Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 58(3); 2024 > Article
-
Review
Interpretation of PD-L1 expression in gastric cancer: summary of a consensus meeting of Korean gastrointestinal pathologists -
Soomin Ahn1
 , Yoonjin Kwak2
, Yoonjin Kwak2 , Gui Young Kwon3
, Gui Young Kwon3 , Kyoung-Mee Kim1
, Kyoung-Mee Kim1 , Moonsik Kim4
, Moonsik Kim4 , Hyunki Kim5
, Hyunki Kim5 , Young Soo Park6
, Young Soo Park6 , Hyeon Jeong Oh7
, Hyeon Jeong Oh7 , Kyoungyul Lee8
, Kyoungyul Lee8 , Sung Hak Lee9
, Sung Hak Lee9 , Hye Seung Lee2
, Hye Seung Lee2
-
Journal of Pathology and Translational Medicine 2024;58(3):103-116.
DOI: https://doi.org/10.4132/jptm.2024.03.15
Published online: April 25, 2024
1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
2Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
3Seoul Clinical Laboratories, Department of Pathology, Yongin, Korea
4Department of Pathology, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, Daegu, Korea
5Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
6Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
7Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea
8Pathology Center, Seegene Medical Foundation, Seoul, Korea
9Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- Corresponding Author: Hye Seung Lee, MD, PhD, Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-740-8269, Fax: +82-2-744-8273, E-mail: hye2@snu.ac.kr
© 2024 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Abstract
- CURRENT PRACTICE OF PD-L1 TESTING IN GASTRIC CANCER
- INTEROBSERVER CONCORDANCE OF PD-L1 COMBINED POSITIVE SCORE
- CONSENSUS MEETING OF KOREAN GASTROINTESTINAL PATHOLOGISTS
