Endocrine 2014; 45: 392-400. ArticlePubMedPDF
- 132. Yoshimura A, Ohnishi S, Orito C, et al. Association of peripheral total and differential leukocyte counts with obesity-related complications in young adults. Obes Facts 2015; 8: 1-16. ArticlePDF
- 133. Erdal E, Inanir M. Platelet-to-lymphocyte ratio (PLR) and plateletcrit (PCT) in young patients with morbid obesity. Rev Assoc Med Bras (1992) 2019; 65: 1182-7. ArticlePubMed
- 134. Dixon JB, O’Brien PE. Obesity and the white blood cell count: changes with sustained weight loss. Obes Surg 2006; 16: 251-7. ArticlePubMed
- 135. Xu X, Su S, Wang X, et al. Obesity is associated with more activated neutrophils in African American male youth. Int J Obes (Lond) 2015; 39: 26-32. ArticlePubMedPDF
- 136. Raghavan V, Gunasekar D, Rao KR. Relevance of haematologic parameters in obese women with or without metabolic syndrome. J Clin Diagn Res 2016; 10: EC11-6. Article
- 137. Brotfain E, Hadad N, Shapira Y, et al. Neutrophil functions in morbidly obese subjects. Clin Exp Immunol 2015; 181: 156-63. ArticlePubMedPMCPDF
- 138. Nieman DC, Henson DA, Nehlsen-Cannarella SL, et al. Influence of obesity on immune function. J Am Diet Assoc 1999; 99: 294-9. ArticlePubMed
- 139. Womack J, Tien PC, Feldman J, et al. Obesity and immune cell counts in women. Metabolism 2007; 56: 998-1004. ArticlePubMedPMC
- 140. Al-Sufyani AA, Mahassni SH. Obesity and immune cells in Saudi females. Innate Immun 2011; 17: 439-50. ArticlePubMedPDF
- 141. Furuncuoglu Y, Tulgar S, Dogan AN, Cakar S, Tulgar YK, Cakiroglu B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study. Eur Rev Med Pharmacol Sci 2016; 20: 1300-6. PubMed
- 142. Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy 2021; 122: 474-88. ArticlePubMed
- 143. Karakaya S, Altay M, Kaplan Efe F, et al. The neutrophil-lymphocyte ratio and its relationship with insulin resistance in obesity. Turk J Med Sci 2019; 49: 245-8. ArticlePubMedPMC
- 144. Kim DJ, Noh JH, Lee BW, et al. The associations of total and differential white blood cell counts with obesity, hypertension, dyslipidemia and glucose intolerance in a Korean population. J Korean Med Sci 2008; 23: 193-8. ArticlePubMedPMC
- 145. Yu JY, Choi WJ, Lee HS, Lee JW. Relationship between inflammatory markers and visceral obesity in obese and overweight Korean adults: an observational study. Medicine (Baltimore) 2019; 98: e14740.PubMedPMC
- 146. Syauqy A, Hsu CY, Rau HH, Chao JC. Association of dietary patterns, anthropometric measurements, and metabolic parameters with C-reactive protein and neutrophil-to-lymphocyte ratio in middle-aged and older adults with metabolic syndrome in Taiwan: a cross-sectional study. Nutr J 2018; 17: 106.ArticlePubMedPMCPDF
- 147. Bahadir A, Baltaci D, Turker Y, et al. Is the neutrophil-to-lymphocyte ratio indicative of inflammatory state in patients with obesity and metabolic syndrome? Anatol J Cardiol 2015; 15: 816-22. ArticlePubMed
- 148. Yilmaz H, Ucan B, Sayki M, et al. Usefulness of the neutrophil-tolymphocyte ratio to prediction of type 2 diabetes mellitus in morbid obesity. Diabetes Metab Syndr 2015; 9: 299-304. ArticlePubMed
- 149. Thavaraputta S, Dennis JA, Ball S, Laoveeravat P, Nugent K. Relation of hematologic inflammatory markers and obesity in otherwise healthy participants in the National Health and Nutrition Examination Survey, 2011-2016. Proc (Bayl Univ Med Cent) 2020; 34: 17-21. ArticlePubMedPMC
- 150. Ip BC, Hogan AE, Nikolajczyk BS. Lymphocyte roles in metabolic dysfunction: of men and mice. Trends Endocrinol Metab 2015; 26: 91-100. ArticlePubMedPMC
