Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 57(6); 2023 > Article
-
Original Article
BRCA-mutated gastric adenocarcinomas are associated with chromosomal instability and responsiveness to platinum-based chemotherapy -
Ji Hyun Oh1
 , Chang Ohk Sung1,2
, Chang Ohk Sung1,2 , Hyung-Don Kim3
, Hyung-Don Kim3 , Sung-Min Chun1,4
, Sung-Min Chun1,4 , Jihun Kim1
, Jihun Kim1
-
Journal of Pathology and Translational Medicine 2023;57(6):323-331.
DOI: https://doi.org/10.4132/jptm.2023.10.22
Published online: November 14, 2023
1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
2Department of Medical Science, Asan Medical Institute of Convergence Science and Technology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
3Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
4Asan Center for Cancer Genome Discovery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- Corresponding Author: Jihun Kim, MD, PhD, Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-uil, Songpa-gu, Seoul 05505, Korea Tel: +82-2-3010-4556, Fax: +82-2-472-7898, E-mail: jihunkim@amc.seoul.kr
© 2023 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Homologous recombination defect is an important biomarker of chemotherapy in certain tumor types, and the presence of pathogenic or likely pathogenic mutations involving BRCA1 or BRCA2 (p-BRCA) mutations is the most well-established marker for the homologous recombination defect. Gastric cancer, one of the most prevalent tumor types in Asia, also harbors p-BRCA mutations.
-
Methods
- To investigate the clinical significance of p-BRCA mutations, we analyzed 366 gastric cancer cases through next-generation sequencing. We determined the zygosity of p-BRCA mutations based on the calculated tumor purity through variant allelic fraction patterns and investigated whether the presence of p-BRCA mutations is associated with platinum-based chemotherapy and a certain molecular subtype.
-
Results
- Biallelic p-BRCA mutation was associated with better response to platinum-based chemotherapy than heterozygous p-BRCA mutation or wild type BRCA genes. The biallelic p-BRCA mutations was observed only in the chromosomal instability subtype, while all p-BRCA mutations were heterozygous in microsatellite instability subtype.
-
Conclusions
- In conclusion, patients with gastric cancer harboring biallelic p-BRCA mutations were associated with a good initial response to platinum-based chemotherapy and those tumors were exclusively chromosomal instability subtype. Further investigation for potential association with homologous recombination defect is warranted.
- Case selection and mutation analysis
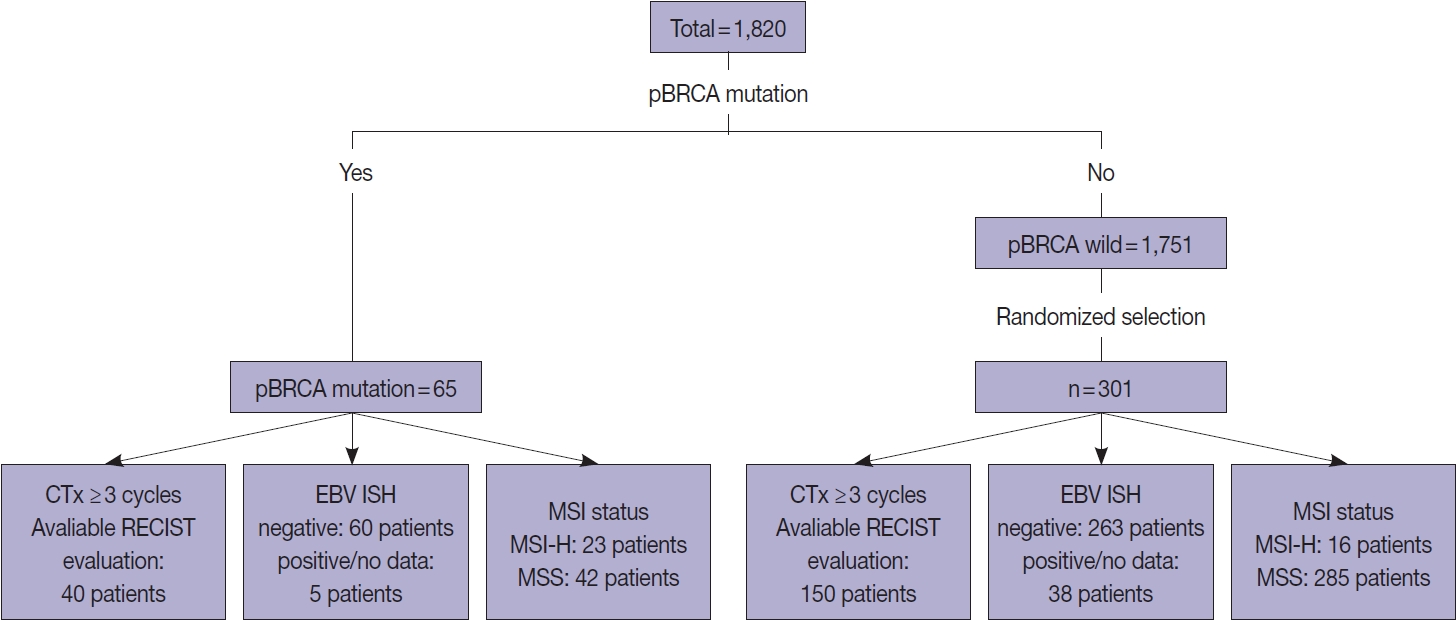
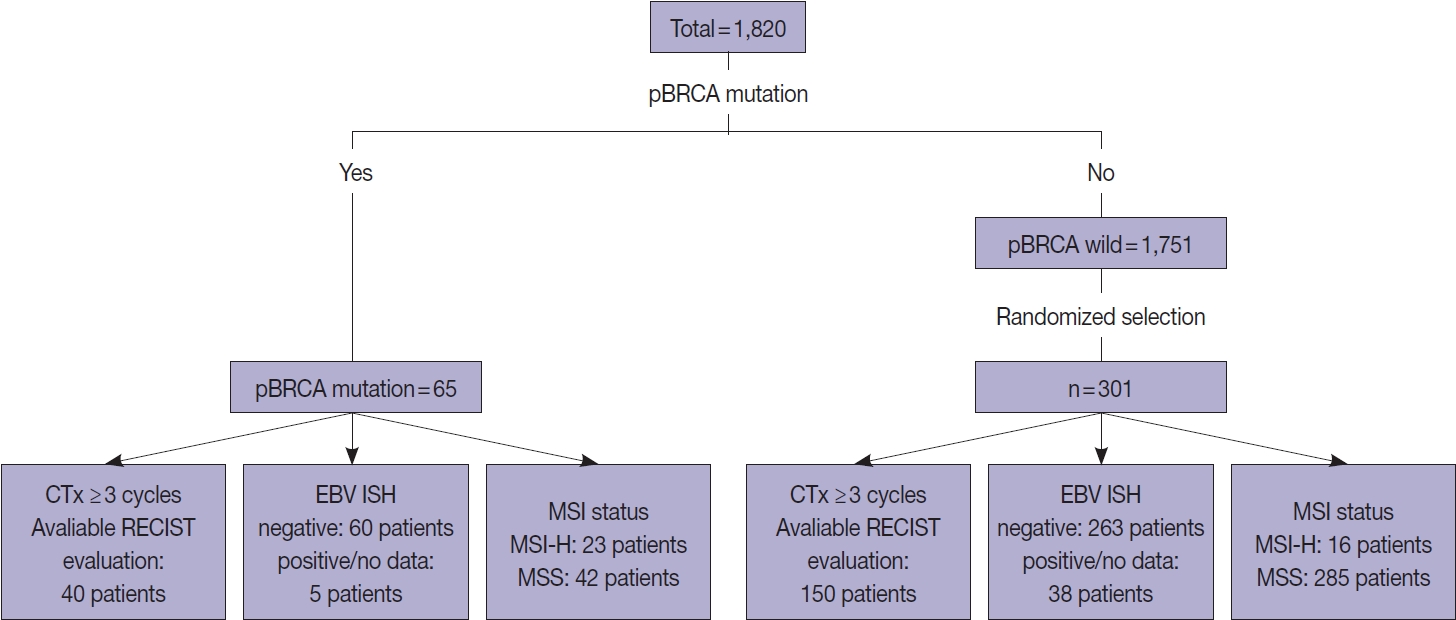
- Overall, 1,820 patients who were pathologically diagnosed with adenocarcinoma of the stomach and underwent clinical NGS of the tumor tissues between January 2017 and July 2022 were initially included. Among them, 65 patients with gastric adenocarcinoma harboring p-BRCA mutations were identified (Fig. 1). The functional effects of BRCA1 or BRCA2 mutations were predicted using the BRCA mutation database of the Department of Pathology, University of Utah (https://arup.utah.edu/database/BRCA/) (Supplementary Table S1). For BRCA wild-type cases, 301 of 1,751 patients with BRCA wild-type tumors were randomly selected. The MSI status was determined by our validated NGS as described previously [16]. Ambiguous cases were confirmed by immunohistochemistry of MLH1, MSH2, MSH6, and PMS2. Finally, Epstein-Barr virus (EBV) in situ hybridization results were reviewed, when available.
- NGS analysis
- The NGS study was performed using our clinically validated OncoPanel AMC v4.3 panel, which is a DNA-based hybrid capture targeted gene panel using the NextSeq 550Dx Sequencing System (Illumina, San Diego, CA, USA) as described previously [16]. Briefly, this panel was approximately 1.2 Mbp with 33524 probes targeting 382 genes, including entire exons of 199 genes, 184 hot spots, and partial introns for eight genes often rearranged in cancer. For DNA extraction, the tumor area was macrodissected from formalin-fixed, paraffin-embedded tissue blocks to achieve > 20% tumor purity. Sequenced reads were processed and variants were called as described previously [17]; briefly, sequenced reads were processed by GATK (ver.4.2.6.1) pipeline, and variant calling was performed using VarDict (ver. 1.6). Common and germline variants from somatic variant candidates were filtered out with the common dbSNP build 141 (found in > 1% of samples), Exome Aggregation Consortium release 0.3.1 (http://exac.broadinstitute.org), and Korean Reference Genome database (http://152.99.75.168/KRGDB) and an in-house panel of normal variants except BRCA1 and BRCA2.
