Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 57(6); 2023 > Article
-
Original Article
Elevated expression of Axin2 in intestinal metaplasia and gastric cancers -
Dong Hui Lee1
 , In Ho Jeong2
, In Ho Jeong2 , Bogun Jang1
, Bogun Jang1
-
Journal of Pathology and Translational Medicine 2023;57(6):315-322.
DOI: https://doi.org/10.4132/jptm.2023.10.12
Published online: November 7, 2023
1Department of Pathology, Jeju National University Hospital, Jeju National University School of Medicine, Jeju, Korea
2Department of Surgery, Jeju National University Hospital, Jeju National University School of Medicine, Jeju, Korea
- Corresponding Author: Bogun Jang, MD, PhD, Department of Pathology, Jeju National University Hospital, Jeju National University School of Medicine, 15 Aran 13-gil, Jeju 63241, Korea Tel: +82-64-717-1410, Fax: +82-64-717-1494, E-mail: bgjang9633@gmail.com
© 2023 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- The Wnt signaling pathway regulates crucial cellular processes, including stem cell development and tissue repair. Dysregulation of this pathway, particularly β-catenin stabilization, is linked to colorectal carcinoma and other tumors. Axin2, a critical component in the pathway, plays a role in β-catenin regulation. This study examines Axin2 expression in normal gastric mucosa and various gastric pathologies.
-
Methods
- Formalin-fixed and paraffin-embedded tissue samples from normal stomach, gastritis, intestinal metaplasia (IM), and gastric carcinoma were collected. Axin2 and β-catenin expression were evaluated using RNA in situ hybridization and immunohistochemistry, respectively. Histo-scores (H-scores) were calculated to quantify expression levels of Axin2. Associations between Axin2 expression and clinicopathological variables were examined.
-
Results
- Axin2 expression was examined in normal stomach, gastritis, and IM tissues. Axin2 expression was mainly observed in the surface and isthmus areas in the normal stomach and gastritis, whereas Axin2 expression was markedly higher at the bases of IM. Axin2 H-scores were significantly elevated in IM (mean ± standard deviation [SD], 87.0 ± 38.9) compared to normal (mean ± SD, 18.0 ± 4.5) and gastritis tissues (mean ± SD, 33.0 ± 18.6). In total, 30% of gastric carcinomas showed higher Axin2 expression. Axin2 expression did not have significant associations with age, sex, Lauren classification, histological differentiation, invasion depth, and lymph node metastasis. However, a strong positive correlation was observed between Axin2 and nuclear β-catenin in gastric carcinomas (p < .001).
-
Conclusions
- Axin2 expression was significantly increased in IM compared to normal and gastritis cases. In addition, Axin2 showed a strong positive association with nuclear β-catenin expression in gastric carcinomas, demonstrating a close relationship with abnormal Wnt/β-catenin signaling pathway.
- Tissue samples
- The samples were collected from patients with gastric carcinomas who underwent surgical resection at Jeju National University Hospital (JNUH) in Jeju, Korea, between 2012 and 2017, including GC (n = 56), IM (n = 10), chronic gastritis (n = 5), and normal (n = 5) gastric samples. Inflammation in the gastric mucosa was graded according to the Sydney classification and we considered the cases as chronic gastritis when inflammation grade is moderate or marked. The classification of histological subtypes of carcinomas was independently determined by two pathologists (D.H.L. and B.J.). For GC cases, we gathered clinical and pathological data, including age, sex, Lauren classification, histological grade, invasion depth, and lymph node metastasis.
- Tissue array construction
- Total five tissue microarrays (TMAs) were constructed; four TMAs included 96 cores from GC cases and 20 cores from noncancerous gastric lesions. In brief, representative tumor portion was carefully selected from hematoxylin and eosin–stained slides through histologic examination. Each representative tumor portion comprised more than 70% of the cell population. The 4-mm diameter core tissues were obtained from individual GC paraffin blocks and non-cancerous block. Then, these core tissues were arranged in a new recipient paraffin block (tissue array block) using a trephine apparatus (SuperBioChips Laboratories, Seoul, Korea).
- Immunohistochemistry and interpretation
- Immunohistochemistry (IHC) targeting β-catenin was conducted on 4-μm sections of the TMAs. IHC was done with the BOND-MAX automated immunostainer and a Bond Polymer Refine Detection kit (Leica Microsystems, Wetzlar, Germany) according to the manufacturer’s guidelines. The primary antibody used was anti–β-catenin (1:800, catalog number 17C2, Novocastra Laboratories, Newcastle, UK). Positive nuclear β-catenin staining was defined as cases where more than 10% of tumor cell nuclei displayed strong staining for β-catenin.
- Axin2 RNA in situ hybridization and interpretation
- To detect Axin2 mRNA, we applied RNAscope kit (Advanced Cell Diagnostics, Hayward, CA, USA) with unstained tissue slides according to the manufacturer’s instructions. Each tissue section underwent pretreatment involving heat and protease application before hybridization with a specific Axin2 probe. A horseradish peroxidase-based signal amplification system was hybridized to the probe, followed by color development using 3,3’-diaminobenzidine tetrahydrochloride. The housekeeping gene ubiquitin C was employed as a positive control, and the bacterial gene DapB served as a negative control. The patterns of brown punctate dots present in nucleus and/or cytoplasm interpreted positive staining. Axin2 expression was quantified according to the five-grade scoring system (grade 0: no staining, grade 1: 1–3 dots/cell, grade 2: 4–10 dots/cell, grade 3: > 10 dots/cell, grade 4: > 15 dots/cell with > 10% of dots in clusters) [13]. The grade and percentage of tumor cells expressing Axin2 were measured, and histoscores (H-scores) were calculated by multiplying the grade (range, 0 to 4) and percentage of Axin2-positive tumor cells (range, 1 to 100), ranging from 0 to 400. For statistical analysis, H-score of 68 was chosen as cutoff value based on the mean value of Axin2 H-score (H-score, 68); the tumor was defined as high when Axin2 H-score is higher than 68.
- Statistical analysis
- Statistical analyses were performed using the SPSS (Statistical Package for the Social Sciences) statistical software ver. 18.0 (SPSS Inc., Chicago, IL, USA) and Prism ver. 9.0.1 (GraphPad Software, San Diego, CA, USA). We analyzed the association of Axin2 with clinicopathological features with the Pearson χ2 test. The correlations between the Axin2 H-scores and β-catenin expressions were evaluated by the Spearman correlation test. Difference was considered significant when p < .05.