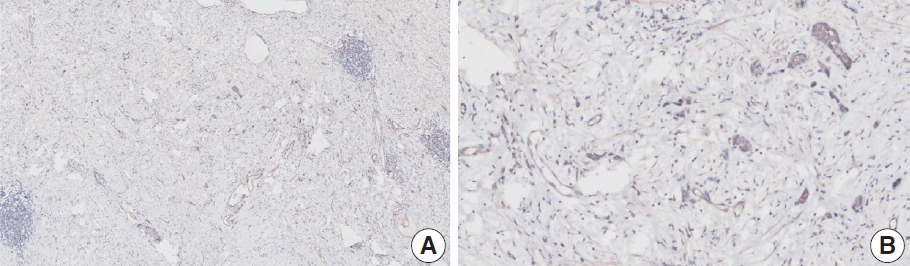
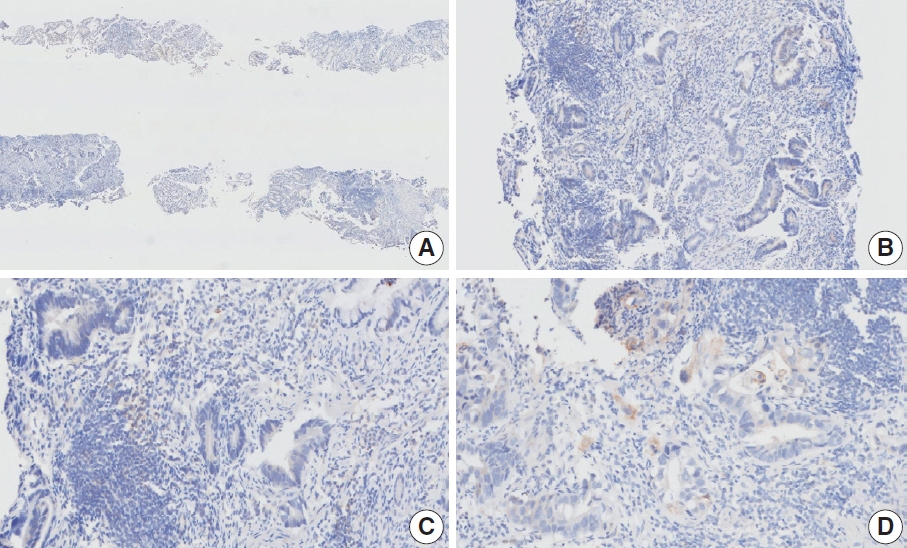
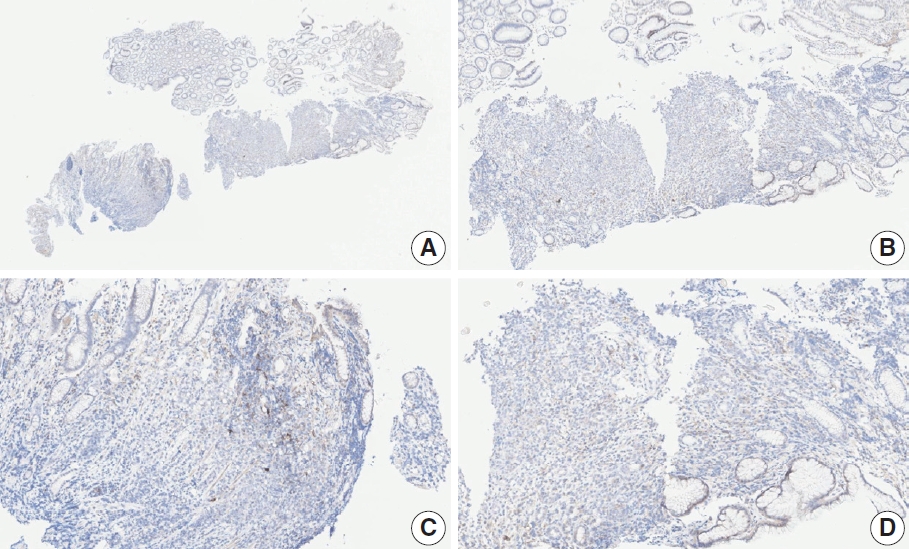
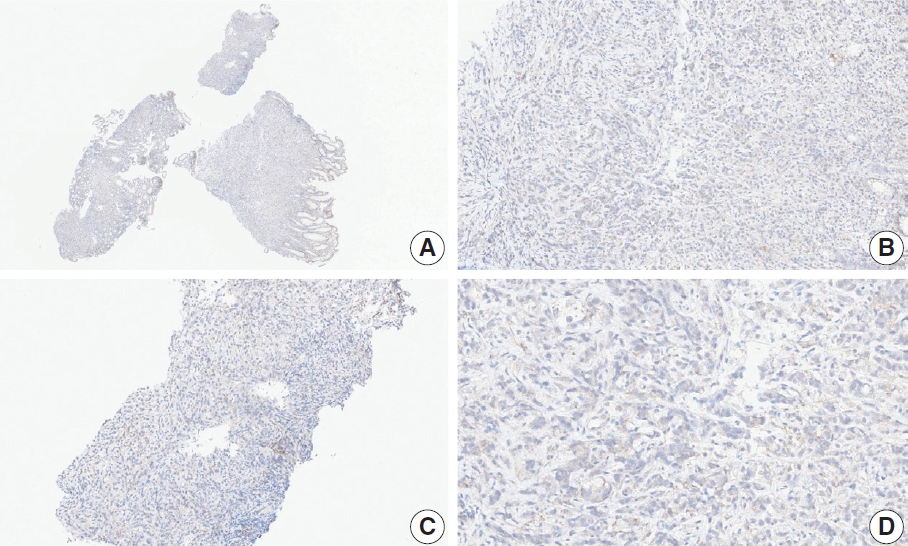
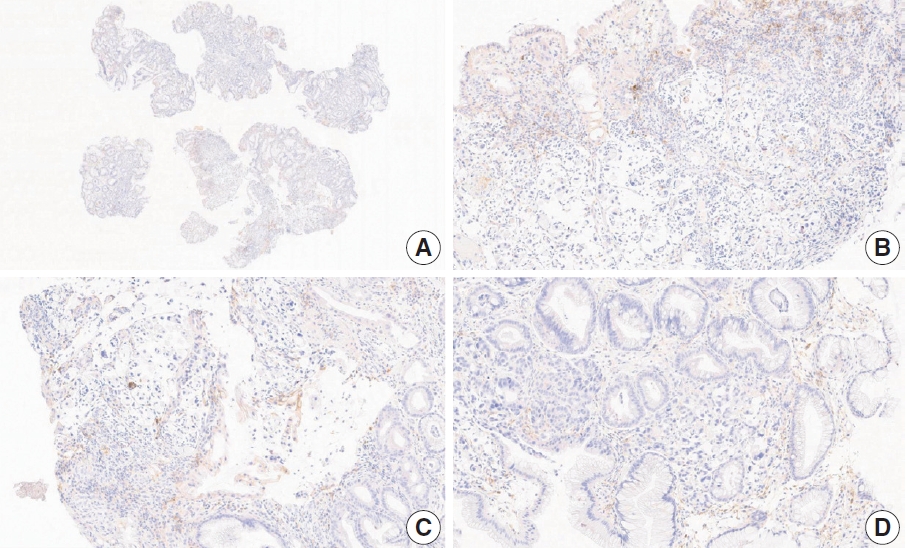
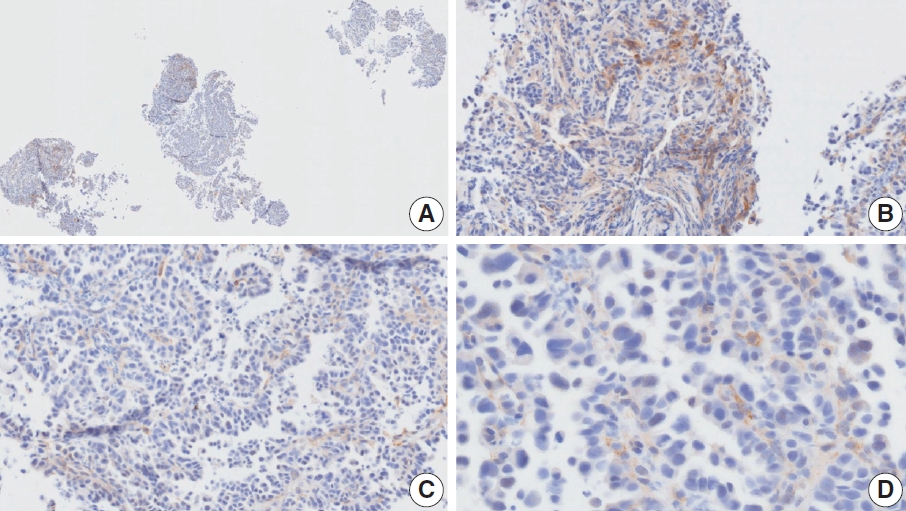
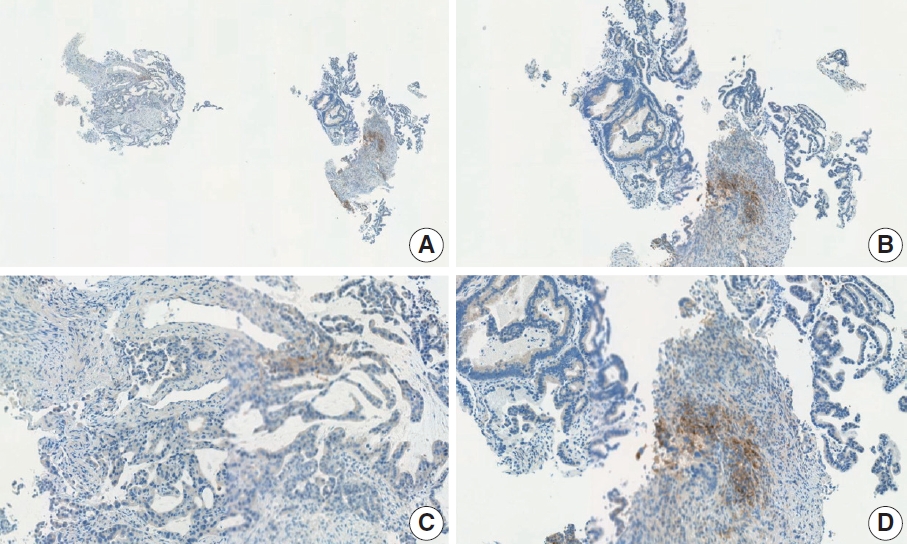
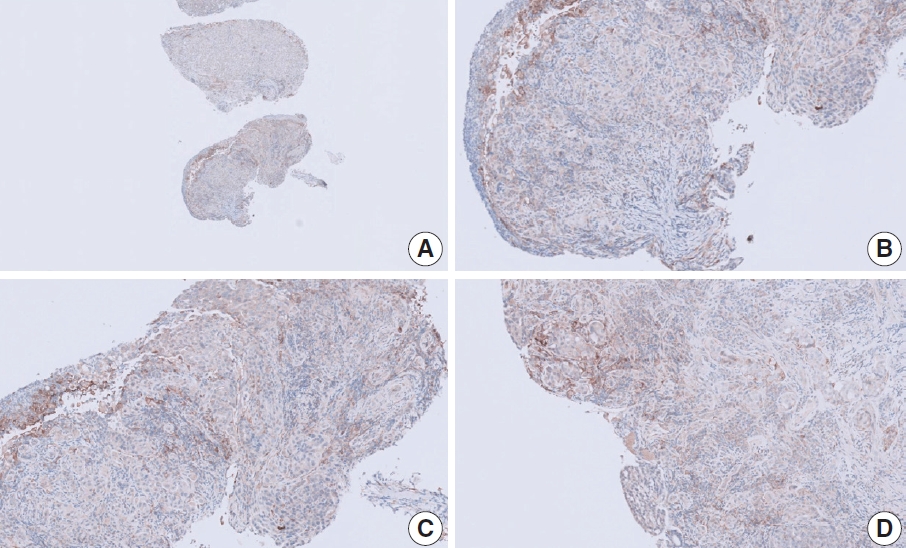
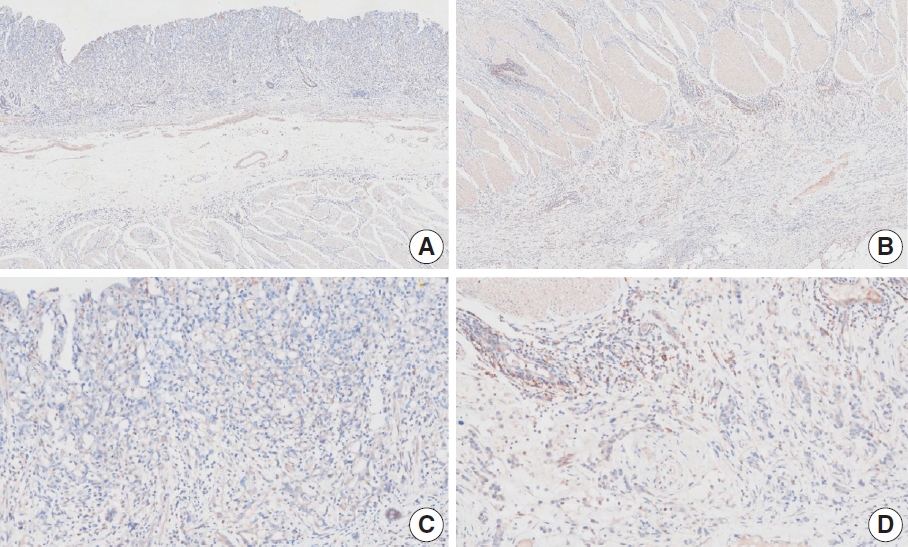
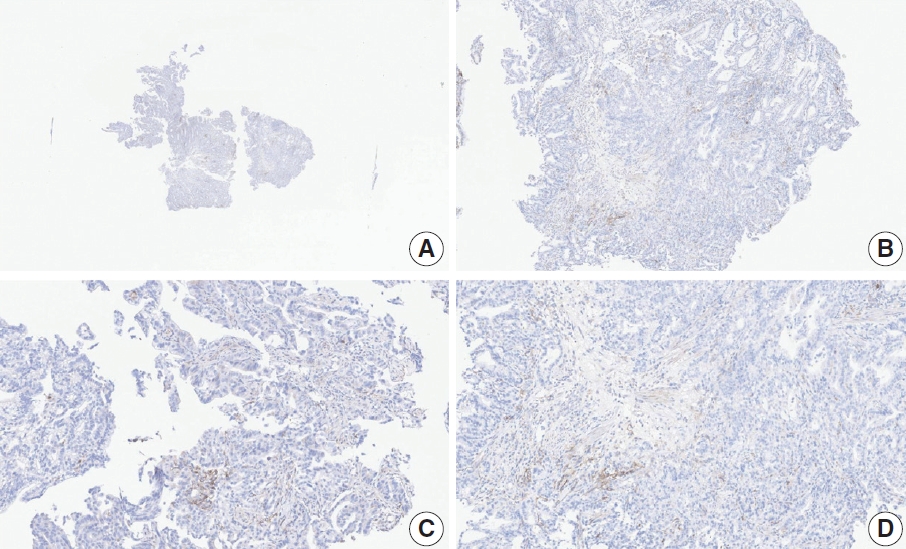
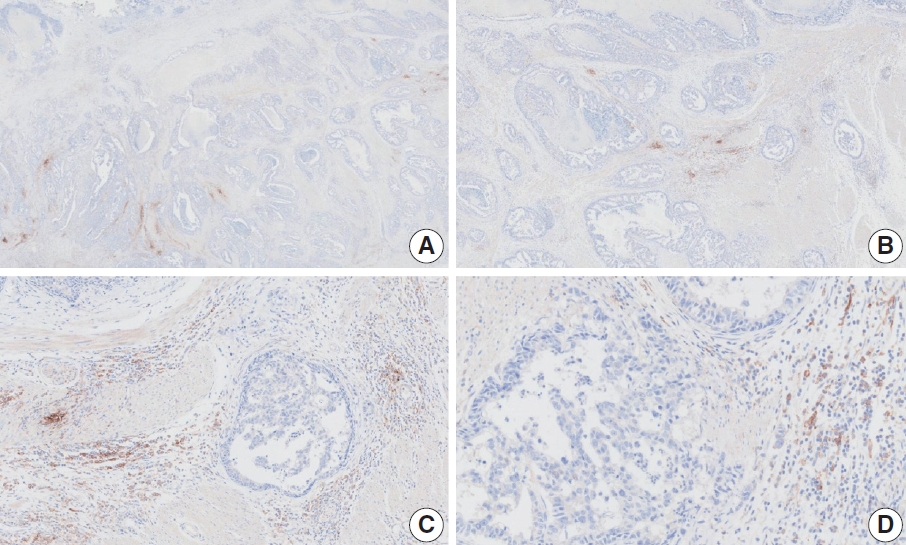
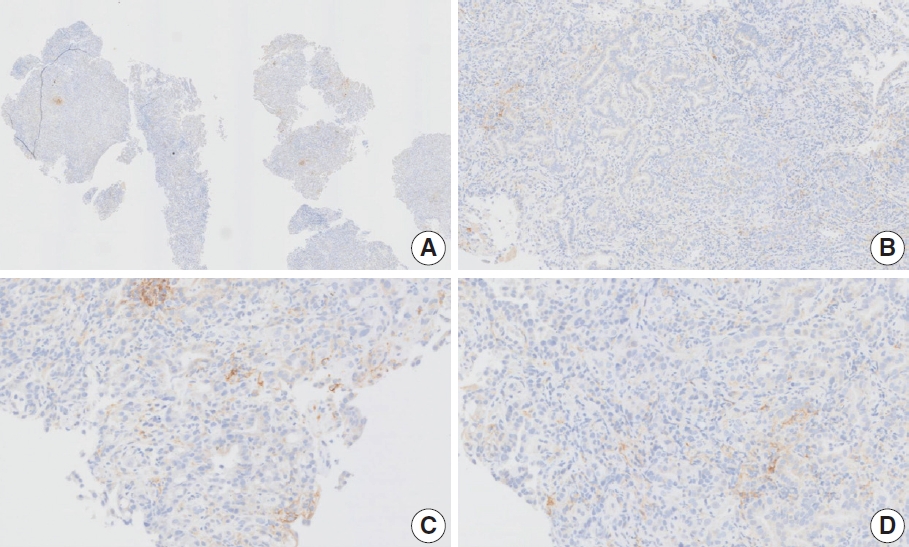
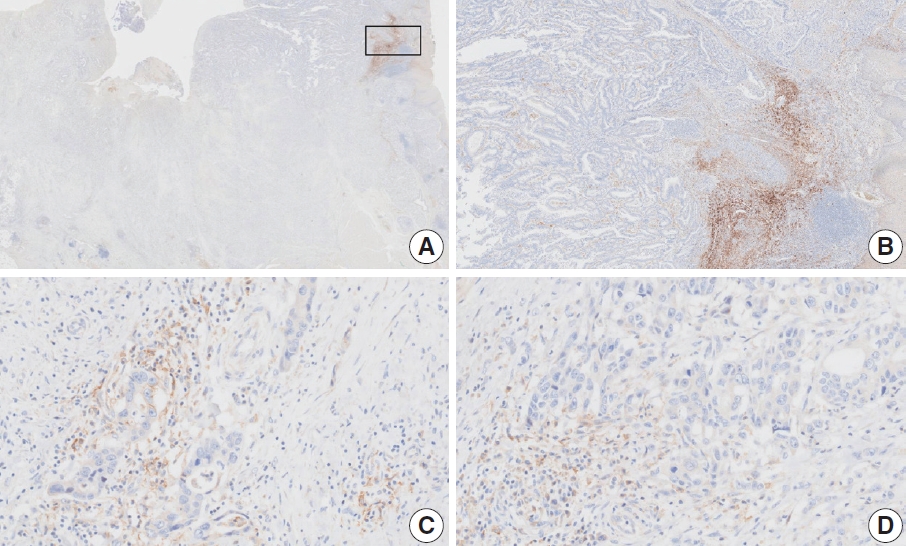