- Two pathologists manually reviewed NGS data to remove false positives or false negatives and curated functional effects of annotated BRCA1 or BRCA2 mutations. All detected single nucleotide variant and Indel types, including synonymous and nonsynonymous mutations in all exonic regions and splice sites, were used to calculate tumor mutation burden (TMB) [18].
- Copy number analysis
- Copy number burden was calculated by the fraction of copy number altered regions across the human genome. The copy number altered regions were detected using CNVkit [19] with default parameters without normal tissue. Then, we classified tumors into CN-high (CIN) and CN-low (genomically stable) based on the relative copy number burden within EBV-negative/microsatellite stable (MSS) tumors. Briefly, we first sorted EBVnegative/MSS tumors in ascending order according to copy number burden, and then tumors with more than 25 percentile values were classified as CN-high tumors. This method was based on the previously published the Cancer Genome Atlas (TCGA) data where the frequency of the CN-high tumors was 72% while that of the CN-low (genomically stable) tumors was 28%, respectively, among the EBV-negative/MSS tumors [15].
- Inference of the zygosity of p-BRCA mutations
- Because BRCA1 and BRCA2 are tumor suppressors, complete loss of protein function might be biologically important. Thus, the zygosity of p-BRCA mutations or the loss of heterozygosity (LOH) of the p-BRCA–mutant allele in tumor tissues was necessary. To achieve this, tumor purity information was re-estimated through an analysis of variant allelic fraction (VAF) patterns of the detected variants rather than through pathologists’ estimation, as described previously [20] with slight modification. Briefly, truncal mutations were carefully chosen. Truncal mutations are well-known oncogenes that are usually heterozygous. However, such oncogenes undergo LOH or mutant allele amplification on some occasions because of tumor-specific biology or selective pressure. Thus, we also considered local copy-number profiles and re-reviewed tumor slides from which DNA had been extracted.
- As a simplified model, if the truncal mutation is heterozygous (or LOH–), tumor purity (T) would be two times of VAF (V): T = 2V. If the truncal mutation is biallelic (or LOH+), tumor purity (T) would be equal to VAF (V): T = V. Based on the calculated tumor purity, the zygosity of p-BRCA mutations could be determined.
- If the reported VAF of the p-BRCA mutation was T/2, such a mutation was thought to be heterozygous (or LOH–). If it was the same as T, the p-BRCA mutation was thought to be biallelic (or LOH+). If the reported VAF of the p-BRCA mutation was similar to T+(1–T)/2, the p-BRCA mutation was suggested to be a germline plus somatic LOH. The distinction between biallelic somatic and germline with or without LOH was not always possible. Thus, we grouped biallelic somatic and germline with or without LOH into the biallelic (or LOH+) category.
- Evaluation of response to platinum-based chemotherapy
- To evaluate responsiveness to platinum-based chemotherapy, we further selected 40 patients with BRCA-mutated gastric cancer and 150 with BRCA wild-type gastric cancer who underwent more than 3 cycles of platinum-based palliative chemotherapy and had valid response evaluation data (Fig. 1). Response to platinum-based chemotherapy was evaluated according to the modified Response Evaluation Criteria in Solid Tumors (RECIST ver. 1.1) [21]. When multiple combinations of chemotherapy were given, the initial response to the first platinum-based chemotherapy was used.
- Statistics
- Chi-square test and Wilcoxon signed-rank test were performed for pairwise comparisons. For nonparametric analysis between groups and variants, the Kruskal-Wallis test was used. The Kaplan-Meier method was performed for survival analyses, and the Mantel-Cox log-rank test was used to evaluate whether certain factors affect survival. p-values of < .05 were considered statistically significant. All statistical analyses and data visualizations were performed using IBM SPSS Statistics ver. 28.0.1 (IBM Corp., Armonk, NY, USA) and R-Studio using R ver. 4.2.2 (RStudio, Boston, MA, USA).