MATERIALS AND METHODS
- Axin2 expression in the normal gastric mucosa with or without inflammation
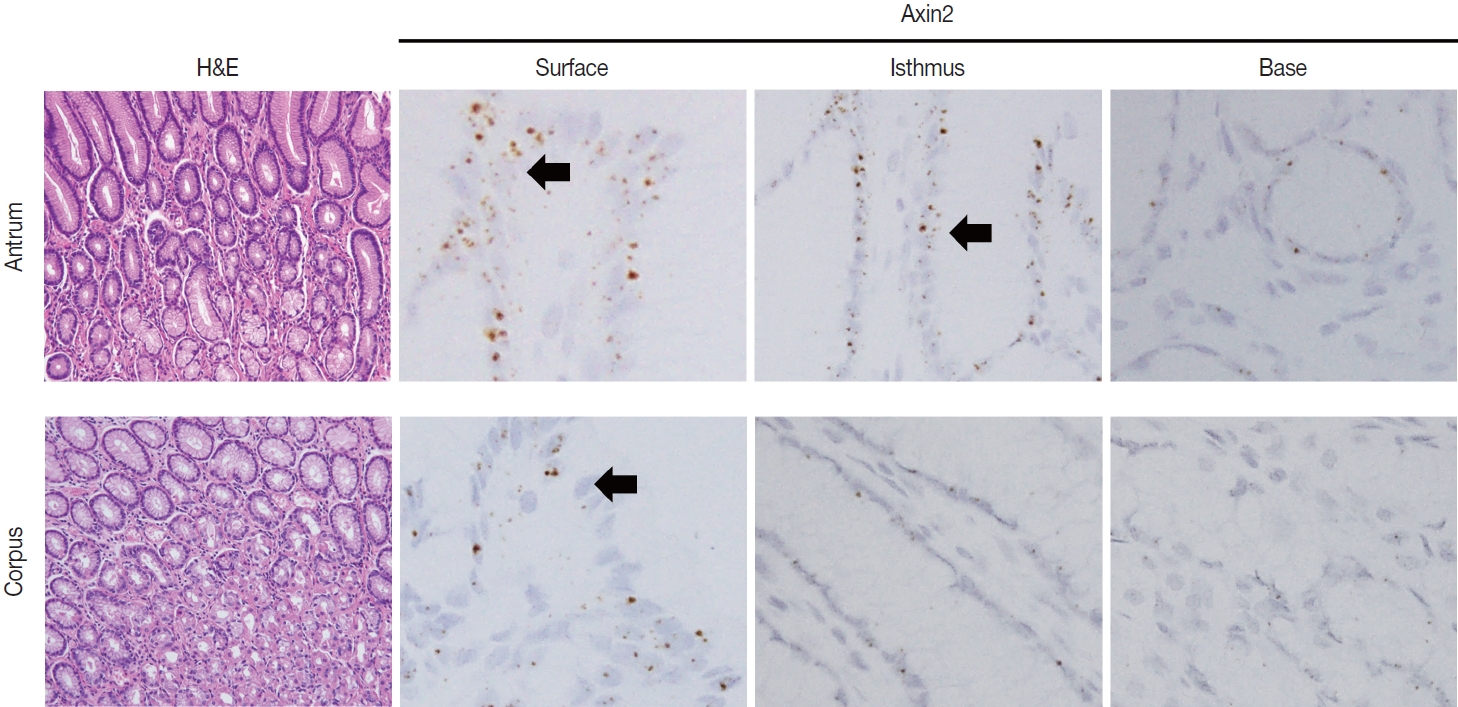
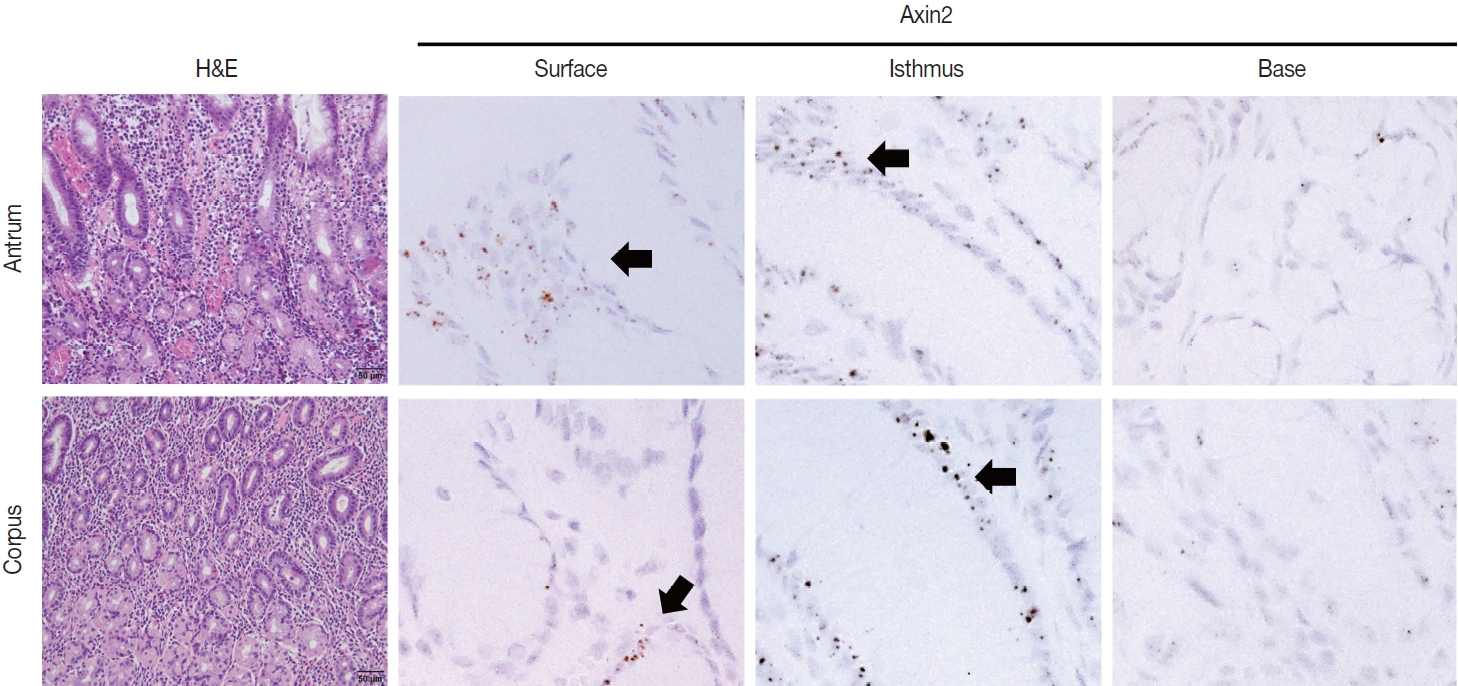
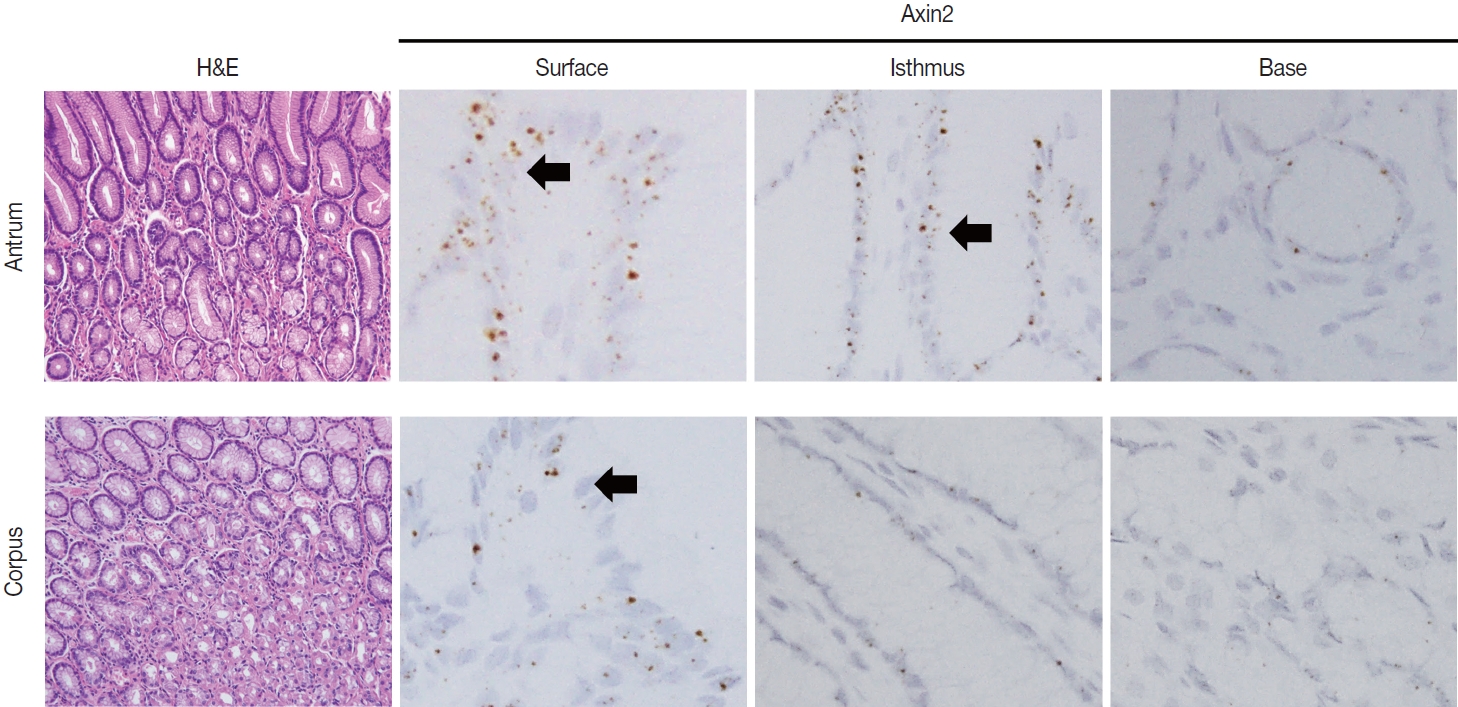
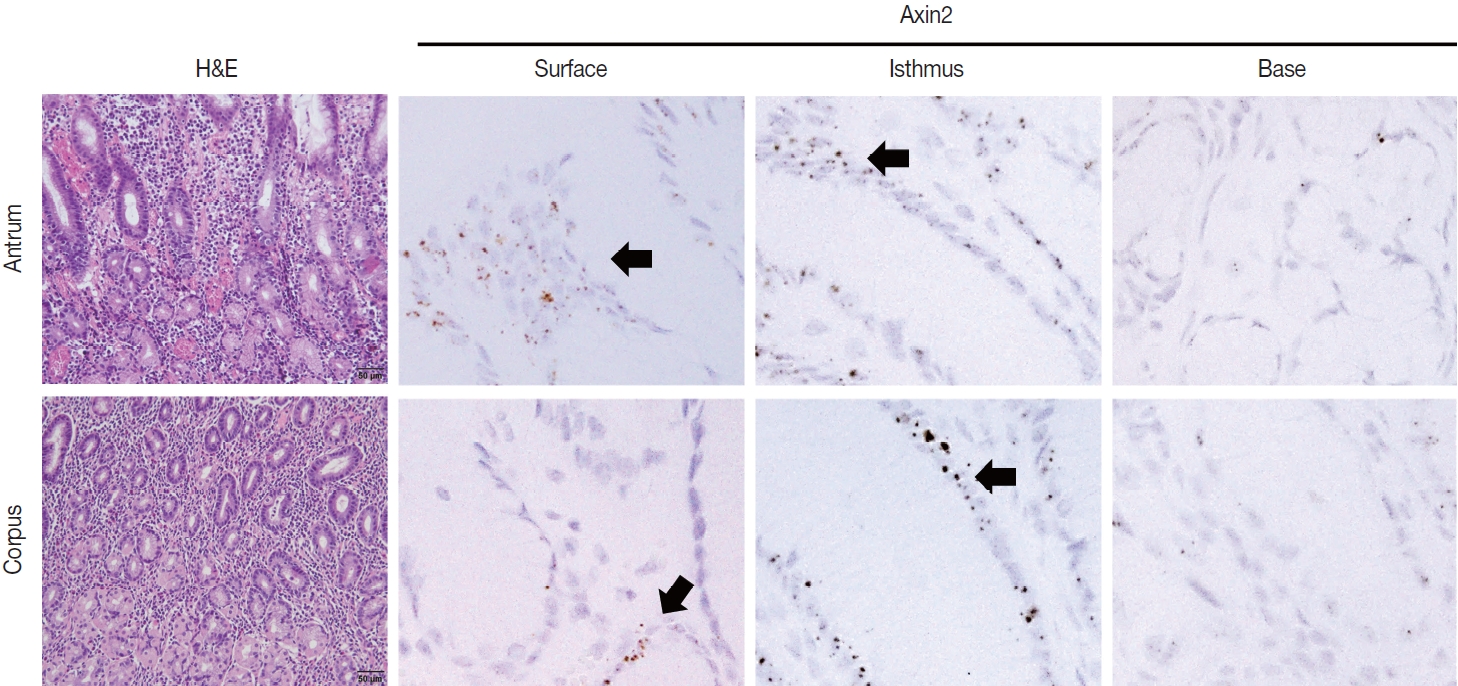
- To examine Axin2 expression in the non-tumorous stomach, we performed RNA in situ hybridization for Axin2 with 12 cases of normal gastric tissues (n = 12, comprising 20 spots): including samples from normal gastric mucosa without inflammation (5 spots), gastritis cases (5 spots), and IM (10 spots). We observed a significant level of Axin2 expression in the normal stomach without inflammation. Axin2 was primarily expressed in the superficial epithelial cells and isthmus areas with an intensity level of 2 or lower (Fig. 1). In contrast, Axin2 expression was rarely observed at the gland bases. Then, we determined Axin2 expression in gastritis cases and observed a similar extent of Axin2 expression to as seen in the normal gastric mucosa (Fig. 2).