- 151. Bahr I, Jahn J, Zipprich A, Pahlow I, Spielmann J, Kielstein H. Impaired natural killer cell subset phenotypes in human obesity. Immunol Res 2018; 66: 234-44. ArticlePubMedPMCPDF
- 152. Rodriguez CP, Gonzalez MC, Aguilar-Salinas CA, Najera-Medina O. Peripheral lymphocytes, obesity, and metabolic syndrome in young adults: an immunometabolism study. Metab Syndr Relat Disord 2018; 16: 342-9. ArticlePubMed
- 153. Sato Mito N, Suzui M, Yoshino H, Kaburagi T, Sato K. Long term effects of high fat and sucrose diets on obesity and lymphocyte proliferation in mice. J Nutr Health Aging 2009; 13: 602-6. ArticlePubMedPDF
- 154. Breznik JA, Foley KP, Maddiboina D, Schertzer JD, Sloboda DM, Bowdish DM. Effects of obesity-associated chronic inflammation on peripheral blood immunophenotype are not mediated by TNF in female C57BL/6J mice. Immunohorizons 2021; 5: 370-83. ArticlePubMedPDF
- 155. Tenorio TR, Farah BQ, Ritti-Dias RM, et al. Relation between leukocyte count, adiposity, and cardiorespiratory fitness in pubertal adolescents. Einstein (Sao Paulo) 2014; 12: 420-4. ArticlePubMedPMC
- 156. Friedrich K, Sommer M, Strobel S, et al. Perturbation of the monocyte compartment in human obesity. Front Immunol 2019; 10: 1874.ArticlePubMedPMC
- 157. Christou KA, Christou GA, Karamoutsios A, et al. Metabolically healthy obesity is characterized by a proinflammatory phenotype of circulating monocyte subsets. Metab Syndr Relat Disord 2019; 17: 259-65. ArticlePubMed
- 158. Subramanian V, Ferrante AW Jr. Obesity, inflammation, and macrophages. Nestle Nutr Workshop Ser Pediatr Program 2009; 63: 151-9. ArticlePubMed
- 159. Bastarrachea RA, Lopez-Alvarenga JC, Bolado-Garcia VE, TellezMendoza J, Laviada-Molina H, Comuzzie AG. Macrophages, inflammation, adipose tissue, obesity and insulin resistance. Gac Med Mex 2007; 143: 505-12. PubMed
- 160. Shehata HM, Murphy AJ, Lee MK, et al. Sugar or fat?: metabolic requirements for immunity to viral infections. Front Immunol 2017; 8: 1311.ArticlePubMedPMC
- 161. Wiedermann U, Garner-Spitzer E, Wagner A. Primary vaccine failure to routine vaccines: why and what to do? Hum Vaccin Immunother 2016; 12: 239-43. ArticlePubMedPMCPDF
- 162. Young KM, Gray CM, Bekker LG. Is obesity a risk factor for vaccine non-responsiveness? PLoS One 2013; 8: e82779.ArticlePubMedPMC
- 163. Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev 2013; 14: 232-44. PubMed
- 164. Wu Y, Potempa LA, El Kebir D, Filep JG. C-reactive protein and inflammation: conformational changes affect function. Biol Chem 2015; 396: 1181-97. ArticlePubMed
- 165. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003; 111: 1805-12. ArticlePubMedPMC
- 166. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol 2018; 9: 754.ArticlePubMedPMC
- 167. Viikari LA, Huupponen RK, Viikari JS, et al. Relationship between leptin and C-reactive protein in young Finnish adults. J Clin Endocrinol Metab 2007; 92: 4753-8. ArticlePubMed
- 168. Hribal ML, Fiorentino TV, Sesti G. Role of C reactive protein (CRP) in leptin resistance. Curr Pharm Des 2014; 20: 609-15. ArticlePubMedPMC
- 169. Liu Y, Liu C, Jiang C, et al. C-reactive protein inhibits high-molecular-weight adiponectin expression in 3T3-L1 adipocytes via PI3K/ Akt pathway. Biochem Biophys Res Commun 2016; 472: 19-25. ArticlePubMed
- 170. Naylor C, Petri WA Jr. Leptin regulation of immune responses. Trends Mol Med 2016; 22: 88-98. ArticlePubMed
- 171. La Cava A. Leptin in inflammation and autoimmunity. Cytokine 2017; 98: 51-8. ArticlePubMedPMC