MATERIALS AND METHODS
- The presence of p-BRCA mutation is not associated with platinum chemotherapy response in gastric carcinoma when LOH of the p-BRCA mutant allele is not taken into consideration
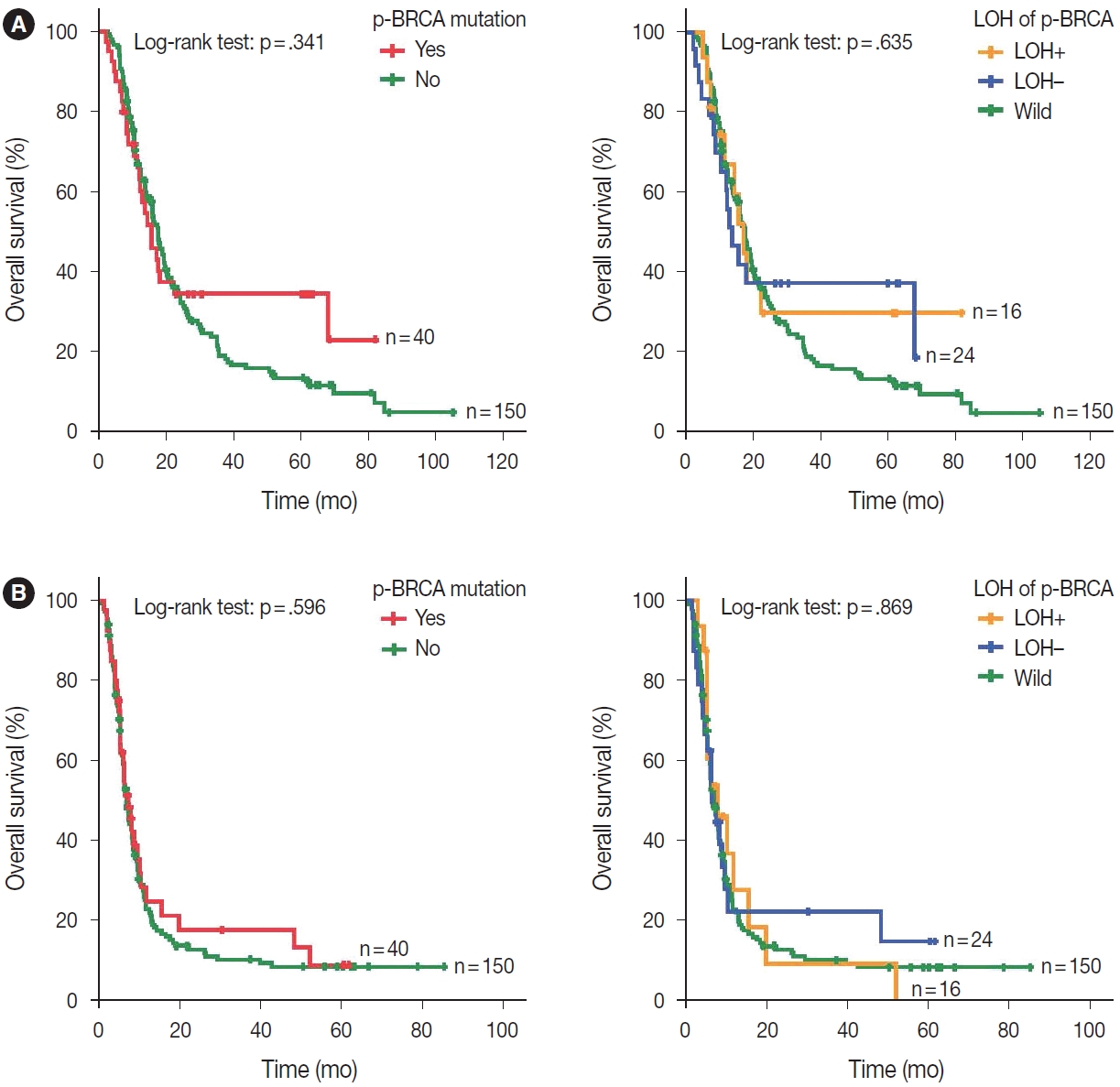
- Most clinicopathological variables for chemotherapy response were comparable between p-BRCA–mutant cases and BRCA wild-type cases (Table 1). Owing to the low prevalence of p-BRCA mutations, the finally selected patients were heterogeneous in terms of initial staging, treatment, and follow-up method. When zygosity of the p-BRCA mutations was not taken into consideration, the presence of p-BRCA mutations was not associated with responsiveness to platinum-based chemotherapy (p = .833) (Supplementary Table S2). In addition, overall survival and progression-free survival were not affected by the presence of p-BRCA mutations (log-rank test, p = .341, and p = .596, respectively) (Fig. 2).
- LOH of the p-BRCA mutant allele is an important predictor of responsiveness to platinum-based chemotherapy in gastric carcinoma
- We hypothesized that biallelic p-BRCA mutations may result in the complete loss of BRCA protein function and hence may show a stronger association with responsiveness to platinumbased chemotherapy. Indeed, patients with gastric cancer harboring p-BRCA mutations accompanied by LOH showed a clear positive correlation with better responsiveness to platinum-based chemotherapy (relative risk, 1.74; 95% confidence interval, 1.136 to 1.531; p = .001) (Table 2). Notably, all gastric cancers showing p-BRCA mutations plus LOH showed objective responses. On multivariate analysis, MSI status, EBV in situ hybridization positivity, ERBB2 status, age, and sex were not associated with platinum-based chemotherapy (data not shown). In contrast to the initial response to platinum-based chemotherapy, overall and progression-free survivals were not different according to the LOH of the p-BRCA mutant allele (Fig. 2).