- Axin2 expression in IM
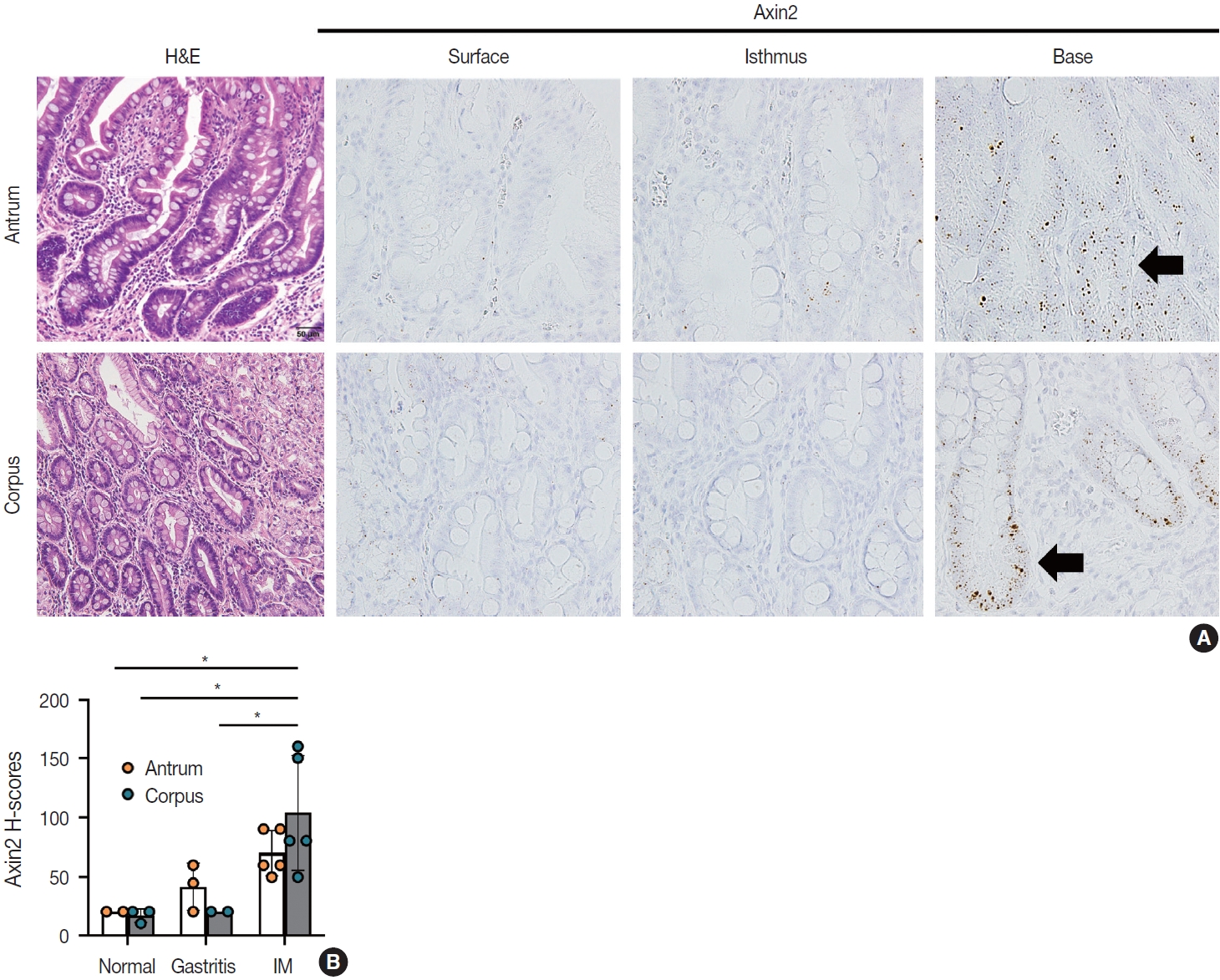
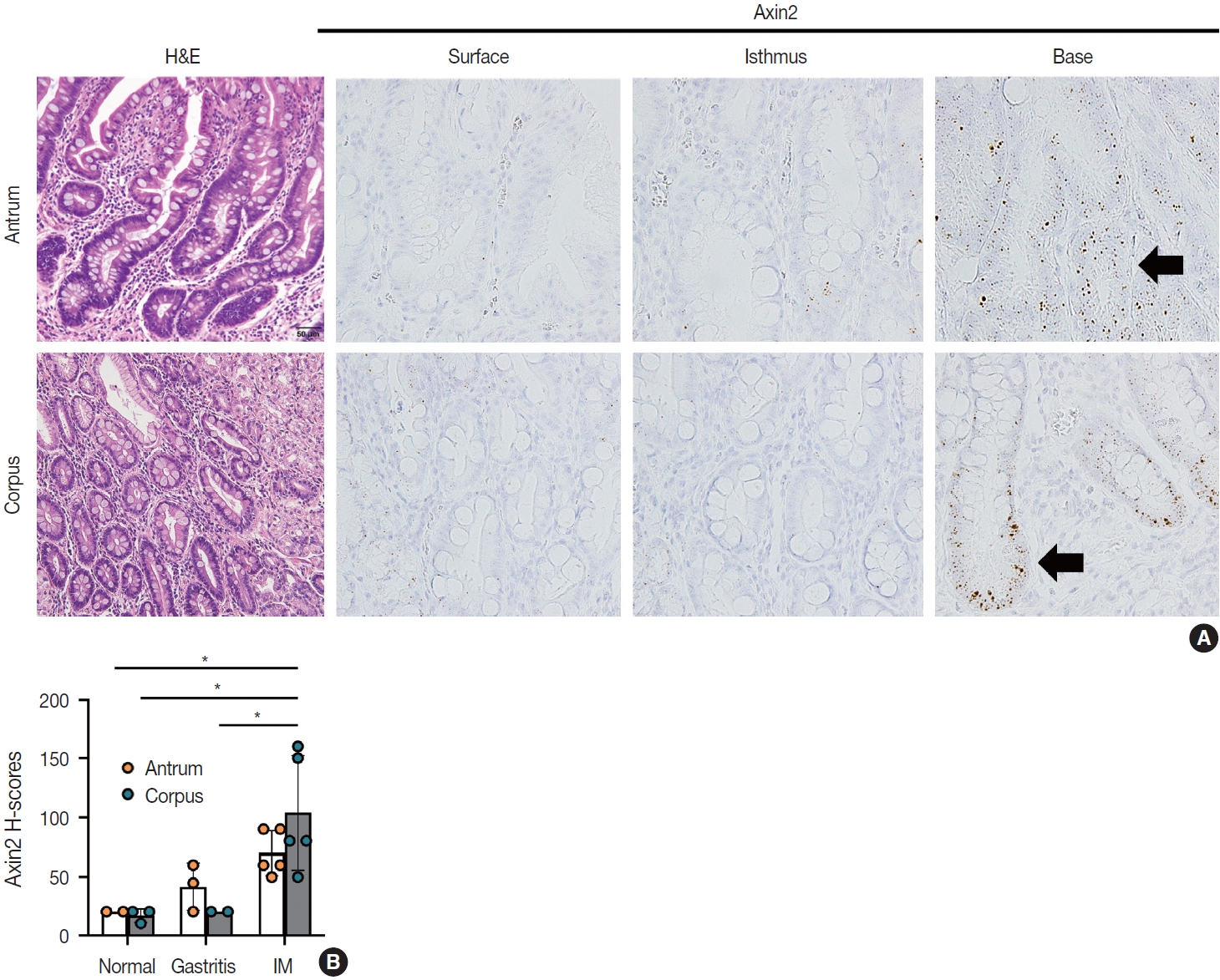
- IM is considered a precancerous lesion of GC. Thus, we evaluated Axin2 expression in IM and found that Axin2 expression was remarkably increased with an intensity level of 3 or higher both in the antrum and corpus. In particular, Axin2 expression was accentuated at the bases of IM glands (Fig. 3A), while the surface and isthmus regions displayed an intensity level of 2. Likewise, goblet cells in the superficial area did not exhibit Axin2 expression, whereas those at the base region displayed an intensity level of 2 to 3. We determined H-scores of Axin2 in 12 nontumorous tissue samples. Axin2 expression was significantly upregulated in IM (H-score: mean ± standard deviation [SD], 87.00 ± 38.88) compared to normal (H-score: mean ± SD, 18.00 ± 4.47) and gastritis (H-score: mean ± SD, 33.00 ± 18.57) cases (Fig. 3B). These findings suggest that Axin2 expression might be associated intestinal differentiation or indicate a development into precancerous stage.