- 172. Dayakar A, Chandrasekaran S, Veronica J, Maurya R. Leptin induces the phagocytosis and protective immune response in Leishmania donovani infected THP-1 cell line and human PBMCs. Exp Parasitol 2016; 160: 54-9. ArticlePubMed
- 173. Baltodano-Calle MJ, Polo-Vasquez JS, Romani-Pozo A, GutarraSaldana D, Guija-Poma E. Leptin as a potential prognostic marker of the severity of COVID-19 infection in obese patients. Nutr Metab Cardiovasc Dis 2022; 32: 743-4. ArticlePubMed
- 174. Smilowitz NR, Kunichoff D, Garshick M, et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J 2021; 42: 2270-9. ArticlePubMedPMCPDF
- 175. Giner-Galvan V, Pomares-Gomez FJ, Quesada JA, et al. C-Reactive protein and serum albumin ratio: a feasible prognostic marker in hospitalized patients with COVID-19. Biomedicines 2022; 10: 1393.ArticlePubMedPMC
- 176. Tonon F, Di Bella S, Giudici F, et al. Discriminatory value of adiponectin to leptin ratio for COVID-19 pneumonia. Int J Endocrinol 2022; 2022: 9908450.ArticlePubMedPMC
- 177. van der Voort PHJ, Moser J, Zandstra DF, et al. Leptin levels in SARS-CoV-2 infection related respiratory failure: a cross-sectional study and a pathophysiological framework on the role of fat tissue. Heliyon 2020; 6: e04696.ArticlePubMedPMC
- 178. Larsson A, Lipcsey M, Hultstrom M, Frithiof R, Eriksson M. Plasma leptin is increased in intensive care patients with COVID-19: an investigation performed in the PronMed-Cohort. Biomedicines 2021; 10: 4.ArticlePubMedPMC
- 179. Blot M, David M, Nguyen M, et al. Are adipokines the missing link between obesity, immune response, and outcomes in severe COVID-19? Int J Obes (Lond) 2021; 45: 2126-31. ArticlePubMedPMCPDF
- 180. Wang J, Xu Y, Zhang X, et al. Leptin correlates with monocytes activation and severe condition in COVID-19 patients. J Leukoc Biol 2021; 110: 9-20. ArticlePubMedPDF
- 181. Honce R, Schultz-Cherry S. A tale of two pandemics: obesity and COVID-19. J Travel Med 2020; 27: taaa097.ArticlePubMedPDF
- 182. Brandao SCS, Godoi E, de Oliveira Cordeiro LH, et al. COVID-19 and obesity: the meeting of two pandemics. Arch Endocrinol Metab 2021; 65: 3-13. PubMed
REFERENCES
Figure & Data
References
Citations

- Nonlinear association between pre-pregnancy body mass index and preterm birth in singleton pregnancies conceived with assisted reproductive technology
Tingting Zhuang, Jingli Sun, Yu Zhang
Frontiers in Nutrition.2026;[Epub] CrossRef - The Human Energy Balance: Uncovering the Hidden Variables of Obesity
Nikolaos Theodorakis, Maria Nikolaou
Diseases.2025; 13(2): 55. CrossRef - Candida infections in COVID-19 patients: A review of prevalence, risk factors, and mortality
Eduardo Franco Tulio, Fabíola Lucini, Allan Carminatti de Lima, Natalia Daiane Garoni Martins do Carmo, Marcelo dos Santos Barbosa, Gleyce Hellen de Almeida de Souza, Luana Rossato
Indian Journal of Medical Microbiology.2025; 55: 100831. CrossRef - Cell therapies for immune-mediated disorders
Natalia Wiewiórska-Krata, Bartosz Foroncewicz, Krzysztof Mucha, Radosław Zagożdżon
Frontiers in Medicine.2025;[Epub] CrossRef - Adipose Tissue in Chagas Disease: A Neglected Component of Pathogenesis
Vitória França dos Santos Pessoa, Mariana Hecht, Nadjar Nitz, Luciana Hagström
Pathogens.2025; 14(4): 339. CrossRef - The Impact of Obesity on Readmission and Healthcare Costs in Patients with Skin and Subcutaneous Tissue Infections
David Suh, Seung-Mi Lee
Risk Management and Healthcare Policy.2025; Volume 18: 1579. CrossRef - SARS-CoV-2 mRNA vaccines confer protection in diet-induced obese mice despite altered immune cell profiles in the lung
Katherine S. Lee, Dylan T. Boehm, Olivia A. Miller-Stump, Nathaniel A. Rader, Melissa Cooper, Holly A. Cyphert, Emel Sen-Kilic, Mariette Barbier, F. Heath Damron