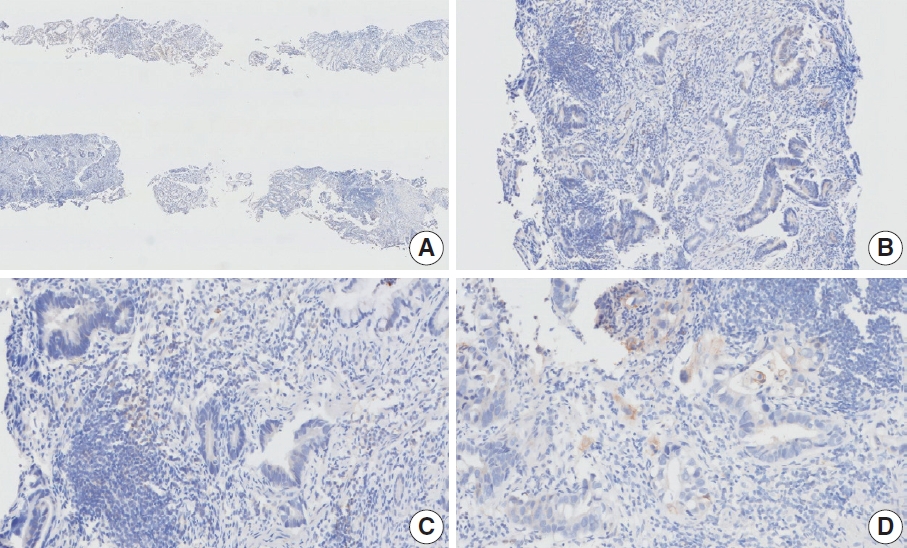
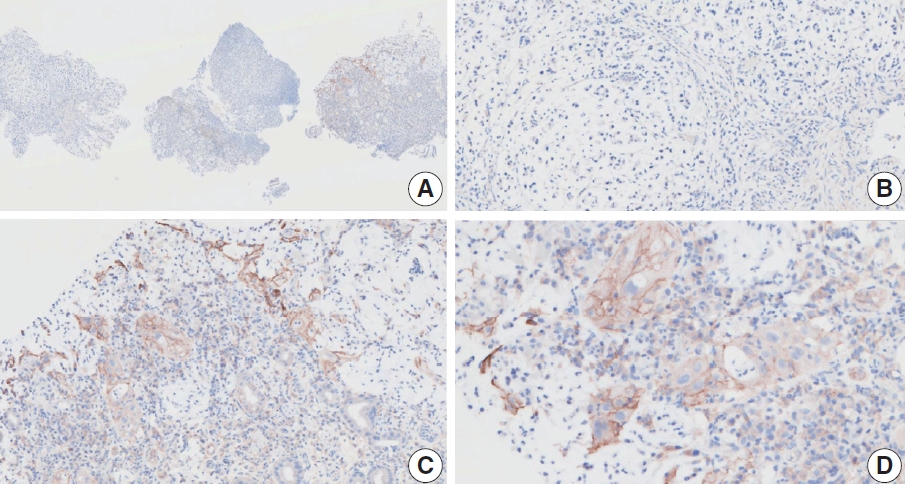
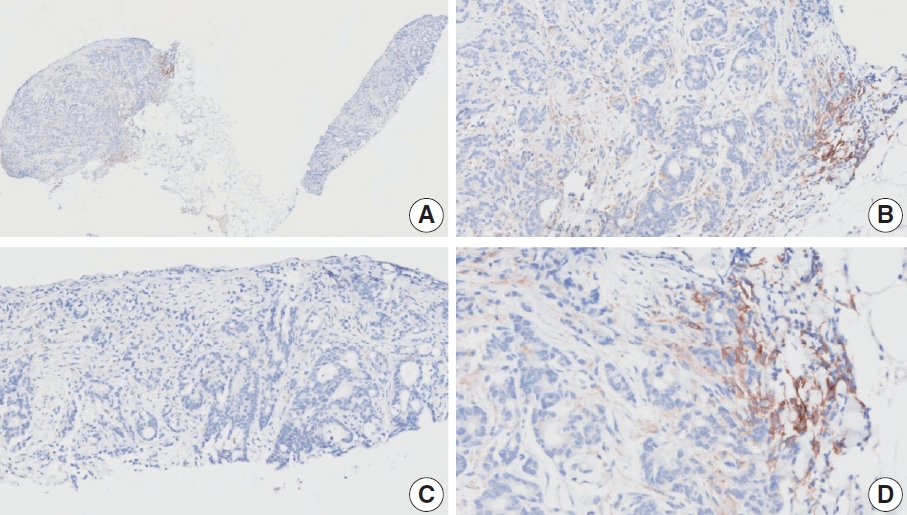
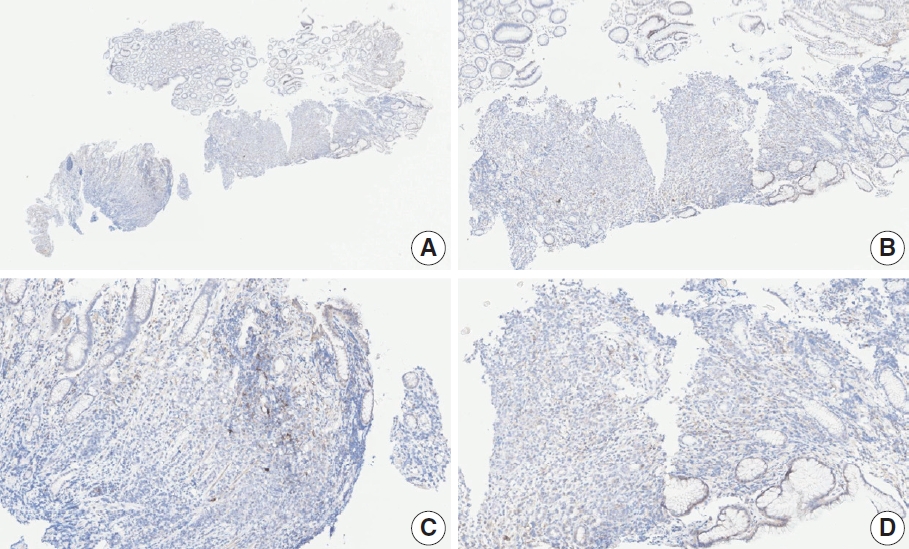
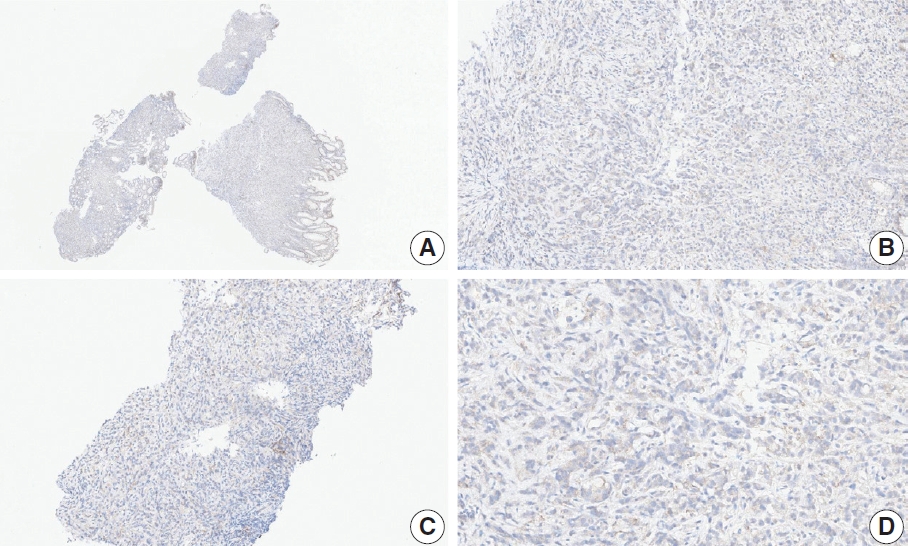
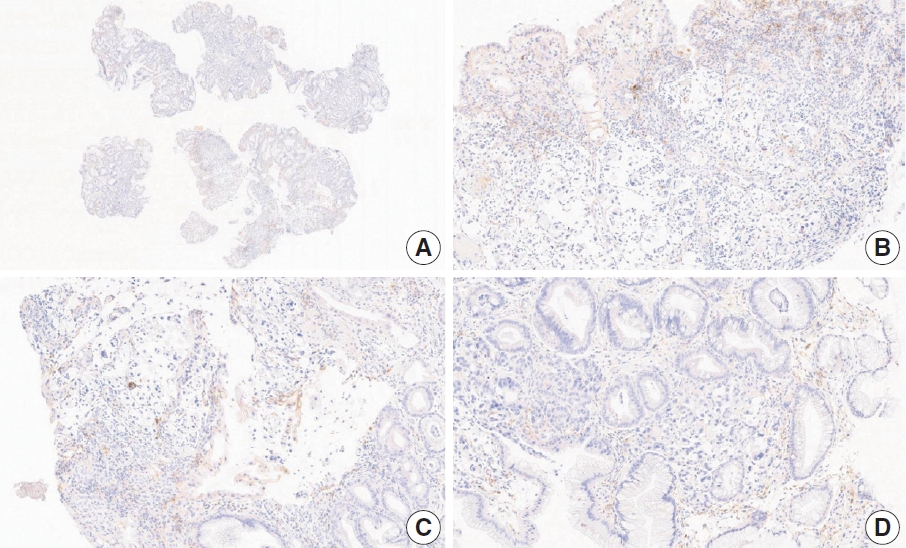
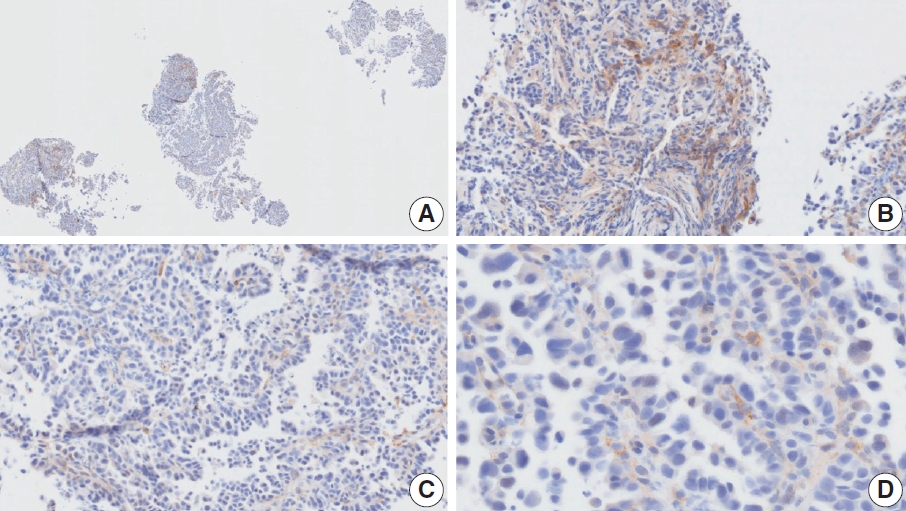
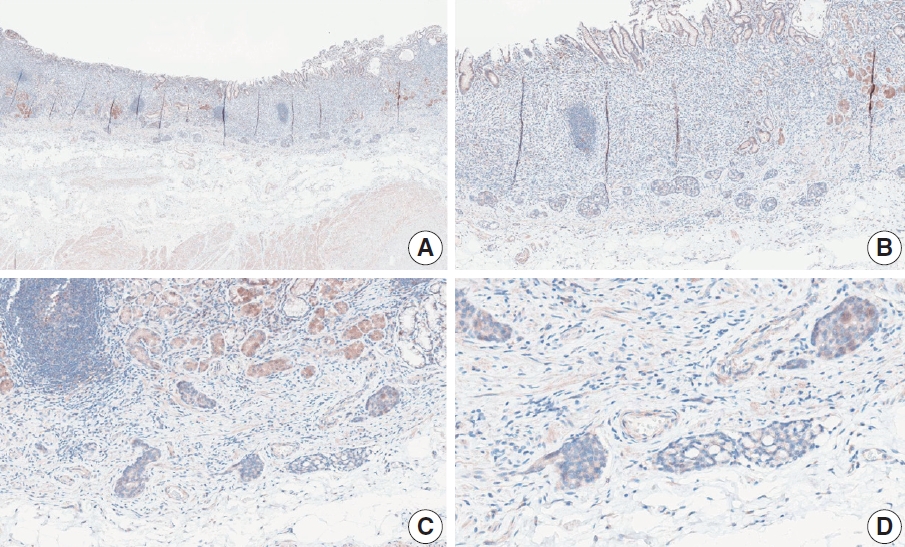
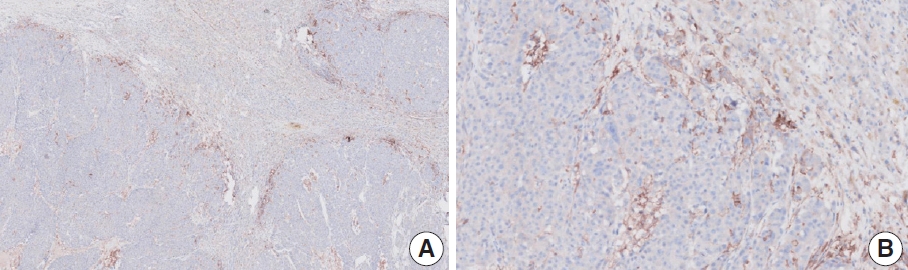
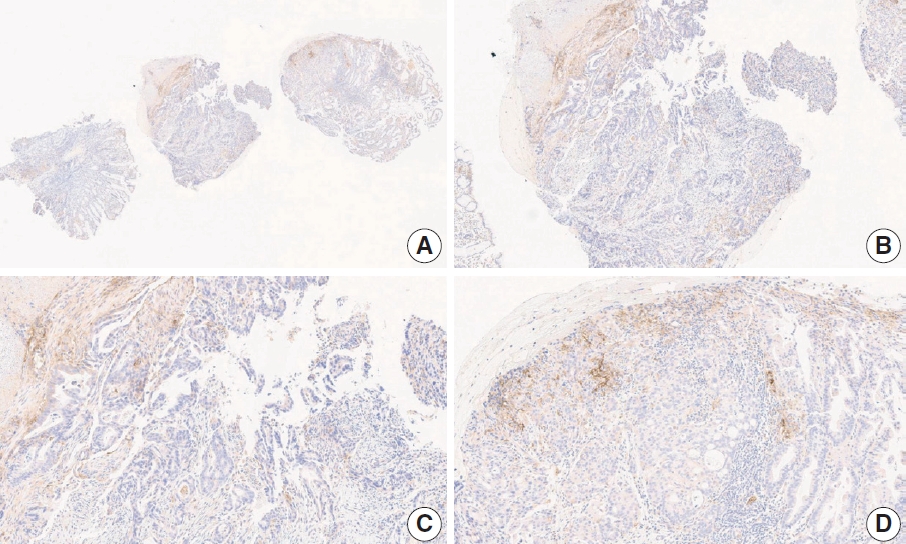
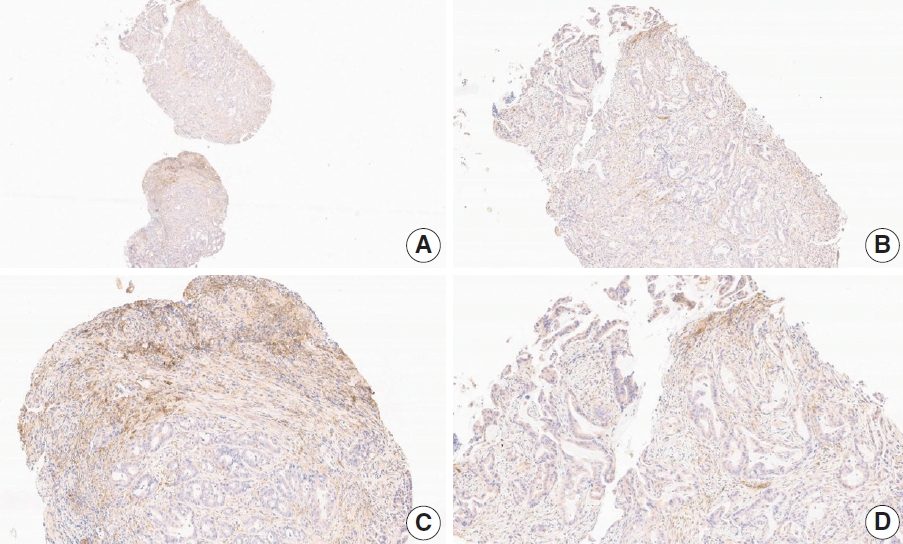
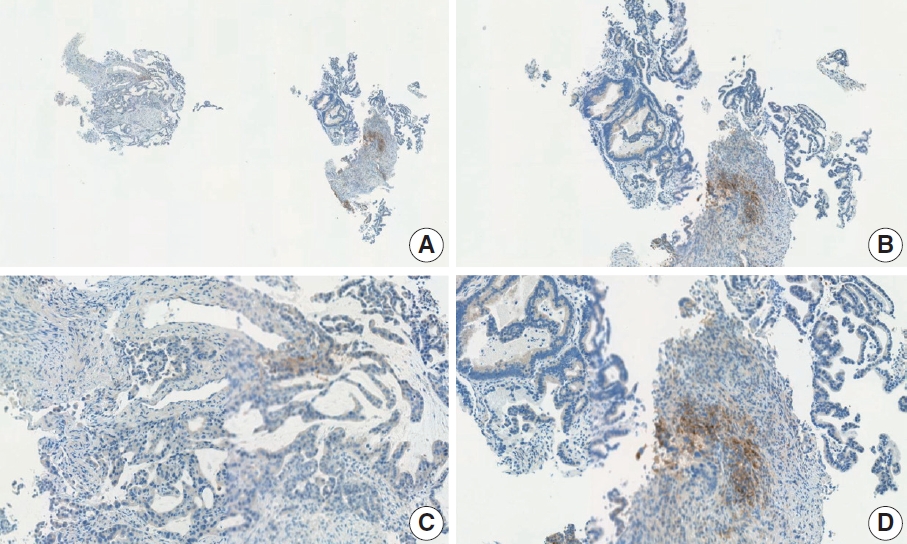
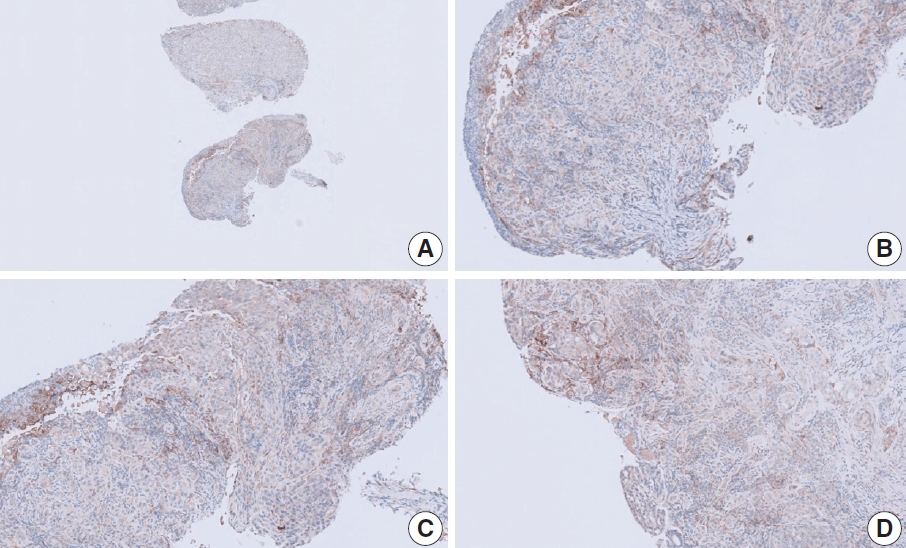