- Associations of Axin2 expression with clinicopathological features in gastric carcinomas
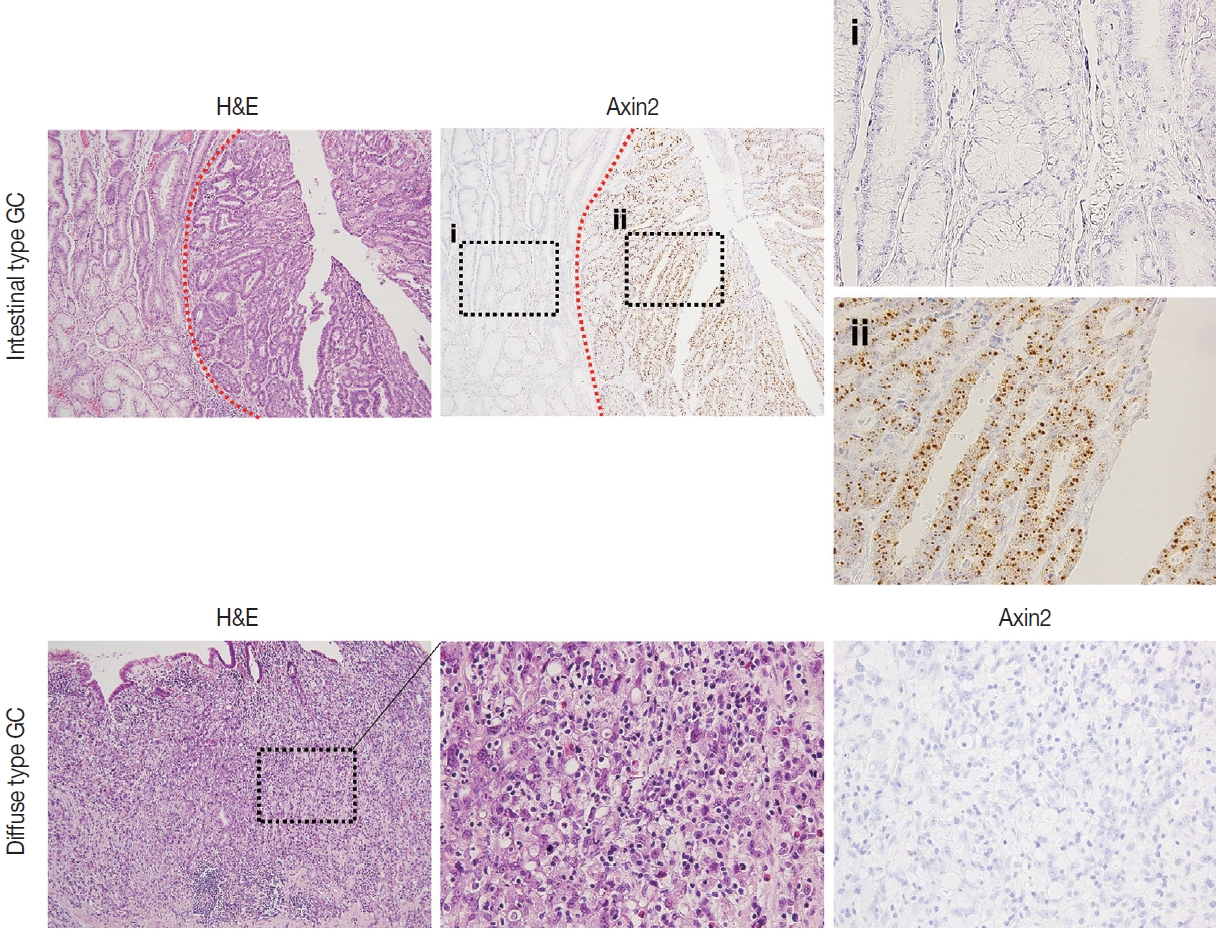
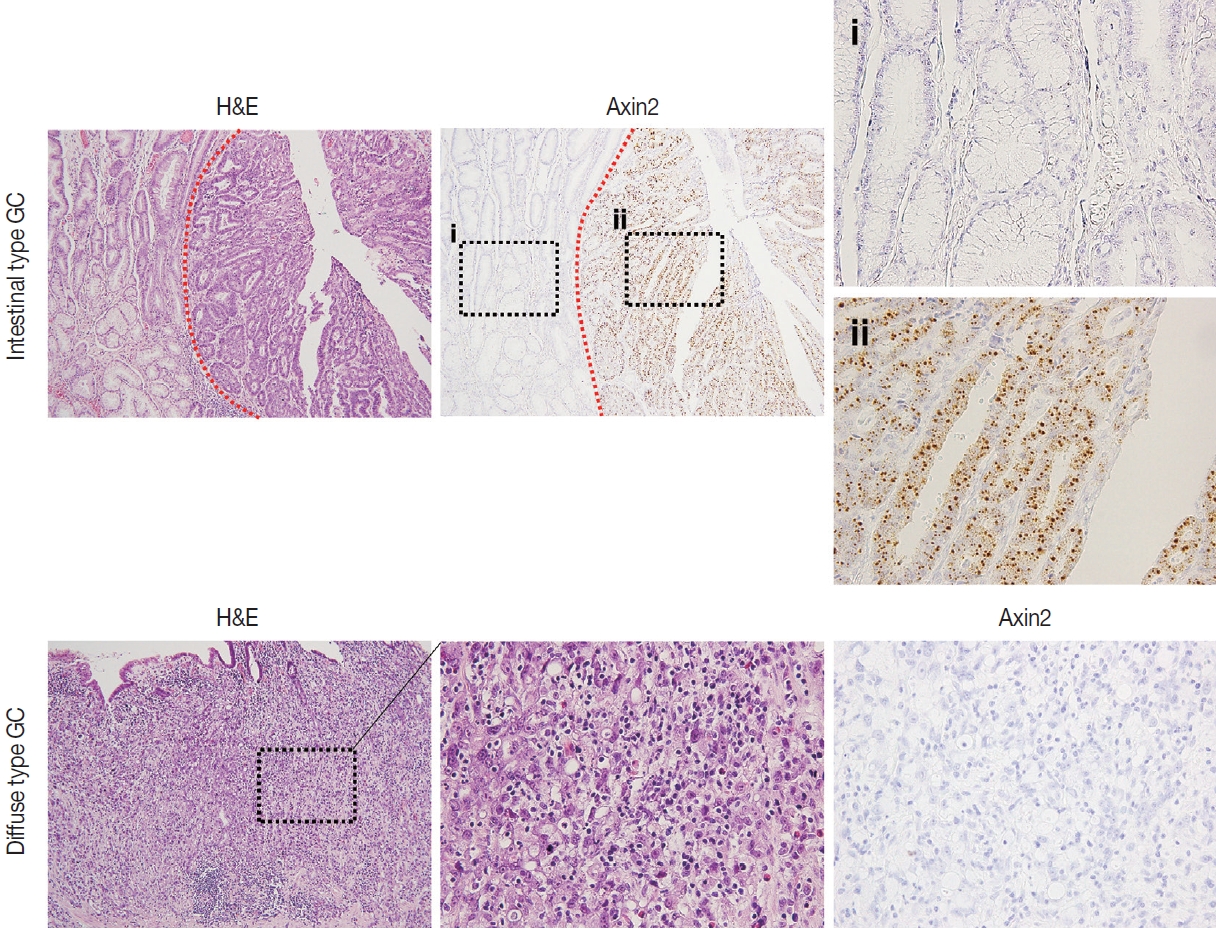
- Next, we examined Axin2 expression in 56 cases of early gastric carcinomas and measured H-scores. Patient characteristics including age, gender, Lauren classification, histological differentiation, invasion depth, and lymph node metastasis is presented in Table 1. We considered Axin2 expression high when Hscores were higher than 68 based on the fact that mean value of H-score was 68. In total, 30% of GC cases showed high Axin2 expression (Table 1). No clinicopathological characteristic was found to be significantly associated with Axin2 expression (Table 1). Although it did not reach the statistical significance, high Axin2 expression was more often observed in GC patients with older age (p = .062), and intestinal-type gastric carcinomas tend to show high Axin2 expression (p = .286) (Fig. 4). Furthermore, lymph node metastasis was not observed in Axin2-high GC (p = .108).