Scientific Reports.2025;[Epub] CrossRef - Obesity promotes urinary tract infection by disrupting bladder focal adhesion kinase signaling
Laura Schwartz, Kristin Salamon, Aaron Simoni, Israel Cotzomi-Ortega, Yuriko Sanchez-Zamora, Sarah Linn-Peirano, Preeti John, Juan de Dios Ruiz-Rosado, Ashley R. Jackson, Xin Wang, John David Spencer
iScience.2025; 28(11): 113862. CrossRef - Theory of the Leaky Intestine: Gender Differences in Intestinal Parasitic Infections, Cytoskeletal Wall Dysfunctions, and Hypertension
Philip Njemanze, Anthonia Chioma Amadi, Joy E. Onuchukwu, Chinwendu C. Darlington, Nneoma E. Ukeje, Clinton O. Mezu, Clara C. Ofoegbu, Chidera Okuh, Chidimma O. Ukaegbu, Linda O. Uzoma, Marvis Amuchie, Faustina N. Ojilere, Lilian C. Mbara, Esther C. Nneke
Qeios.2024;[Epub] CrossRef - The potential of DNA methylation markers in the study of obesity
A. F. Nikolaeva, K. O. Petrova, O. V. Vasyukova, R. M. Guseinova, I. R. Minniakhmetov, R. I. Khusainova, N. G. Mokrysheva, V. O. Sigin
Obesity and metabolism.2024; 20(4): 301. CrossRef - Dysbiosis of the gut microbiota and its effect on α-synuclein and prion protein misfolding: consequences for neurodegeneration
Nasir Uddin Mahbub, Md Minarul Islam, Seong-Tshool Hong, Hea-Jong Chung
Frontiers in Cellular and Infection Microbiology.2024;[Epub] CrossRef - Sarcoma Size and Limb Dimensions Predict Complications, Recurrence, and Death in Patients with Soft Tissue Sarcoma in the Thigh: A Multidimensional Analysis
Rami Elmorsi, Luis Camacho, David D. Krijgh, Gordon S. Tilney, Heather Lyu, Raymond S. Traweek, Russell G. Witt, Margaret S. Roubaud, Christina L. Roland, Alexander F. Mericli
Annals of Surgical Oncology.2024; 31(8): 5421. CrossRef - Theory of the Leaky Intestine: Sex Differences in Intestinal Parasitic Infections, Cytoskeletal Wall Dysfunctions, and Hypertension
Philip Njemanze, Anthonia Chioma Amadi, Joy E. Onuchukwu, Chinwendu C. Darlington, Nneoma E. Ukeje, Clinton O. Mezu, Clara C. Ofoegbu, Chidera Okuh, Chidimma O. Ukaegbu, Linda O. Uzoma, Marvis Amuchie, Faustina N. Ojilere, Lilian C. Mbara, Esther C. Nneke
Qeios.2024;[Epub] CrossRef - Cellular immune response to a single dose of live attenuated hepatitis a virus vaccine in obese children and adolescents
Tanatchabhorn Soponkanabhorn, Narissara Suratannon, Supranee Buranapraditkun, Chomchanat Tubjareon, Sittichoke Prachuapthunyachart, Sutha Eiamkulbutr, Voranush Chongsrisawat
Heliyon.2024; 10(16): e36610. CrossRef - Increased Fruit and Vegetable Consumption Prevents Dysregulated Immune and Inflammatory Responses in High-Fat Diet-Induced Obese Mice
Weimin Guo, Dayong Wu, Lijun Li, Erin D Lewis, Simin Nikbin Meydani
The Journal of Nutrition.2024; 154(10): 3144. CrossRef - Sex differences in the inflammation-depression link: A systematic review and meta-analysis
Dana A. Jarkas, Ally H. Villeneuve, Ayeila Z.B. Daneshmend, Paul J. Villeneuve, Robyn J. McQuaid
Brain, Behavior, and Immunity.2024; 121: 257. CrossRef - The Strong Effect of Propolis in Suppressing NF-κB, CysC, and ACE2 on a High-fat Diet
Muhammad Reza Primaguna, Haerani Rasyid, Makbul Aman, Syakib Bakri, Hasyim Kasim, Harun Iskandar, Ressy Dwiyanti, Ade Rifka Junita, Ridwan Ridwan, Rizki Amelia Noviyanthi, Nur Indah Purnamasar, Mochammad Hatta
Biomedical and Pharmacology Journal.2024; 17(3): 1539. CrossRef - How market integration impacts human disease ecology
Lev Kolinski, Tyler M Barrett, Randall A Kramer, Charles L Nunn