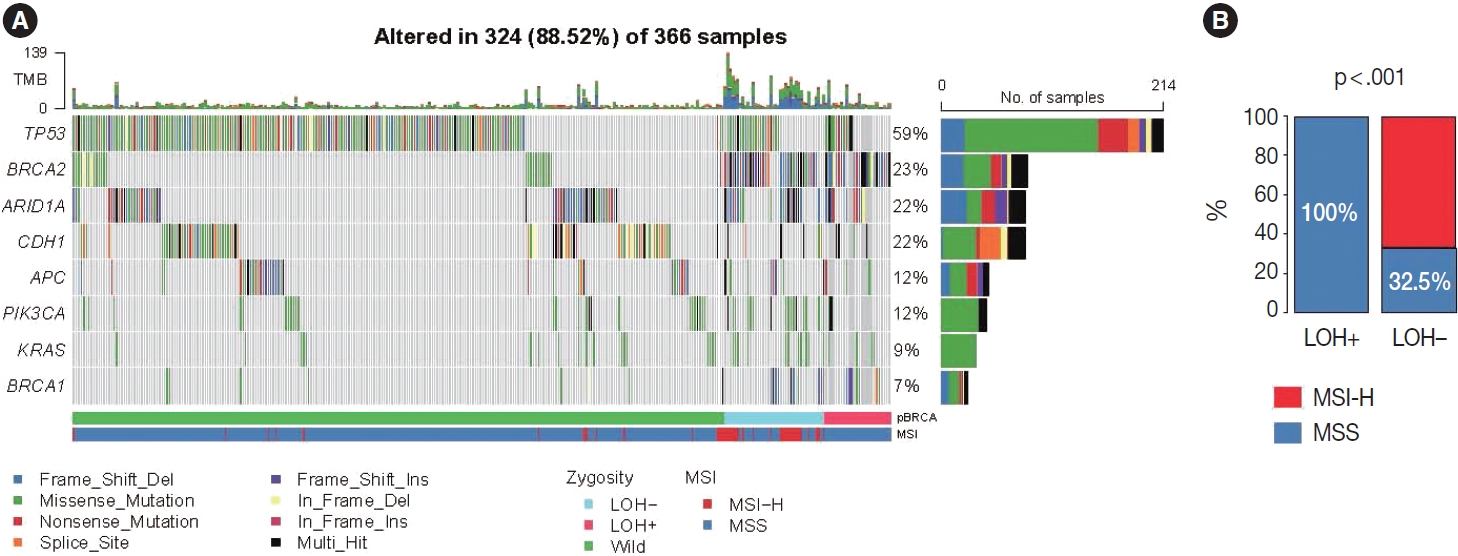
- The simultaneous p-BRCA mutation and LOH of the mutant allele is associated with increased copy number burden and CIN molecular subtype
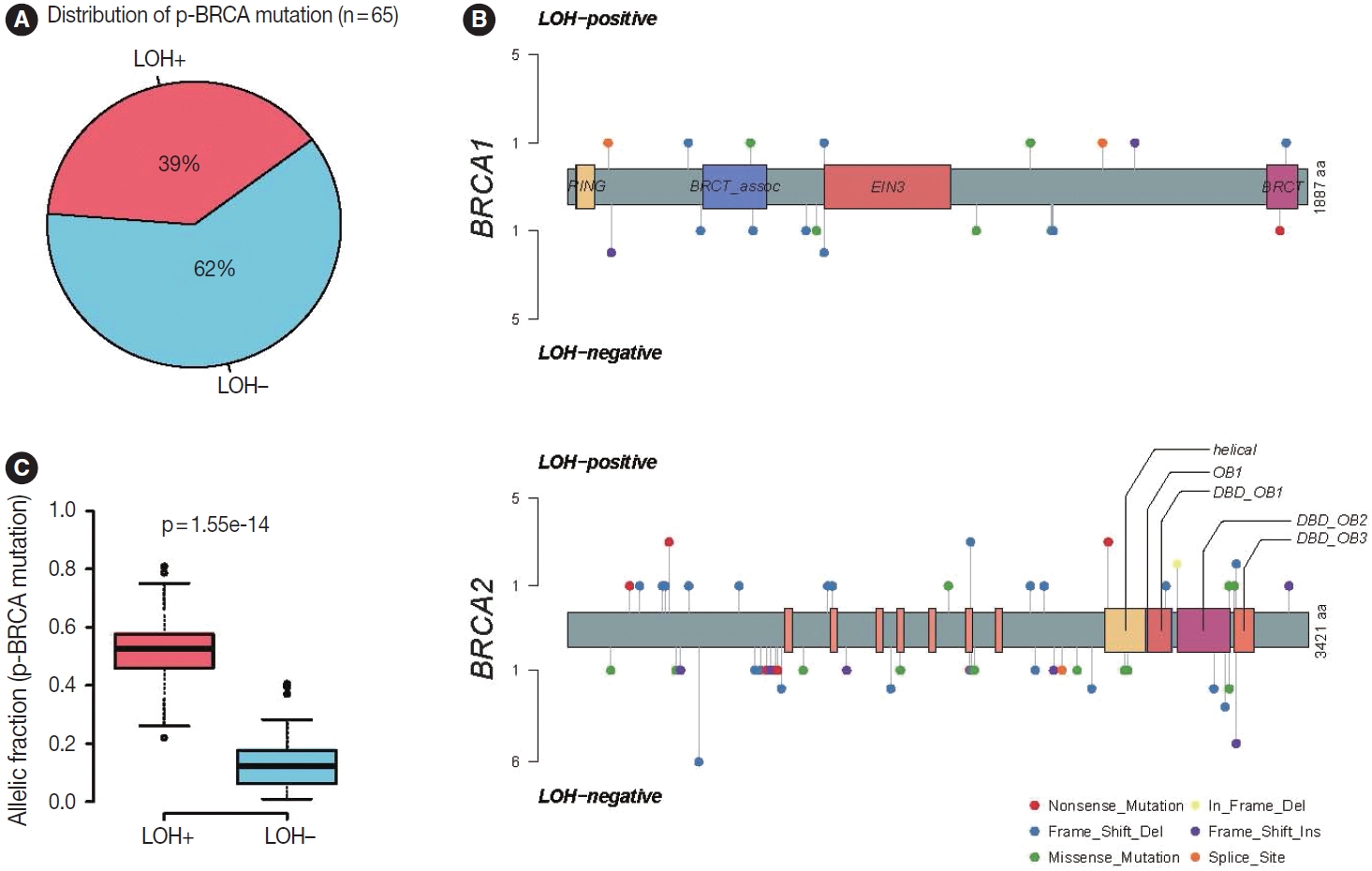
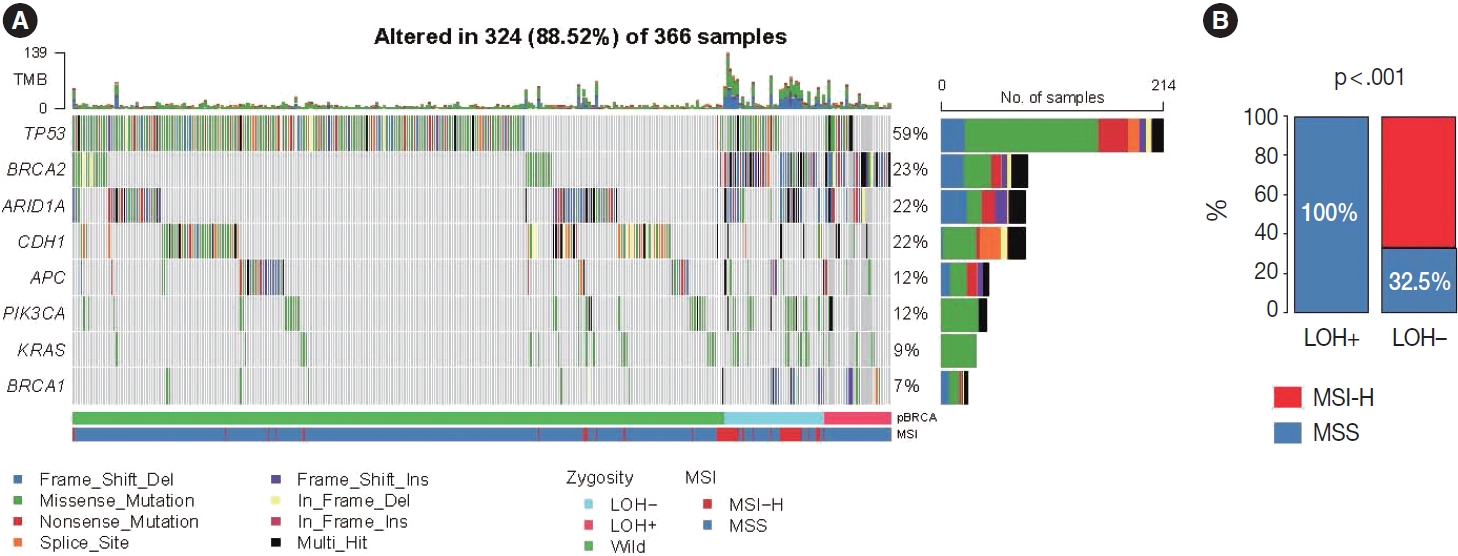
- Based on the association with responsiveness to platinumbased chemotherapy, we hypothesized that gastric cancers harboring p-BRCA mutation and LOH of the mutant allele might be associated with HRD and might be a good candidate for PARP inhibitor therapy. Approximately 40% of the p-BRCA mutations were accompanied by the LOH (Fig. 3A). Positions of observed mutations were scattered throughout the entire exonic area without any striking hotspot as usual tumor suppressors (Fig. 3B). VAFs were significantly higher in LOH+ group as expected (Fig. 3C). Genomic landscape of the included patients was generally consistent with the previously published large scale sequencing studies with TP53 mutations being most frequent followed by ARID1A and CDH1 mutations (Fig. 4A) [15,22]. The relatively frequent BRCA2 mutations were due to selection bias related to study design and BRCA2 mutations were far more frequent than BRCA1 mutations. Interestingly, all p-BRCA mutations, that were accompanied by LOH, were found in MSS tumors, while the vast majority of the p-BRCA mutations without LOH were in MSI-high (MSI-H) tumors (p < .001) (Fig. 4B). Genetic alterations involving homologous recombination (HR)–related genes other than the BRCA1 and BRCA2 were observed in 117 samples (32%) but they did not show mutual exclusivity from those of BRCA1 and BRCA2 alterations (Supplementary Fig. S1).
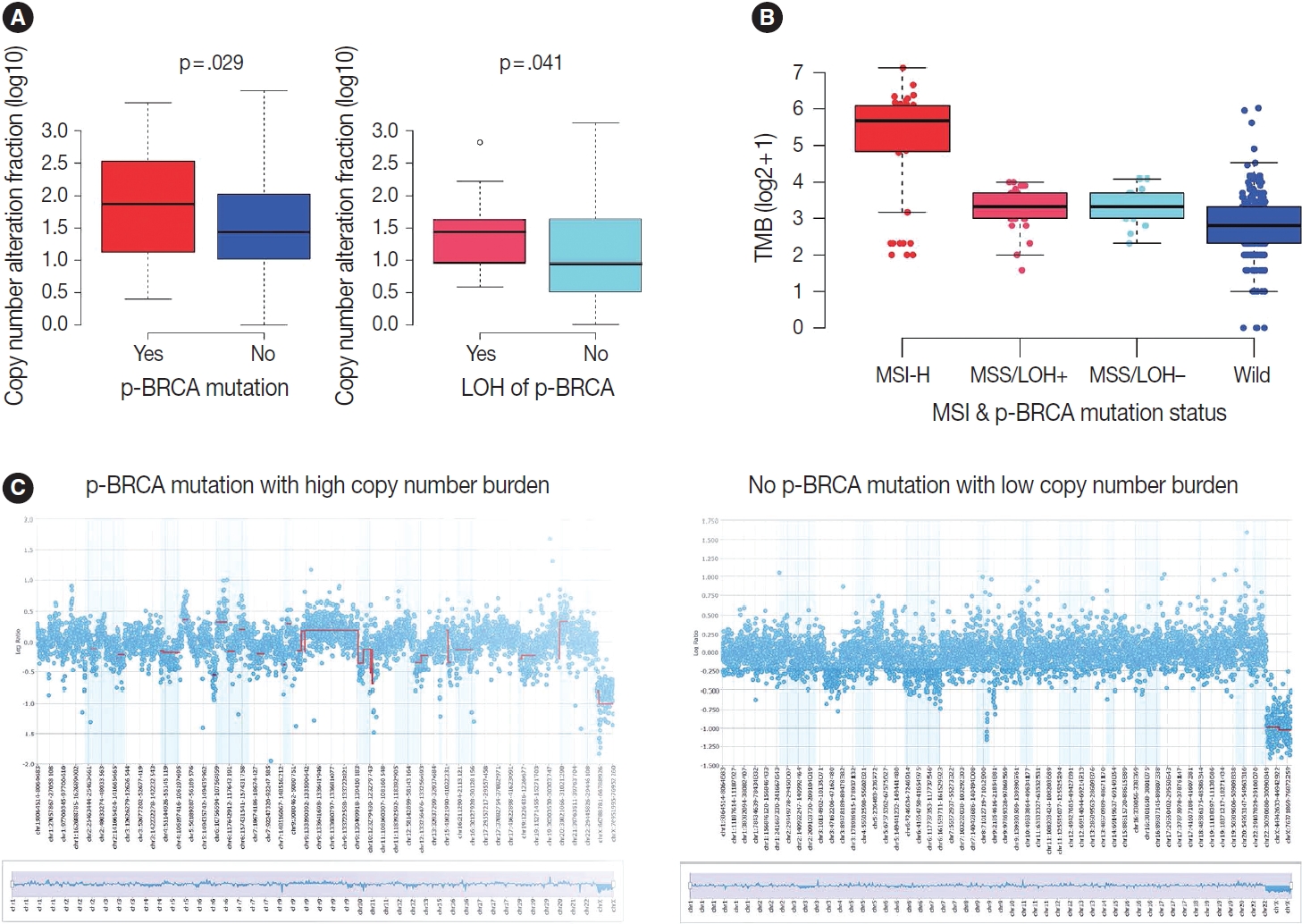
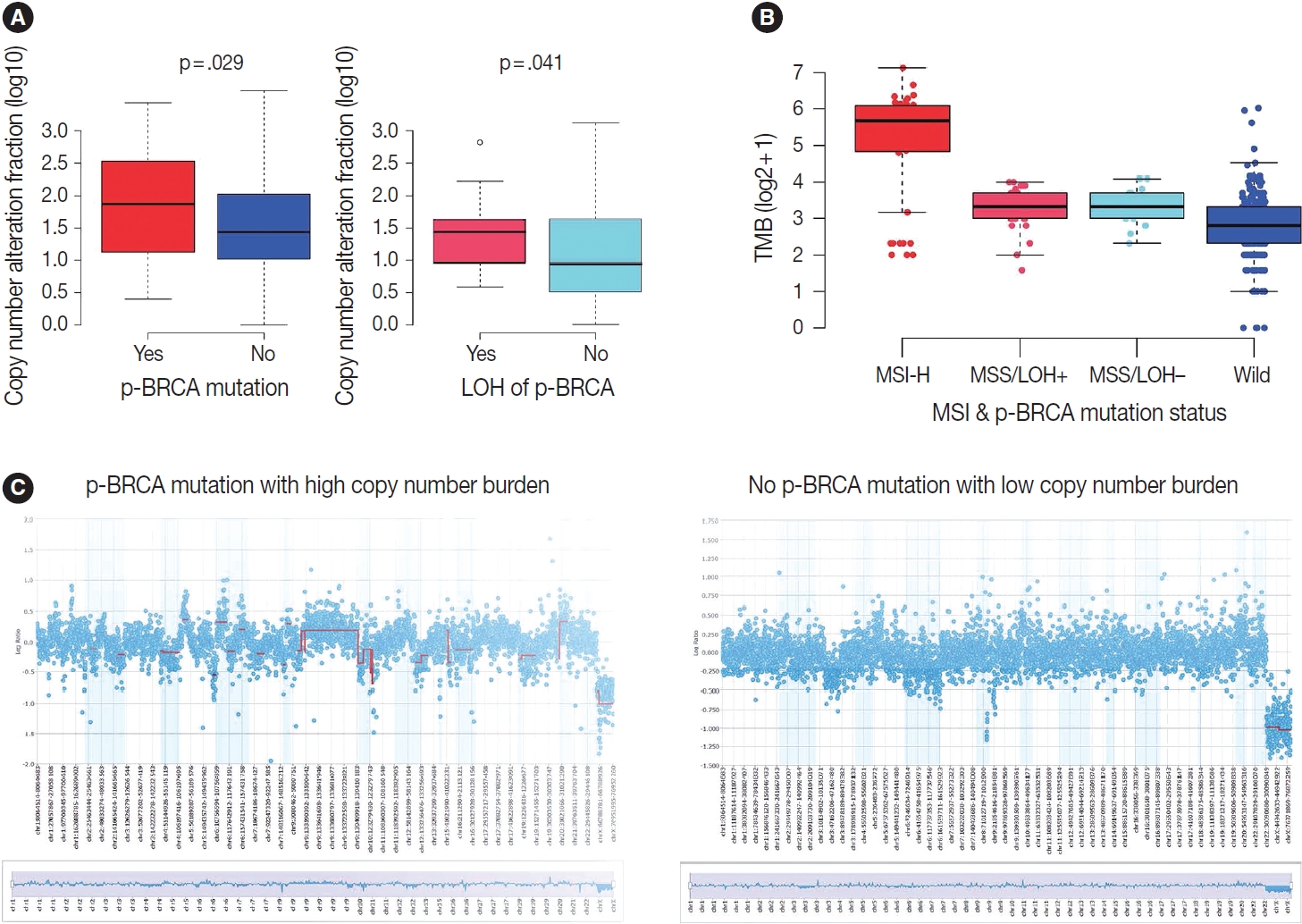