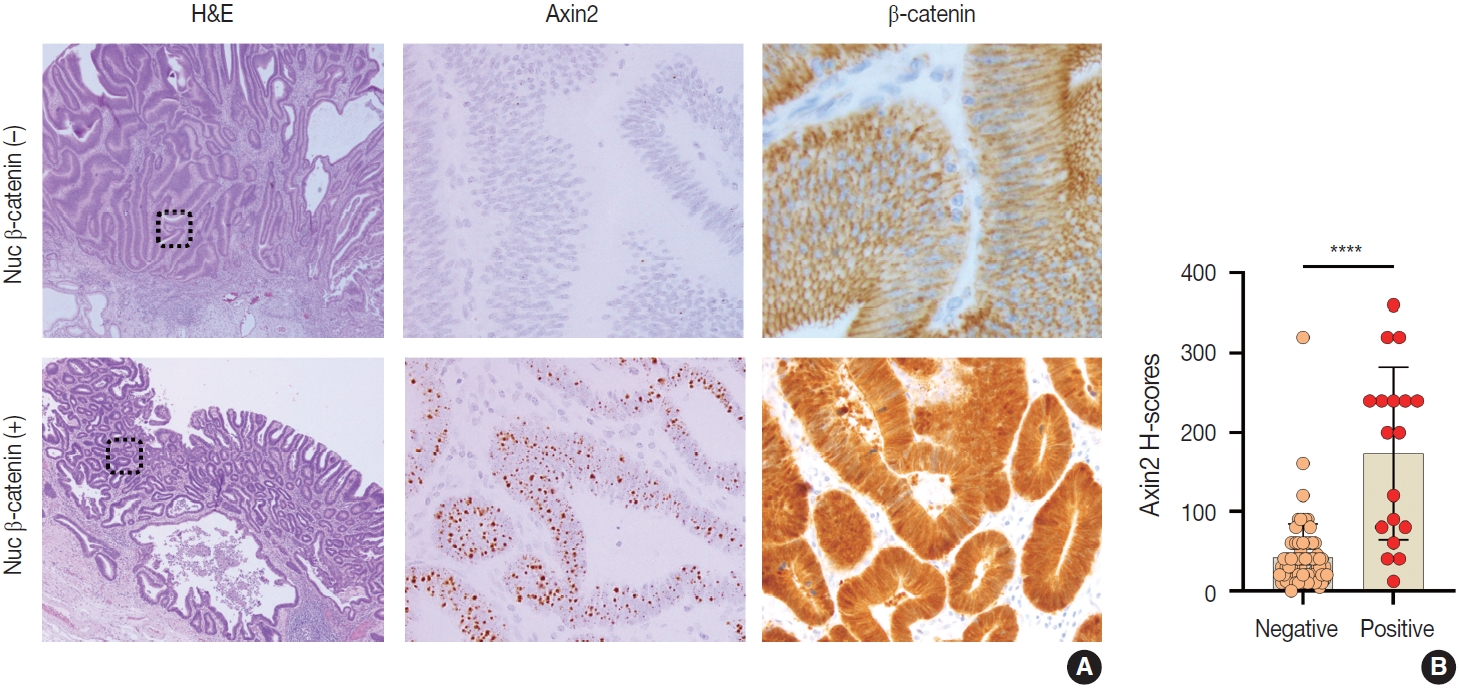
- Nuclear β-catenin expression in gastric carcinomas and its correlation with Axin2
- Nuclear β-catenin represents abnormal activation of Wnt/β-catenin pathway. Since Axin2 is one of the target genes of Wnt pathway, we hypothesized that Axin2 expression might be associated with nuclear β-catenin expression. Therefore, we performed IHC for β-catenin on GC cases and found that 12 cases (21%) of GC exhibited nuclear β-catenin expression (Table 1). Notably, Axin2 expression exhibited a strong positive association with nuclear β-catenin (p < .001) (Table 1). Representative images are presented in Fig. 5A. Among 44 cases with negative nuclear β-catenin, 37 cases (84%) displayed low Axin2 H-scores (mean ± SD, 78.00 ± 77.04), while seven cases (16%) exhibited high Axin2 H-scores. Conversely, in the subset of 12 cases featuring positive nuclear β-catenin, two cases (17%) demonstrated low Axin2 H-scores, while the remaining 10 cases (83%) exhibited high Axin2 H-scores (mean ± SD, 179.40 ± 87.09). Axin2 H-scores were significantly higher in GC with nuclear β-catenin expression (Fig. 5B), clearly indicating that abnormally enhanced Wnt/b-catenin activity might be associated with elevated Axin2 expression.
RESULTS
- When comparing the expression of Axin2 among normal, gastritis, and IM, normal and gastritis samples demonstrated H-scores below the average in the surface and isthmus regions, respectively. Conversely, IM predominantly exhibited elevated H-scores, particularly at the base of glands (Fig. 5). The absence of a substantial increase in Axin2 expression in gastritis suggests that mild to moderate gastritis does not induce activation of Wnt/β-catenin pathway. In contrast, Axin2 expression was significantly increased in cases of IM. We believe that this is the first study that has demonstrated the upregulation of Axin2 in IM. Considering that Axin2 is normally highly expressed in the crypt bases of normal intestines, Axin2 upregulation in IM seems to be associated with the transition into intestinal phenotype. This observation appears to align with previous research indicating that the progression of IM increases the susceptibility to dysplasia and carcinoma in the stomach [14]. However, further studies are required to fully understand the implications of Axin2 in IM, for example, whether it is involved in the progression of IM into dysplasia or gastric carcinoma. Despite the relatively limited number of IM cases in the present study, our results provided a connection between the IM and Axin2 expression.