Evolution, Medicine, and Public Health.2024; 12(1): 229. CrossRef - Molecular Pathways Linking High-Fat Diet and PM2.5 Exposure to Metabolically Abnormal Obesity: A Systematic Review and Meta-Analysis
Sagrario Lobato, Víctor Manuel Salomón-Soto, Claudia Magaly Espinosa-Méndez, María Nancy Herrera-Moreno, Beatriz García-Solano, Ernestina Pérez-González, Facundo Comba-Marcó-del-Pont, Mireya Montesano-Villamil, Marco Antonio Mora-Ramírez, Claudia Mancilla
Biomolecules.2024; 14(12): 1607. CrossRef - Anti-Obesity Effects of Marine Macroalgae Extract Caulerpa lentillifera in a Caenorhabditis elegans Model
Kawita Chumphoochai, Preeyanuch Manohong, Nakorn Niamnont, Montakan Tamtin, Prasert Sobhon, Krai Meemon
Marine Drugs.2023; 21(11): 577. CrossRef - Obesity and consequent changes in the body
Bojana Kisić, Dragana Puhalo-Sladoje, Dijana Mirić, Dragiša Rašić, Tatjana Novaković
Praxis medica.2022; 51(3): 35. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


Fig. 1.
Fig. 2.
| Study | Patients’ details | Finding |
|---|---|---|
| Ndako et al. (2021); Nigeria [29] | 180 Diabetic patients and 100 non-diabetics controls | Higher risk of HBV infection among type 2 diabetic patients than non-diabetics |
| Iovanescu et al. (2015); Romania [30] | 246 Patients with chronic liver disease (136 chronic viral hepatitis, 110 viral liver cirrhosis) | A significant association between diabetes mellitus and HCV-induced chronic liver disease |
| Cheng et al. (2006); Hong Kong [31] | 2,838 Type 2 diabetes patients | HBV-infected patients had earlier onset of diabetes, higher frequency of retinopathy, and increased risk of end-stage renal disease than non-HBV–infected patients |
| Vírseda Chamorro et al. (2006); Spain [32] | 305 Patients who came for HCV assessment | A relationship between HCV infection and type 2 diabetes |
| Arao et al. (2003); Japan [33] | 866 Patients with chronic viral disease (707 HCV-infected and 159 HBV-infected) | HCV infection was closely associated with diabetes, and cirrhosis was an independent risk factor for diabetes |
| Dworzanski et al. (2019); Poland [34] | 173 Diabetic patients and 50 persons without diabetes | Prevalence of EBV, HPV, and EBV+HPV co-infection was significantly higher in diabetic patients than those without diabetes |
| Karjala et al. (2011); USA [35] | Data from the National Health and Examination and Nutritional Examination Survey (NHANES) 2007–2008 | Obesity was significantly associated with HSV-1 infection |
| Fernandez-Real et al. (2007); Spain [36] | 74 Healthy middle-aged men from the general population | Significant positive relation between HSV-1 titer and fat mass |
| Sun et al. (2003); China [37] | 1,244 Inpatients (408 with dyslipidemia and 836 controls) | Prevalence of HSV-2 seropositivity was significantly higher in patients with dyslipidemia. BMI, diabetes, and hypertension were more common in patients with dyslipidemia than those without |
| Woelfle et al. (2022); Germany [38] | From the German population–based KORA cohort (pre-diabetes, n = 1,257) | HSV-2 and CMV were associated with pre-diabetes incidence |
| Yoo et al. (2019); Korea [39] | 576 Adults with CMV diseases | Type 2 diabetes cases had a higher incidence of CMV diseases |
| Chen et al. (2012); Netherlands [40] | 549 Participants | CMV seropositive subjects were more likely to have type 2 diabetes |
| Roberts and Cech (2005); USA [41] | 113 Hemodialysis patients (83 type 2 diabetes and 30 controls) | A higher seroprevalence of anti-CMV IgG among diabetes patients |
| Chiu et al. (1997); Canada [42] | Endarterectomy specimens from 76 patients with carotid artery stenosis and 20 normal carotid artery and aortic tissue autopsy specimens | CMV was detected in carotid atherosclerotic plaques from 27 cases (35.5%) |