- Next, we focused on the copy number burden, namely, relative genomic regions showing copy number alterations of both loss and gain because it may serve as a surrogate for HRD score. Indeed, gastric cancers harboring p-BRCA mutations showed a higher copy number burden than those without p-BRCA mutations (p = .029) (Fig. 5A). Furthermore, within p-BRCA mutated tumors, tumors with concomitant LOH of the mutant allele were associated with a higher copy number burden than those without LOH (p = .041) (Fig. 5A). Then, when we classified cases with all required data, such as MSI status, EBV in situ hybridization result, and relative copy number burden, into the previously published four molecular subtypes, all gastric cancers harboring p-BRCA mutations with LOH belonged to CIN subtype while those harboring p-BRCA mutations without LOH were highly enriched in MSI-H subtype (Table 3) [15]. For TMB, MSI-H subtype showed a higher TMB as expected, and p-BRCA mutated tumors showed a trend toward higher TMB than nonmutated tumors within MSS tumors (Fig. 5B). The representative copy number plots were depicted in Fig. 5C.
RESULTS
- In this study, we showed that gastric cancer harboring p-BRCA mutations and LOH of the mutant allele is highly enriched in CIN molecular subtype and is associated with platinum-based chemotherapy. Although we could not directly evaluate HRD score and responsiveness to PARP inhibition, our findings suggest that patients with gastric cancer harboring biallelic p-BRCA mutations constitute a distinct, genetically defined subgroup that shows HRD phenotype and, hence might be a potential candidate for PARP inhibitor therapy. Considering the fact that molecularly targeted therapies are pretty limited in gastric cancer, our findings shed light on potential PARP inhibitor therapy for patients with gastric cancer harboring biallelic p-BRCA mutations. In addition, the absence of improvement in progressionfree survival by platinum-based chemotherapy implies that the PARP inhibitor therapy would be tried in an adjuvant setting rather than initially metastatic disease like high-grade serous carcinoma.