- Axin2 is a direct target gene of Wnt/β-catenin. It exerts control over the cytoplasmic levels of β-catenin by facilitating its degradation [1]. In the absence of Wnt signaling, the amino terminus of β-catenin undergoes phosphorylated by CK1 and GSK-3β, leading to its destruction through multi-protein destruction complex composed of APC, GSK-3β, Axin1, and conductin/Axin2. This process results in the proteasomal degradation of β-catenin [15,16]. In the nucleus, β-catenin bonds with TCF to activate expression of various genes including Axin2. Axin2 participates in the negative feedback regulation of Wnt/β-catenin signaling pathway by promoting the degradation of β-catenin [17]. Consistent with this role, Axin2 has been demonstrated to act as a tumor suppressor [16]. Carcinomas arising due to abnormalities in the Wnt/β-catenin signaling pathway exhibit increased expression of nuclear β-catenin [18]. Therefore, we suggest that increased Axin2 expression in gastric carcinomas is likely derived from the enhanced Wnt/β-catenin signaling pathway. Furthermore, it might be meaningful to explore the potential of high Axin2 expression as a prognostic factor for gastric car-cinoma, similar to abnormal β-catenin nuclear expression [19].
- One disappointing aspect of our findings is the absence of a discernible correlation between the expression level of Axin2 and the differentiation status of gastric carcinomas. This observation implies that the differentiation of GC might be governed by mechanisms independent of abnormal activation of Wnt/β-catenin signaling pathway. As previously established, well-differentiated (WD) carcinoma and poorly differentiated (PD) carcinoma follow distinct carcinogenetic pathways [20]. While WD carcinoma is primarily associated with factors such as H. pylori infection, hTERT-positive stem cell hyperplasia, and genetic instability, PD carcinoma is linked to events like chromosome 17p’s loss of heterozygosity (LOH), mutation or LOH of p53, and mutation or loss of E-cadherin [20]. Our initial hypothesis suggested that Axin2 might play a role in the differentiation process; however, our results did not yield substantial evidence to substantiate this notion.
- This study has certain inherent limitations. Firstly, we did not simultaneously assess other components of the Canonical Wnt/β-catenin signaling pathway. The progression of cancer cells can occur through specific proteins other than β-catenin and Axin2. Further study is required to better understand the interactions between Axin2 and other essential proteins in Wnt signaling pathway. Secondly, there exists an imbalance in the number of cancer tissues exhibiting high Axin2 expression compared to low expression. Ideally, a more balanced distribution would be desirable. However, in our experimental setup, the ratio of patients with Axin2-high GC to those with Axin2-low GC was approximately 1:2. Consequently, future experiments with a greater number of Axin2-high GC could yield results differing from those obtained in this study. Furthermore, the evaluation of Helicobacter pylori infection and the noncanonical Wnt/β-catenin signaling pathway was not included in our study. Prior research has reported an increase in nuclear β-catenin and TCF/LEF transactivation due to H. pylori [21,22]. Hence, exploring the potential association between H. pylori infection and Axin2’s negative feedback could offer meaningful insights.
- In summary, we discovered that Axin2 expression is significantly increased in IM compared to normal gastric tissue and gastritis, in line with the fact that IM is a precancerous stage for dysplasia or cancer progression. The expression level of Axin2 in GC was also associated with the nuclear expression of β-catenin, demonstrating the intimate relationship between Axin2 and Wnt/β-catenin signaling pathway. Further research is required to explore the potential of Axin2 as a prognostic marker in GC.
DISCUSSION
Ethics Statement
This study was approved by the ethics committee of the Institutional Review Board of JNUH (IRB 2021-07-007) and was conducted in accordance with the Declaration of Helsinki. Informed consent from the patients was exempted by IRB approval.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author contributions
Conceptualization: DHL, IHJ, BJ. Data curation: DHL, IHJ, BJ. Formal analysis: BJ. Investigation: DHL. Supervision: BJ. Visualization: DHL. Writing—original draft: DHL. Writing—review & editing: BJ. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
| Total |
Axin2 |
p-valuea | |||
|---|---|---|---|---|---|
| Low | High | ||||
| No. of patients | 56 (100) | 39 (70.0) | 17 (30.0) | ||
| Age (yr) | .062 | ||||
| < 65 | 20 (35.7) | 17 (85.0) | 3 (15.0) | ||
| ≥ 65 | 36 (64.3) | 22 (61.1) | 14 (38.9) | ||
| Sex | .449 | ||||
| Female | 19 (33.9) | 27 (73.0) | 10 (27.0) | ||
| Male | 37 (66.1) | 12 (63.2) | 7 (36.8) | ||
| Lauren classification | .286 | ||||
| Intestinal | 46 (82.1) | 30 (65.2) | 16 (34.8) | ||
| Diffuse | 8 (14.3) | 7 (87.5) | 1 (12.5) | ||
| Mixed | 2 (3.6) | 2 (100) | 0 | ||
| Histological differentiation | .792 | ||||
| Well | 11 (19.6) | 7 (63.6) | 4 (36.4) | ||
| Moderate | 34 (60.7) | 23 (67.6) | 11 (32.4) | ||
| Poor | 6 (10.7) | 5 (83.3) | 1 (16.7) | ||
| PCC | 5 (8.9) | 4 (80.0) | 1 (20.0) | ||
| Invasion depth | .867 | ||||
| Mucosa | 14 (25.0) | 10 (71.4) | 4 (28.6) | ||
| Submucosa | 42 (75.0) | 29 (69.0) | 13 (31.0) | ||
| LN metastasis | .108 | ||||
| Negative | 35 (85.4) | 24 (68.6) | 11 (31.4) | ||
| Positive | 6 (14.6) | 6 (100) | 0 | ||
| Nuclear β-catenin | < .001 | ||||
| Negative | 44 (78.6) | 37 (84.1) | 7 (15.9) | ||
| Positive | 12 (21.4) | 2 (16.6) | 10 (83.4) | ||
- 1. Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell 2012; 149: 1192-205. ArticlePubMed
- 2. Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 2014; 15: 19-33. ArticlePubMedPDF
- 3. Haseeb M, Pirzada RH, Ain QU, Choi S. Wnt signaling in the regulation of immune cell and cancer therapeutics. Cells 2019; 8: 1380.ArticlePubMedPMC
- 4. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009; 17: 9-26. PubMedPMC