| Reinholdt et al. (2021); Denmark [43] | Male population (n = 2,528,756), nationwide registry-based cohort study | Increased incidence rate of HPV-related anogenital intraepithelial neoplasia and cancer among men with diabetes than non-diabetic men |
| Sobti et al. (2019); UK [44] | 210 Patients with HNSCC | Prevalence of developing HPV-16–positive HNSCC was 3.79 times higher in diabetic patients than in those without diabetes. Moreover, diabetes was a risk factor for a poorer prognosis |
| Slama et al. (2021); USA [45] | 1,584 Men with pre-diabetes (793 with HIV, 791 without HIV), over a median 12-year follow-up | 40% higher risk for the development of diabetes among men with HIV |
| Kubiak et al. (2021); South Africa [46] | 1,369 Persons with HIV | Among adults with HIV, diabetes and pre-diabetes were common |
| Hema et al. (2021); Burkina Faso [47] | 4,259 Patients in a cross-sectional study | Prevalence of diabetes and hypertension was higher among persons with HIV on ART than the general population |
| Jeremiah et al. (2020); Tanzania [48] | 1,947 Adults (336 with HIV on ART, 956 with HIV ART-naïve, 655 without HIV) | Prevalence of diabetes was high, particularly among HIV-infected ART-naïve persons |
| Study | Study design | Finding |
|---|---|---|
| Ayers et al. (2017) [87] | Injection of β-synuclein fibrils in M83 transgenic mice |
Injection of α-synuclein fibrils via these peripheral routes in M83 mice induced a robust α-synuclein pathology in the central nervous system. |
| Betemps et al. (2014) [88] | Transgenic M83 mice were inoculated intracerebrally in the striato-cortical area |
Disease acceleration following intracerebral inoculation suggests that disease propagation involves a prion-like mechanism. |
| Boluda et al. (2015) [89] | Intracerebral injection of Alzheimer’s disease brain extracts enriched in pathological tau in young mutant P301S tau transgenic mice (PS19) |
At 1-month post-injection, inoculated Alzheimer’s disease-tau in young PS19 mice induced tau pathology predominantly in neuronal perikarya (neuron cell body). With longer post-injection survival periods of up to 6 months, tau pathology spread to different brain regions distant from the inoculated sites. |
| Guo et al. (2016) [90] | 2–3-Month-old C57BL6 and C57BL6/C3H F1 mice were intracerebrally inoculated with different tau fibrils; 15–19-month-old C57BL6 mice were injected with Alzheimer’s disease-tau. | Intracerebral inoculation of tau fibrils purified from Alzheimer’s disease brains, but not synthetic tau fibrils, resulted in the formation of abundant tau inclusions in the brain of non-transgenic mice. |
| Lam et al. (2021) [91] | The posterior cingulate cortex |
After 21 months, amyloid beta (Aβ) and tau pathologies developed in all Alzheimer-inoculated animals (n = 12) while no control brain extract-inoculated animals (n = 6) developed such lesions. |
| Morales et al. (2015) [92] | Brain extracts from 18–20 months old tg2576 mice |
Administration of misfolded Aβ significantly accelerated amyloid deposition in young mice. |
| Study | Subject | Important finding |
|---|---|---|
| Udoh et al. (2020); Nigeria [105] | Cross-sectional study of 208 diabetic patients | Diabetic patients were reservoirs of asymptomatic Plasmodium falciparum. |
| Wyss et al. (2017); Sweden [106] | Retrospective observational study on 937 adults with malaria | Comorbidities, specifically obesity and diabetes, were risk factors for severe malaria in adults diagnosed with Plasmodium falciparum. |