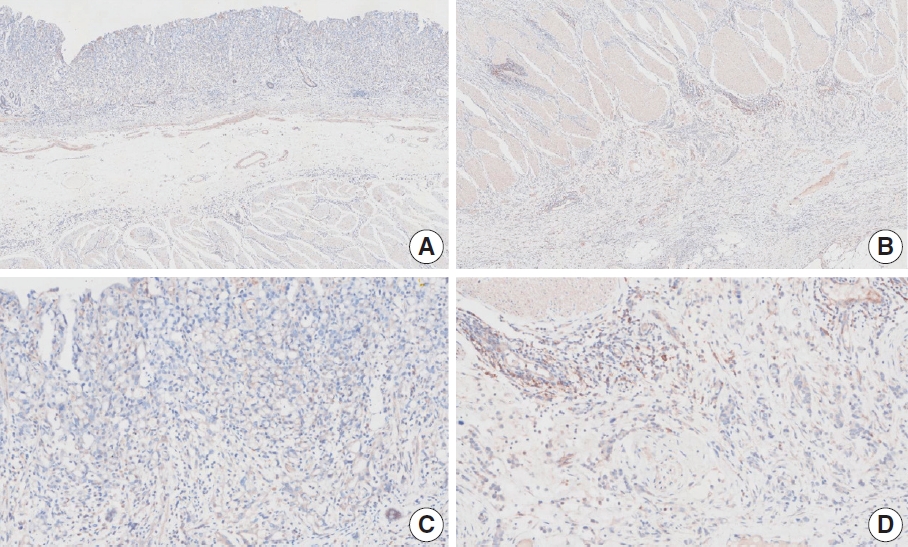
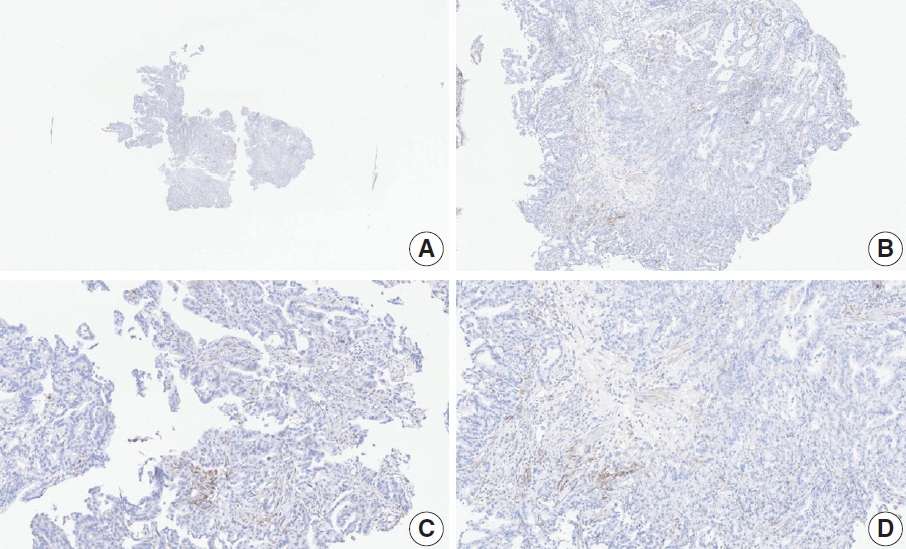
- CASES WITH CONSENSUS COMBINED POSITIVE SCORE <5
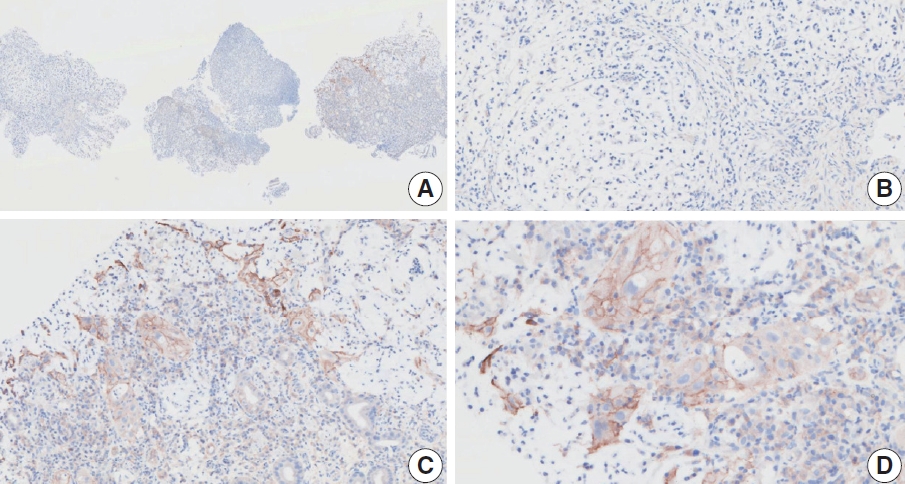
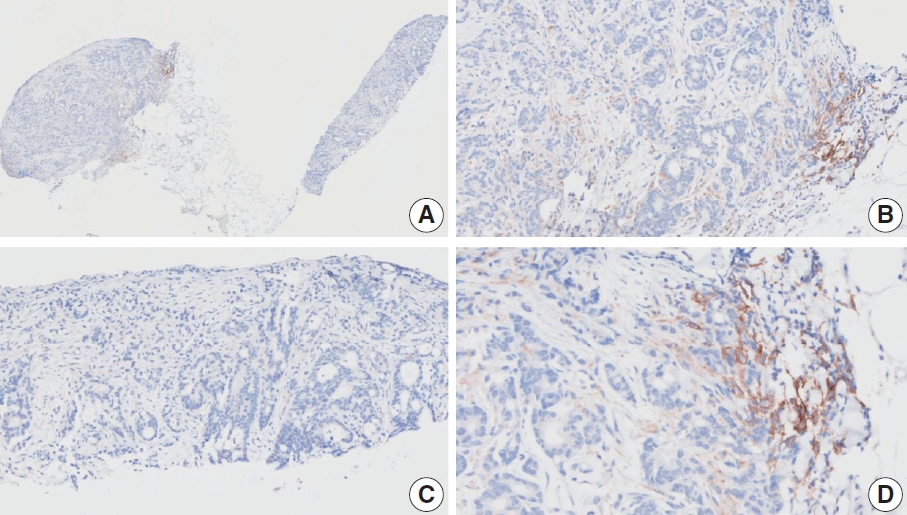
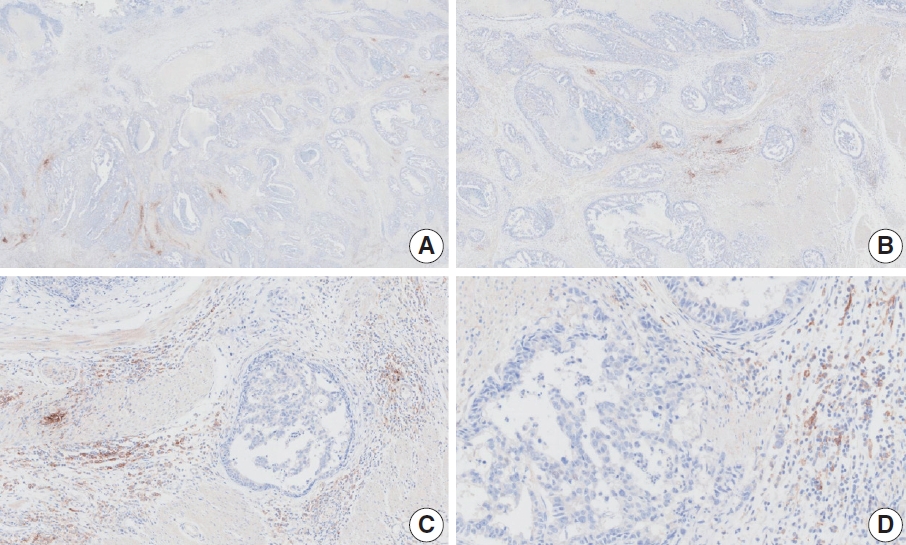
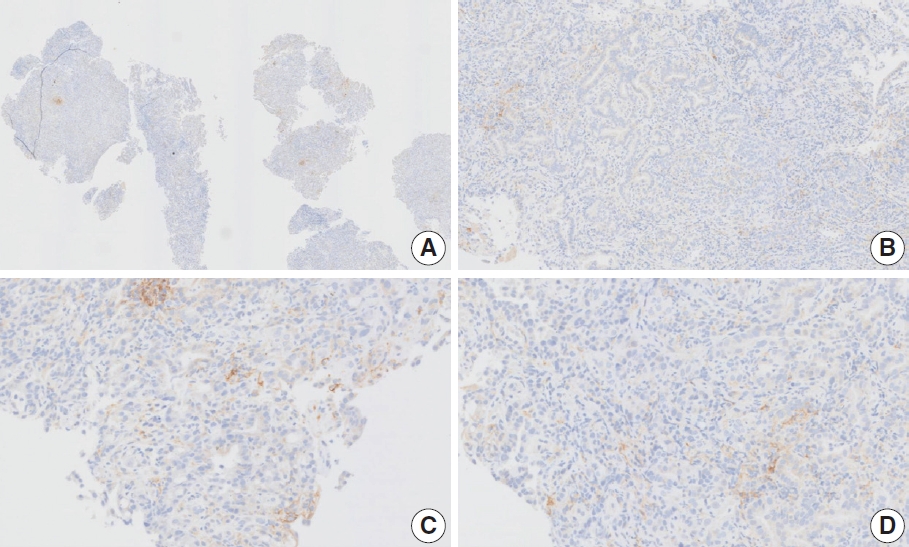
- CASES WITH CONSENSUS COMBINED POSITIVE SCORE ≥5
- CONCLUSION
- Supplementary Information
- NOTES
- REFERENCES
Figure & Data
References
Citations

- Organ Preservation for Gastroesophageal Junction and Gastric Cancers: Ready for Primetime?