- In terms of selecting patients who would benefit from PARP inhibitor therapy, the most validated criterion is the presence of p-BRCA mutations. However, it has not been clearly defined whether the p-BRCA mutations should be accompanied by the LOH of the mutant allele. We found that the biological significance of p-BRCA mutation differs depending on the general genomic context. Patients with gastric cancer harboring p-BRCA mutations and LOH of the mutant allele showed a clear positive correlation with responsiveness to platinum-based chemotherapy, while those with gastric cancer harboring heterozygous p-BRCA mutations were more like those with BRCA wild-type tumors. Furthermore, the vast majority of the heterozygous p-BRCA mutations were found in microsatellite-unstable tumors, and all p-BRCA mutations found in MSI-H gastric cancers were heterozygous. It can be explained by the biological characteristics of the MSI-H tumors that frameshift insertion or deletion mutations rapidly accumulate during tumor evolution, and those “passenger” p-BRCA mutations may hit quite large BRCA1 or BRCA2 genes [23]. As such, most p-BRCA mutations in MSI-H gastric cancers are just passengers that are not associated with the HRD phenotype. Finally, concomitant LOH of the p-BRCA mutant allele represents selection pressure toward HRD and may lead to complete loss of BRCA protein function and HRD phenotype. Indeed, the strong association between responsiveness to platinum-based chemotherapy and p-BRCA mutation plus LOH remained significant within the MSS subgroup (p = .005) (Supplementary Table S3).
- Although inference of zygosity was done according to the methods described previously [20], it can be sometimes inaccurate because of inherent limitations of tumor-only sequencing. Therefore, whole exome sequencing on matched tumor-normal pairs might be required for more accurate inference.