| Danquah et al. (2010); Ghana [107] | Case-control study of 946 diabetic patients and 520 controls | Patients with type 2 diabetes had a 46% increased risk for infection with Plasmodium falciparum. |
| Vizzoni et al. (2018); Brazil [108] | Cross-sectional study of 619 patients with Chagas disease | Elderly patients had a high frequency of hypertension and other comorbidities such as diabetes and dyslipidemia. |
| dos Santos et al. (1999); Brazil [109] | Cross-sectional study of female patients with Chagas disease (n = 362) and controls (n = 285) | Diabetes/hyperglycemia was more prevalent in patients with the cardiac form of Chagas disease than in controls, or patients with gastrointestinal problems or the asymptomatic form of the disease. |
| Soltani et al. (2021); Iran [110] | Case-control study of 105 diabetic patients and 150 controls | Chronic Toxoplasma gondii infection was significantly associated with diabetes. |
| Li et al. (2018); China [111] | Case-control study of 1,200 diabetic patients (type 1, 2, and gestational) and 1,200 matched controls | Diabetic patients had a significantly higher Toxoplasma gondii seroprevalence than controls. |
| Reeves et al. (2013); Germany [112] | 999 Randomly selected adults | Obese persons had significantly higher Toxoplasma gondii seropositivity than non-obese individuals. |
| Machado et al. (2018); Brazil [113] | Descriptive study of 156 diabetic individuals | Frequencies of Giardia lamblia were higher in individuals with type 2 diabetes than those without. |
| Sisu et al. (2021); Ghana [114] | Cross-sectional study of 152 diabetes patients | Diabetes patients appeared susceptible to infections with Giardia lamblia, Entamoeba hystolytica, and Cryptosporidium parvum. |
| Akinbo et al. (2013); Nigeria [115] | 150 Diabetic patients and 30 controls | Diabetes was significantly associated with intestinal parasitic infections (like Entamoeba histolytica). |
| Alemu et al. (2018); Ethiopia [116] | Cross-sectional study of 215 diabetic patients | Intestinal parasites were found more frequently in diabetic patients compared to data from other similar studies. Cryptosporidium parvum was the parasite found with the highest frequency. |
HBV, hepatitis B virus; HCV, hepatitis C virus; EBV, Epstein-Barr virus; HPV, human papillomavirus; HSV, herpes simplex virus; BMI, body mass index; CMV, cytomegalovirus; HNSCC, head and neck squamous cell carcinoma; HIV, human immunodeficiency virus; ART, antiretroviral therapy.
The M83 transgenic mouse model overexpresses A53T mutated human α-synuclein protein, which is connected with buildup of pathognomonic Ser129-phosphorylated α-synuclein in the central nervous system. Abnormal accumulation of misfolded α-synuclein is linked to synucleinopathies including Parkinson’s disease; Striato-cortical area: The corpus striatum (subcortical basal ganglia) and the adjacent cerebral cortex in the forebrain region; PS19 transgenic mouse expresses the P301S mutant form of human microtubule-associated protein tau. This hyper-phosphorylated and insoluble protein accumulates in the brain; Posterior cingulate cortex: Situated at the posterior part of the cingulate gyrus in the medial part of the inferior parietal lobe, above the posterior end of the corpus callosum; The Tg2576 mouse model overexpresses a mutant form of amyloid precursor protein (APP695SWE, found in early-onset familial Alzheimer’s disease), which has the double mutation- APPK670M/671L. The most common neurodegenerative diseases: Alzheimer’s disease and Parkinson’s disease.

 E-submission
E-submission