- 5. Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev 1999; 13: 1768-73. ArticlePubMedPMC
- 6. Qian L, Mahaffey JP, Alcorn HL, Anderson KV. Tissue-specific roles of Axin2 in the inhibition and activation of Wnt signaling in the mouse embryo. Proc Natl Acad Sci U S A 2011; 108: 8692-7. ArticlePubMedPMC
- 7. Liu C, He X. Destruction of a destructor: a new avenue for cancer therapeutics targeting the Wnt pathway. J Mol Cell Biol 2010; 2: 70-3. ArticlePubMedPMC
- 8. Taniguchi K, Roberts LR, Aderca IN, et al. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene 2002; 21: 4863-71. ArticlePubMedPDF
- 9. Shimizu Y, Ikeda S, Fujimori M, et al. Frequent alterations in the Wnt signaling pathway in colorectal cancer with microsatellite instability. Genes Chromosomes Cancer 2002; 33: 73-81. ArticlePubMed
- 10. Ishiguro H, Tsunoda T, Tanaka T, Fujii Y, Nakamura Y, Furukawa Y. Identification of AXUD1, a novel human gene induced by AXIN1 and its reduced expression in human carcinomas of the lung, liver, colon and kidney. Oncogene 2001; 20: 5062-6. ArticlePubMedPDF
- 11. Mai M, Qian C, Yokomizo A, Smith DI, Liu W. Cloning of the human homolog of conductin (AXIN2), a gene mapping to chromosome 17q23-q24. Genomics 1999; 55: 341-4. ArticlePubMed
- 12. Pan KF, Liu WG, Zhang L, You WC, Lu YY. Mutations in components of the Wnt signaling pathway in gastric cancer. World J Gastroenterol 2008; 14: 1570-4. ArticlePubMedPMC
- 13. Neau L, Lorin C, Frentzel S, Hoeng J, Iskandar A, Leroy P, et al. Optimization of a novel in situ hybridization technology on 3D organotypic cell cultures. Appl In Vitro Toxicol 2019; 5: 75-85. Article
- 14. Jencks DS, Adam JD, Borum ML, Koh JM, Stephen S, Doman DB. Overview of current concepts in gastric intestinal metaplasia and gastric cancer. Gastroenterol Hepatol (N Y) 2018; 14: 92-101. PubMedPMC
- 15. Ying Y, Tao Q. Epigenetic disruption of the WNT/ss-catenin signaling pathway in human cancers. Epigenetics 2009; 4: 307-12. Article
- 16. Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 2002; 22: 1172-83. PubMedPMC
- 17. Bernkopf DB, Hadjihannas MV, Behrens J. Negative-feedback regulation of the Wnt pathway by conductin/axin2 involves insensitivity to upstream signalling. J Cell Sci 2015; 128: 33-9. ArticlePubMedPDF
- 18. Chiurillo MA. Role of the Wnt/beta-catenin pathway in gastric cancer: an in-depth literature review. World J Exp Med 2015; 5: 84-102. PubMedPMC
- 19. Li LF, Wei ZJ, Sun H, Jiang B. Abnormal beta-catenin immunohistochemical expression as a prognostic factor in gastric cancer: a meta-analysis. World J Gastroenterol 2014; 20: 12313-21. PubMedPMC
- 20. Tahara E. Genetic pathways of two types of gastric cancer. IARC Sci Publ 2004; (157):327-49. PubMed
- 21. Murata-Kamiya N, Kurashima Y, Teishikata Y, et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the betacatenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene 2007; 26: 4617-26. ArticlePubMedPDF
- 22. Sokolova O, Bozko PM, Naumann M. Helicobacter pylori suppresses glycogen synthase kinase 3beta to promote beta-catenin activity. J Biol Chem 2008; 283: 29367-74. PubMedPMC
REFERENCES
Figure & Data
References
Citations

- A review of potential mechanisms and treatments of gastric intestinal metaplasia
Yueyao Wu, Kehan Zhang, Yichao Zheng, Haifeng Jin
European Journal of Gastroenterology & Hepatology.2025; 37(4): 383. CrossRef - Refining NTRK Fusion Detection in Papillary Thyroid Carcinoma Through Pan-TRK Immunohistochemistry and Histopathologic Features
Hyun Lee, Sue Youn Kim, Ji Min Park, Seung-Hyun Jung, Ozgur Mete, Chan Kwon Jung
Endocrine Pathology.2025;[Epub] CrossRef - AXIN2 variants, tooth agenesis, and cancer risk: a systematic review
Nutthakarn Ratanasereeprasert, Narin Intarak, Chayanit Chaweewannakorn, Mushriq Abid, Anand Marya, Sung-dae Cho, Thantrira Porntaveetus
BMC Oral Health.2025;[Epub] CrossRef - Discovery of Atirmociclib (PF-07220060): A Potent and Selective CDK4 Inhibitor
Gary M. Gallego, Cynthia Palmer, Suvi Orr, Louise Bernier, Ping Chen, Sujin Cho-Schultz, Judith G. Deal, Klaus Dress, Martin Edwards, Mehran Jalaie, Eric Johnson, Robert Kania, John C. Kath, Jennifer Lafontaine, Sacha Ninkovic, Neal Sach, Hong Shen, Lars
Journal of Medicinal Chemistry.2025; 68(24): 26085. CrossRef - Listening to the Past, Shaping the Future: A Data-mining Based and Visual Analysis of Five Decades of Gastric Carcinogenesis Research
Tai Zhang, Xudong Tang
Biological Procedures Online.2025;[Epub] CrossRef - Postbiotics Combination Synergises the Antiproliferative Effects of Doxorubicin in Gastric Cancer Cells: A Cellular and Molecular Deep Dive
Radwa A. Eladwy, Mohamed Fares, Muhammad A. Alsherbiny, Dennis Chang, Chun-Guang Li, Deep Jyoti Bhuyan
International Journal of Molecular Sciences.2025; 27(1): 362. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure





Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
| Total | Axin2 |
p-value |
|||
|---|---|---|---|---|---|
| Low | High | ||||
| No. of patients | 56 (100) | 39 (70.0) | 17 (30.0) | ||
| Age (yr) | .062 | ||||
| < 65 | 20 (35.7) | 17 (85.0) | 3 (15.0) | ||
| ≥ 65 | 36 (64.3) | 22 (61.1) | 14 (38.9) | ||
| Sex | .449 | ||||
| Female | 19 (33.9) | 27 (73.0) | 10 (27.0) | ||
| Male | 37 (66.1) | 12 (63.2) | 7 (36.8) | ||
| Lauren classification | .286 | ||||
| Intestinal | 46 (82.1) | 30 (65.2) | 16 (34.8) | ||
| Diffuse | 8 (14.3) | 7 (87.5) | 1 (12.5) | ||
| Mixed | 2 (3.6) | 2 (100) | 0 | ||
| Histological differentiation | .792 | ||||
| Well | 11 (19.6) | 7 (63.6) | 4 (36.4) | ||
| Moderate | 34 (60.7) | 23 (67.6) | 11 (32.4) | ||
| Poor | 6 (10.7) | 5 (83.3) | 1 (16.7) | ||
| PCC | 5 (8.9) | 4 (80.0) | 1 (20.0) | ||
| Invasion depth | .867 | ||||
| Mucosa | 14 (25.0) | 10 (71.4) | 4 (28.6) | ||
| Submucosa | 42 (75.0) | 29 (69.0) | 13 (31.0) | ||
| LN metastasis | .108 | ||||
| Negative | 35 (85.4) | 24 (68.6) | 11 (31.4) | ||
| Positive | 6 (14.6) | 6 (100) | 0 | ||
| Nuclear β-catenin | < .001 | ||||
| Negative | 44 (78.6) | 37 (84.1) | 7 (15.9) | ||
| Positive | 12 (21.4) | 2 (16.6) | 10 (83.4) | ||
Values are presented as number (%). PCC, poorly cohesive carcinoma; LN, lymph node. Pearson chi-square.

 E-submission
E-submission