Winta Mehtsun, Lola Van Doosselaere, Ugwuji N. Maduekwe
American Society of Clinical Oncology Educational Book.2026;[Epub] CrossRef - Deep Learning Analysis Based on Dual-energy CT-Derived Iodine Map for Predicting PD-L1 Expression in Gastric Cancer: A Multicenter Study
Lihong Chen, Yuncong Zhao, Xiaomin Tian, Deye Zeng, Yongxiu Tong, Haiping Xu, Yaru You, Caiming Weng, Sen Lin, Keru Chen, Yilin Chen, Yunjing Xue
Academic Radiology.2026;[Epub] CrossRef - Adjuvant immunotherapy in patients with resected gastric and oesophagogastric junction cancer following preoperative chemotherapy with high risk for recurrence (ypN+ and/or R1): European Organisation of Research and Treatment of Cancer (EORTC) 1707 VESTIG
F. Lordick, M.E. Mauer, G. Stocker, C.A. Cella, I. Ben-Aharon, G. Piessen, L. Wyrwicz, G. Al-Haidari, T. Fleitas-Kanonnikoff, V. Boige, R. Lordick Obermannová, U.M. Martens, C. Gomez-Martin, P. Thuss-Patience, V. Arrazubi, A. Avallone, K.K. Shiu, P. Artru
Annals of Oncology.2025; 36(2): 197. CrossRef - PD-L1 as a Biomarker in Gastric Cancer Immunotherapy
Yunjoo Cho, Soomin Ahn, Kyoung-Mee Kim
Journal of Gastric Cancer.2025; 25(1): 177. CrossRef - PD-L1 importance in malignancies comprehensive insights into the role of PD-L1 in malignancies: from molecular mechanisms to therapeutic opportunities
Mojdeh Soltani, Mohammad Abbaszadeh, Hamed Fouladseresht, Mark J. M. Sullman, Nahid Eskandari
Clinical and Experimental Medicine.2025;[Epub] CrossRef - CLDN18.2 expression in gastroesophageal adenocarcinoma: prevalence, heterogeneity, and prognostic implications in Spanish patients
Carolina Martinez-Ciarpaglini, María Ortega, Sandra Pérez-Buira, Aitana Bolea, Beatriz Casado Guerra, Carmen Herencia Bellido, Paula Tornero Piñero, Dolores Naranjo-Hans, Brenda Palomar, Hernán Quiceno, Amanda Sardón Fernández, Ariadna Torner Calvo, Feder
Virchows Archiv.2025; 487(6): 1337. CrossRef - Distinct clinicopathological and survival profiles of CLDN18.2 and PD-L1 expression in advanced gastric cancer and gastroesophageal junction adenocarcinoma
D.R. Castillo, M. Guo, P. Shah, M. Hazeltin, D. Tai, F. Al-Manaseer, S. Mlamba, D. Perez, S. Yeremian, S. Guzman, R. Mannan, C. Crook, C. Lau, N. Tawar, G. Brar, M. Raoof, Y. Woo, S.P. Wu, D. Li
ESMO Gastrointestinal Oncology.2025; 10: 100261. CrossRef - Best Practice PD-L1 Staining and Interpretation in Gastric Cancer Using PD-L1 IHC PharmDx 22C3 and PD-L1 IHC PharmDx 28-8 Assays, with Reference to Common Issues and Solutions
Soomin Ahn, Inwoo Hwang, Yuyeon Kim, Somin Lee, Yunjoo Cho, So Young Kang, Deok Geun Kim, Jeeyun Lee, Kyoung-Mee Kim
Biomedicines.2025; 13(11): 2824. CrossRef - Intraperitoneal immune microenvironment and efficacy of intraperitoneal chemotherapy in patients with gastric cancer and peritoneal metastasis
Tomoya Nakanishi, Motohiro Imano, Masashi Kohda, Hiroaki Kato, Naoko Kounami, Atsushi Yamada, Masuhiro Terada, Yoko Hiraki, Osamu Shiraishi, Atsushi Yasuda, Masayuki Shinkai, Takushi Yasuda
Scientific Reports.2025;[Epub] CrossRef - PD-L1 thresholds predict efficacy of immune checkpoint inhibition in first-line treatment of advanced gastroesophageal adenocarcinoma. A systematic review and meta-analysis of seven phase III randomized trials
V. Formica, C. Morelli, L. Fornaro, S. Riondino, M. Rofei, E. Fontana, E.C. Smyth, M. Roselli, H.-T. Arkenau
ESMO Open.2024; 9(11): 103967. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
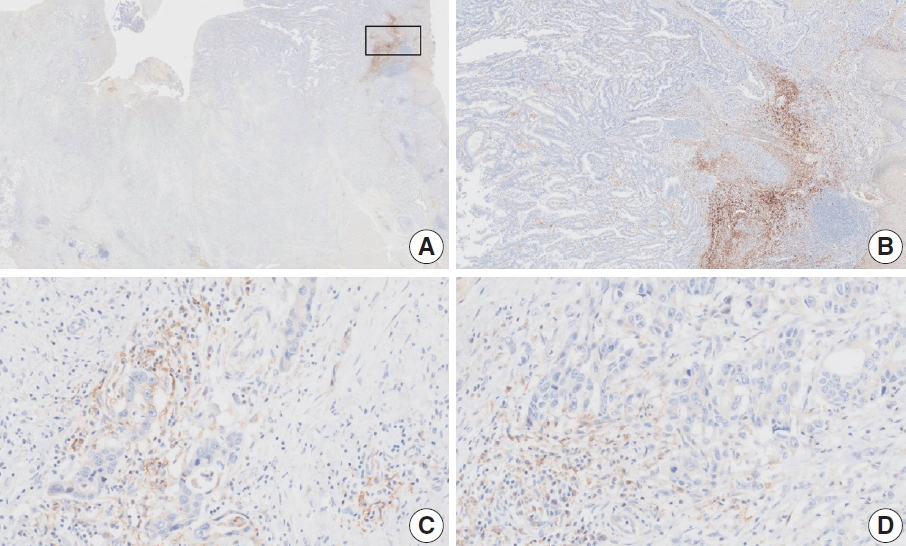
- Figure



















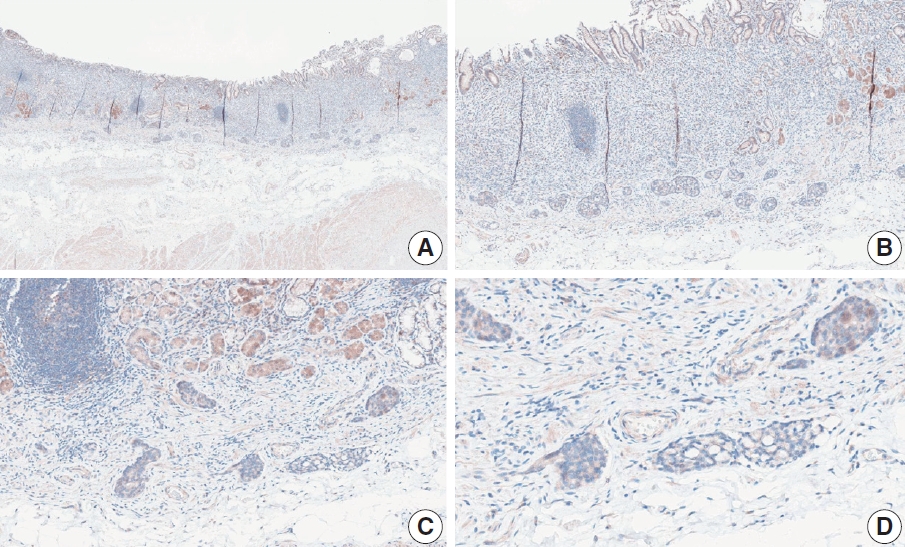
Fig. 1.
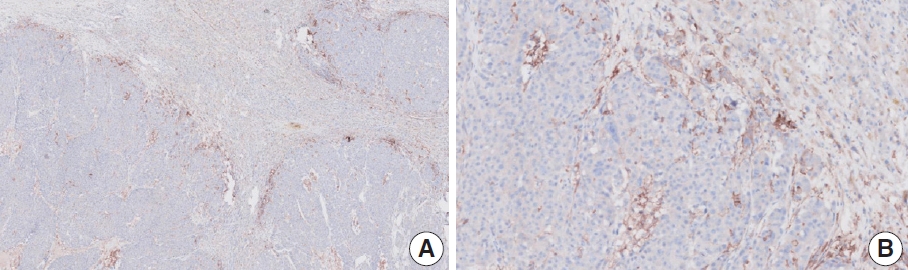
Fig. 2.