- To further define molecular subgroups that can serve as a target population for HR-targeted therapies, we tried to classify our cases into the previously published four molecular subtypes [15] and found that gastric cancers harboring p-BRCA mutations plus LOH are exclusively CIN subtypes. Thus, we propose that potential targets for HR-targeted therapies should be screened in the CIN subtype of gastric cancers. During the assignment of molecular subtypes, we assumed that our study population is similar to that of the TCGA project rather than performing unsupervised clustering. We admit that our assumption may not be true because our sample set has a profound selection bias due to the case-control study design. External validation using independent sample sets with complete multi-omics and chemotherapy response data might be necessary to overcome this limitation. In addition, we could not directly measure HRD score for various reasons, and we used relative copy number burden as a surrogate marker. This may be a fundamental limitation because not all aneuploidies are associated with the HRD phenotype. Furthermore, we chose responsiveness to platinum-based chemotherapy as a clinical outcome because no patient has received PARP inhibitor therapy. To overcome those limitations, further studies involving direct measurement of HRD score or randomized clinical trials of PARP inhibitor therapies in genomically defined patient populations are needed.
- Because our study population has a selection bias and potential confounders, the prognostic significance is hard to determine. In a multivariate analysis including tumor stage, p-BRCA mutation, MSI status, ERBB2 status, and age as covariates, only tumor stage was independently associated with overall survival (data not shown). Notably, the p-BRCA mutation status plus LOH did not affect progression-free survival despite its association with good responses to platinum-based chemotherapy. Based on this observation, we propose that the efficacy of platinum-based chemotherapy in gastric cancer harboring biallelic p-BRCA mutations may be limited in the presence of measurable disease and that the potential use of PARP inhibition should be considered in an adjuvant setting after curative resection like high-grade serous carcinoma [24].
- Because gastric carcinomas with p-BRCA mutation plus LOH are relatively infrequent, an efficient way of patient selection is needed. Our data suggest that potential candidates for PARP inhibitor therapy should be searched in the CIN subtype of gastric cancer because all gastric cancers harboring p-BRCA mutation and LOH of the mutant allele were exclusively found in the CIN subtype. However, identification of the CIN subtype is not easy in routine clinical practice. In this sense, the identification of histologic characteristics of those tumors might be helpful. Although we could not clearly define morphologic characteristics of gastric carcinomas with p-BRCA mutation plus LOH, those tumors tended to have an expansile tumor border, central necrosis, peri-tumoral Crohn-like lymphoid aggregates, and solid, cribriform, or papillary patterns (Supplementary Fig. S2). An artificial intelligence-based approach on a larger cohort may provide useful tools for the identification of those cases on a mor-phologic basis.
- Taken together, patients with gastric cancer harboring p-BRCA mutations plus LOH of the mutant allele were associated with a good initial response to platinum-based chemotherapy, and such patients might be effectively identified by screening gastric cancers of CIN molecular subtype. Because responsiveness to platinum-based chemotherapy are known to be associated with HRD and responsiveness to PARP inhibitors, further investigations for HRD in gastric cancer tissue through validated methods and randomized clinical trials involving PARP inhibitors are warranted in the future.
DISCUSSION
Supplementary Information
Supplementary Table S1.
Supplementary Table S2.
Supplementary Table S3.
Supplementary Fig. S1.
Supplementary Fig. S2.
Ethics Statement
All procedures performed in the current study were approved by the Ethics Committee of Asan Medical Center, Seoul, Korea (IRB no. 2022-0956). Informed consent was waived by the Institutional Review Board.
Availability of Data and Material
Due to ethical limitations, the data analyzed in this study is not included in this manuscript. Under reasonable request, the corresponding author can provide the necessary information.
Code Availability
Not applicable.
Author contributions
Conceptualization: JK. Data curation: JHO, COS, HDK, SMC. Formal analysis: JK, COS, SMC. Investigation: JHO, HDK, SMC. Methodology: JK, COS, HDK. Project administration: JK. Software: SMC, COS. Supervision: JK. Validation: JK, COS. Visualization: COS, JHO. Writing—original draft: JHO. Writing—review & editing: JK, COS, JHO. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
Acknowledgments

| Response |
p-BRCA mutations and LOH status |
p-value | ||
|---|---|---|---|---|
| LOH+ | LOH– | Wild | ||
| Complete response | 1 (6.3) | 1 (4.2) | 5 (3.3) | .001a |
| Partial response | 15 (93.8) | 8 (33.3) | 86 (57.3) | |
| Stable disease | 0 | 12 (50.0) | 52 (34.7) | |
| Progressive disease | 0 | 3 (12.5) | 7 (4.7) | |
| Total | 16 | 24 | 150 | |
| Response |
p-BRCA mutations and LOH status |
p-value | ||
|---|---|---|---|---|
| LOH+ | LOH– | Wild | ||
| Complete response | 1 (6.3) | 1 (4.2) | 5 (3.3) | .001a |
| Partial response | 15 (93.8) | 8 (33.3) | 86 (57.3) | |
| Stable disease | 0 | 12 (50.0) | 52 (34.7) | |
| Progressive disease | 0 | 3 (12.5) | 7 (4.7) | |
| Total | 16 | 24 | 150 | |
- 1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet 2020; 396: 635-48. ArticlePubMed
- 2. Park SH, Kang MJ, Yun EH, Jung KW. Epidemiology of gastric cancer in Korea: trends in incidence and survival based on Korea Central Cancer Registry data (1999-2019). J Gastric Cancer 2022; 22: 160-8. ArticlePubMedPMCPDF
- 3. Johnston FM, Beckman M. Updates on management of gastric cancer. Curr Oncol Rep 2019; 21: 67.ArticlePubMedPDF
- 4. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11-20. ArticlePubMed
- 5. Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019; 393: 1948-57. PubMed
- 6. Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2022; 20: 167-92. PubMed
- 7. Yan D, Dai H. FOLFOX regimen in the patients with locally advanced or metastatic gastric cancer. Zhonghua Zhong Liu Za Zhi 2009; 31: 217-9. PubMed