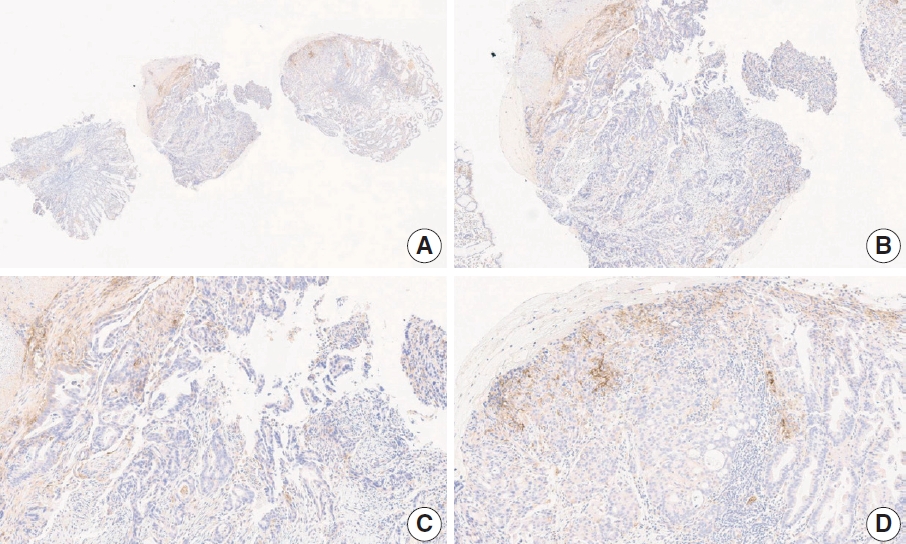
Fig. 3.
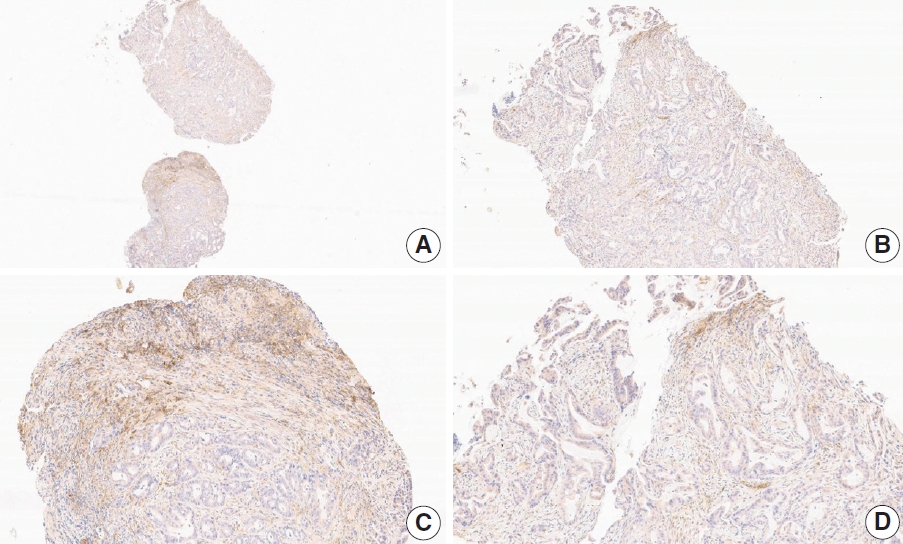
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
Fig. 8.
Fig. 9.
Fig. 10.
Fig. 11.
Fig. 12.
Fig. 13.
Fig. 14.
Fig. 15.
Fig. 16.
Fig. 17.
Fig. 18.
Fig. 19.
| CheckMate-649 | KEYNOTE-811 | KEYNOTE 859 | RATIONALE-305 | |
|---|---|---|---|---|
| Candidates | HER2-negative GC | HER2-positive GC | HER2-negative GC | HER2-negative GC |
| Drug | Nivolumab | Pembrolizumab | Pembrolizumab | Tislelizumab |
| PD-L1 assay | 28-8 pharmDx | 22C3 pharmDx | 22C3 pharmDx | SP263 |
| Antibody supplier | Dako (Agilent) | Dako (Agilent) | Dako (Agilent) | Ventana (Roche) |
| Antibody species | Rabbit mAb | Mouse mAb | Mouse mAb | Rabbit mAb |
| Scoring | CPS | CPS | CPS | TAP |
| Cutoff | 5 | 1 | TBD | 5% |
| US Food and Drug Administration | Approved (on Apr 16, 2021) | Approved (on Aug 29, 2023) | Approved (on Nov 16, 2023) | Not yet |
| Korean Ministry of Food and Drug | Approved (on Sep 1, 2023 | Approved (on Dec 19, 2023) | Not yet | Not yet |
| PD-L1 assay | Cutoff | No. of observers | No. of cases | Sample type | Interobserver agreement | Fleiss kappa value | Reference |
|---|---|---|---|---|---|---|---|
| 22C3 PharmDx | CPS ≥ 1 | 3 | 68 | Not mentioned | OPA 96.6% | - | [13] |
| 22C3 PharmDx | CPS ≥ 1 | 120 | 20 (day1), 25 (day2) | Resection | OPA 90.6% | 0.828 | [21] |
| 22C3 PharmDx | CPS value | 5 | 55 | Tissue microarray | ICC 0.387 (lower 95% CI, 20.9%) | CPS ≥ 1 0.389 | [22] |
| SP263 | CPS value | 5 | 55 | Tissue microarray | ICC 0.349 (lower 95% CI, 13.5%) | CPS ≥ 1 0.256 | |
| 22C3 PharmDx | CPS ≥ 1 | 14 | 112 | Biopsy | OPA 31.48% (95% CI, 22.72–40.24) | 0.477 | [18] |
| ICC 0.484 (95% CI, 0.403–0.571) | - | ||||||
| CPS ≥ 10 | 14 | 112 | Biopsy | OPA 67.59% (95% CI, 58.77–76.42) | 0.607 | ||
| ICC 0.604 (95% CI, 0.584–0.624) | - | ||||||
| CPS ≥ 20 | 14 | 112 | Biopsy | OPA 83.33% (95% CI, 76.3–90.36) | 0.626 | ||
| ICC 0.629 (95% CI, 0.562–0.698) | - | ||||||
| 28-8 PharmDx | CPS value | 12 | 100 | Biopsy | ICC 0.45 (95% CI, 0.38–0.53) | - | [19] |
| 22C3 PharmDx | CPS value | 12 | 100 | Biopsy | ICC 0.55 (95% CI, 0.47–0.63) | - |
| Case No. | Institution | Vendor/file format | Specimen type | Interobserver agreement | Consensus CPS |
|---|---|---|---|---|---|
| 1 | A | Philips/.isyntax | Resection | Good |
0 |
| 2 | A | Philips/.isyntax | Biopsy | Poor | 2 |
| 3 | A | Philips/.isyntax | Biopsy | Poor | Not determined |
| 4 | A | Philips/.isyntax | Resection | Poor | 5 |
| 5 | A | Philips/.isyntax | Resection | Good | 5 |
| 6 | A | Philips/.isyntax | Biopsy | Poor | 3 |
| 7 | B | Leica Biosystems/.svs | Biopsy | Poor | 2 |
| 8 | B | Leica Biosystems/.svs | Biopsy | Poor | 5 |
| 9 | B | Leica Biosystems/.svs | Biopsy | Poor | 2 |
| 10 | C | Leica Biosystems/.svs | Biopsy | Good | ≥ 5 |
| 11 | C | Leica Biosystems/.svs | Biopsy | Poor | 3 |
| 12 | C | Leica Biosystems/.svs | Biopsy | Good | ≥ 5 |
| 13 | D | Philips/.isyntax | Resection | Poor | 5 |
| 14 | D | Philips/.isyntax | Biopsy | Poor | 5 |
| 15 | D | Philips/.isyntax | Biopsy | Good | 2 |
| 16 | D | Philips/.isyntax | Resection | Poor | 5 |
| 17 | D | Philips/.isyntax | Biopsy | Poor | 2 |
| 18 | E | 3DHistech/.MRXS | Biopsy | Good | 5 |
| 19 | F | Philips/.isyntax | Biopsy | Good | ≥ 5 |
| 20 | G | Leica Biosystems/.svs | Resection | Poor | 0 |
PD-L1, programmed death-ligand 1; HER2, human epidermal growth factor receptor 2; GC, gastric cancer; CPS, combined positive score; TBD, to be determined; TAP, tumor area positivity.
PD-L1, programmed death-ligand 1; CPS, combined positive score; OPA, overall percentage agreement; ICC, intraclass correlation coefficient; CI, confidence interval.
CPS, combined positive score. Interobserver agreement was defined as good when more than 80% of pathologists agreed on CPS 5 cutoff.

 E-submission
E-submission