- 8. Patel TH, Cecchini M. Targeted therapies in advanced gastric cancer. Curr Treat Options Oncol 2020; 21: 70.ArticlePubMedPDF
- 9. Zhang J, Willers H, Feng Z, et al. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol Cell Biol 2004; 24: 708-18. ArticlePubMedPMCPDF
- 10. Mylavarapu S, Das A, Roy M. Role of BRCA mutations in the modulation of response to platinum therapy. Front Oncol 2018; 8: 16.ArticlePubMedPMC
- 11. Kim D, Nam HJ. PARP inhibitors: clinical limitations and recent attempts to overcome them. Int J Mol Sci 2022; 23: 8412.ArticlePubMedPMC
- 12. Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature 2019; 571: 576-9. ArticlePubMedPMCPDF
- 13. Noh JM, Choi DH, Baek H, et al. Associations between BRCA mutations in high-risk breast cancer patients and familial cancers other than breast or ovary. J Breast Cancer 2012; 15: 283-7. ArticlePubMedPMC
- 14. Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021; 19: 77-102. PubMed
- 15. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202-9. ArticlePubMedPMCPDF
- 16. Kim JE, Chun SM, Hong YS, et al. Mutation burden and I index for detection of microsatellite instability in colorectal cancer by targeted next-generation sequencing. J Mol Diagn 2019; 21: 241-50. ArticlePubMed
- 17. Kim M, Lee C, Hong J, et al. Validation and clinical application of ONCOaccuPanel for targeted next-generation sequencing of solid tumors. Cancer Res Treat 2023; 55: 429-41. ArticlePubMedPDF
- 18. Buchhalter I, Rempel E, Endris V, et al. Size matters: Dissecting key parameters for panel-based tumor mutational burden analysis. Int J Cancer 2019; 144: 848-58. ArticlePubMedPDF
- 19. Talevich E, Shain AH, Botton T, et al. CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput Biol 2016; 12: e1004873.ArticlePubMedPMC
- 20. Siegmund SE, Manning DK, Davineni PK, Dong F. Deriving tumor purity from cancer next generation sequencing data: applications for quantitative ERBB2 (HER2) copy number analysis and germline inference of BRCA1 and BRCA2 mutations. Mod Pathol 2022; 35: 1458-67. ArticlePubMedPDF
- 21. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228-47. ArticlePubMed
- 22. Sanchez-Vega F, Mina M, Armenia J, et al. Oncogenic signaling pathways in The Cancer Genome Atlas. Cell 2018; 173: 321-37. PubMedPMC
- 23. Wodarz D, Newell AC, Komarova NL. Passenger mutations can accelerate tumour suppressor gene inactivation in cancer evolution. J R Soc Interface 2018; 15: 20170967.ArticlePubMedPMCPDF
- 24. Oza AM, Cibula D, Benzaquen AO, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol 2015; 16: 87-97. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Risk prediction criteria for the primary hepatic perivascular epithelioid cell tumour family, including angiomyolipoma: analysis of 132 cases with a literature review
Youngeun Yoo, Jihun Kim, In Hye Song
Histopathology.2025; 86(6): 979. CrossRef - Presence of RB1 or Absence of LRP1B Mutation Predicts Poor Overall Survival in Patients with Gastric Neuroendocrine Carcinoma and Mixed Adenoneuroendocrine Carcinoma
In Hye Song, Bokyung Ahn, Young Soo Park, Deok Hoon Kim, Seung-Mo Hong
Cancer Research and Treatment.2025; 57(2): 492. CrossRef - Predictive value of homologous recombination-related gene mutations in survival outcomes of first-line nivolumab plus chemotherapy for gastric cancer
Yuna Lee, Hyung-Don Kim, Sun Young Lee, Hyungeun Lee, Jaewon Hyung, Meesun Moon, Jinho Shin, Young Soo Park, Tae Won Kim, Min-Hee Ryu
Gastric Cancer.2025; 28(6): 1158. CrossRef - Association of RAD51 expression with response to neoadjuvant treatment and prognosis in locally advanced gastric cancer
Serhat Sekmek, Serhat Ozan, Fahriye Tugba Kos, Hayriye Tatli Dogan, Mehmet Akif Parlar, Didem Sener Dede
Expert Review of Anticancer Therapy.2025; 25(12): 1433. CrossRef - Artificial intelligence algorithm for neoplastic cell percentage estimation and its application to copy number variation in urinary tract cancer
Jinahn Jeong, Deokhoon Kim, Yeon-Mi Ryu, Ja-Min Park, Sun Young Yoon, Bokyung Ahn, Gi Hwan Kim, Se Un Jeong, Hyun-Jung Sung, Yong Il Lee, Sang-Yeob Kim, Yong Mee Cho
Journal of Pathology and Translational Medicine.2024; 58(5): 229. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure





Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
| Variable | p-BRCA mutation |
p-value | ||
|---|---|---|---|---|
| Yes (n = 65) | No (n = 301) | |||
| Age (mean) | 61.3 | 58.5 | .213 | |
| Sex | .047 | |||
| Male | 51 (78.3) | 198 (65.7) | ||
| Female | 14 (21.7) | 103 (34.3) | ||
| Initial stage | .401 | |||
| I | 8 (11.6) | 13 (4.4) | ||
| II | 5 (8.7) | 33 (10.8) | ||
| III | 14 (23.2) | 82 (26.9) | ||
| IV | 38 (56.5) | 173 (57.9) | ||
| ERBB2 IHC positivity | 2 (3.1) | 40 (13.3) | .019 | |
| Resectable at the time of diagnosis | 27 (43.5) | 129 (42.4) | .845 | |
| Recurrence/metastasis after surgery | 16 (30.3) | 97 (32.7) | .228 | |
| Histologic diagnosis | .076 | |||
| Adenocarcinoma, NOS | 52 (80.0) | 240 (79.7) | ||
| Signet ring cell/poorly cohesive carcinoma | 6 (9.2) | 50 (16.6) | ||
| Mucinous adenocarcinoma | 1 (1.5) | 4 (1.3) | ||
| Medullary carcinoma with lymphoid stroma | 2 (3.1) | 3 (1.0) | ||
| Other special variants | 4 (6.2) | 4 (1.3) | ||
| Lauren classification | .114 | |||
| Intestinal | 20 (30.8) | 93 (30.9) | ||
| Diffuse | 12 (18.5) | 68 (22.6) | ||
| Mixed | 2 (3.1) | 62 (20.6) | ||
| Indeterminate | 31 (47.7) | 78 (25.9) | ||
| Response | p-BRCA mutations and LOH status |
p-value | ||
|---|---|---|---|---|
| LOH+ | LOH– | Wild | ||
| Complete response | 1 (6.3) | 1 (4.2) | 5 (3.3) | .001 |
| Partial response | 15 (93.8) | 8 (33.3) | 86 (57.3) | |
| Stable disease | 0 | 12 (50.0) | 52 (34.7) | |
| Progressive disease | 0 | 3 (12.5) | 7 (4.7) | |
| Total | 16 | 24 | 150 | |
| Response | p-BRCA mutations and LOH status |
p-value | ||
|---|---|---|---|---|
| LOH+ | LOH– | Wild | ||
| Complete response | 1 (6.3) | 1 (4.2) | 5 (3.3) | .001 |
| Partial response | 15 (93.8) | 8 (33.3) | 86 (57.3) | |
| Stable disease | 0 | 12 (50.0) | 52 (34.7) | |
| Progressive disease | 0 | 3 (12.5) | 7 (4.7) | |
| Total | 16 | 24 | 150 | |
Values are presented as number (%) unless otherwise indicated. NGS, next-generation sequencing; p-BRCA mutation, pathogenic or likely pathogenic
Values are presented as number (%). p-BRCA mutation, pathogenic or likely pathogenic Kruskal-Wallis test.
Values are presented as number (%). p-BRCA mutation, pathogenic or likely pathogenic BRCA1 or BRCA2 gene mutation; LOH, loss of heterozygosity. Kruskal-Wallis test.

 E-submission
E-submission