Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(1); 2012 > Article
-
Case Report
A Soft Tissue Perineurioma and a Hybrid Tumor of Perineurioma and Schwannoma - Ji Young Park, Nam Jo Park, Sang Pyo Kim, Kun Young Kwon, Sang Sook Lee
-
Korean Journal of Pathology 2012;46(1):75-78.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.1.75
Published online: February 23, 2012
Department of Pathology, Keimyung University School of Medicine, Daegu, Korea.
- Corresponding Author: Sang Sook Lee, M.D. Department of Pathology, Keimyung University School of Medicine, 1095 Dalgubeoldae-ro, Dalseo-gu, Daegu 704-701, Korea. Tel: +82-53-580-3811, Fax: +82-53-580-3823, sangsook@dsmc.or.kr
• Received: January 21, 2011 • Revised: April 5, 2011 • Accepted: April 7, 2011
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Perineuriomas are composed of differentiated perineurial cells. Perineuriomas have been recently recognized by the immunoreactivity for epithelial membrane antigen (EMA). Microscopically, perineuriomas show proliferation of spindle cells with wavy nuclei and delicate elongated bipolar cytoplasmic processes. The tumor cells are usually negative for the S-100 protein. Ultrastructurally, perineurial cells reveal slender, nontapered processes containing pinocytic vesicles and discontinuous basal lamina. Interestingly, hybrid tumors of benign peripheral nerve sheath tumor (PNST) have been recently reported by using immunohistochemical and ultrastructural investigations. Herein, we report a case of soft tissue perineurioma arising in the skin of a 56-year-old female; another case of a hybrid tumor of perineurioma and schwannoma in the posterior mediastinum occurred in a 53-year-old male, which is the first case of the hybrid PNST tumor reported in Korea.
- Case 1
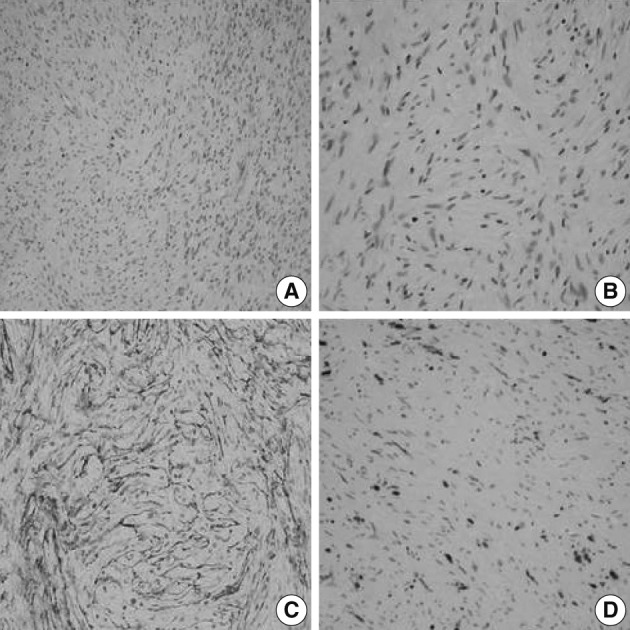
- A 56-year-old female presented with a palpable skin nodule on the anterior chest wall. The patient was not associated with neurofibromatosis type 1 or 2. Excision of the skin nodule was performed. The nodule was well-defined, ovoid, pale tan, and rubbery firm, and measured 2.2×1.5×1.5 cm. On microscopic examination, the nodule was well-circumscribed and composed of slender spindle cells arranged in loose storiform or short fascicular patterns. The tumor cells had wavy nuclei and long streamer-like cytoplasmic processes (Fig. 1A, B). No mitosis or necrosis was found. The tumor cells were immunohistochemically diffusely and strongly positive for EMA and claudin-1. The S-100 protein was only focally positive (Fig. 1C, D). An electron microscopic study failed to reveal any detailed findings due to inappropriately preserved formalin-fixed paraffin-embedded tissue.
- Case 2
- A 53-year-old male visited our out-patient clinic for the evaluation of the posterior mediastinal mass, incidentally found during a regular health check-up. No remarkable medical history was noted. Thoracoscopic excision of the mass was performed. The gross specimen consisted of multiple fragments of pale tan, translucent, and relatively firm soft tissue, measuring 5.0×5.2×2.0 cm in aggregates.
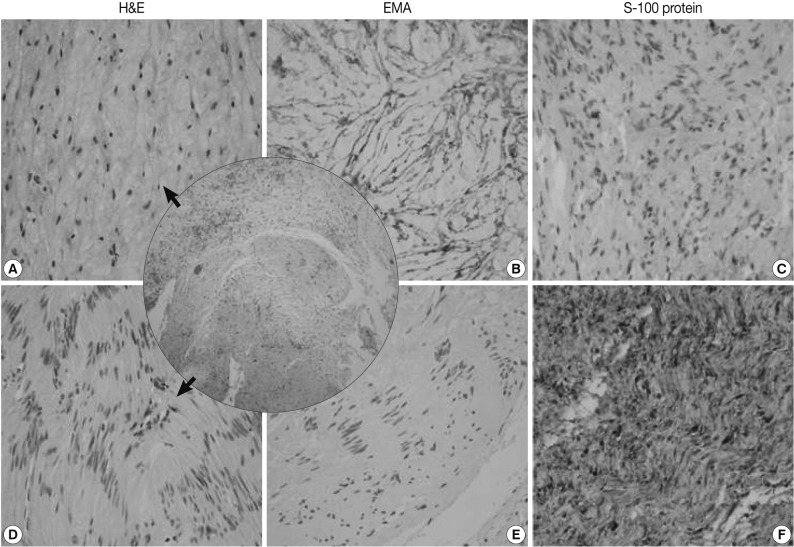
- The mediastinal mass, microscopically, revealed two distinct spindle cell areas. The majority of the mass was composed of spindle cells arranged in short fascicular or parallel cords in a fibromyxoid matrix as seen in soft tissue perineuriomas. The spindle cells had wavy nuclei and elongated bipolar cytoplasmic processes (Fig. 2A). Another spindle cell area was noted, manifesting as a typical schwannoma, including Antoni A and B areas, nuclear palisading, and hyalinized blood vessels (Fig. 2D). Nuclear atypia, necrosis or mitosis was not observed in both areas. The perineurioma-like areas constituted about 80-85% of the tumor volume.
- The perineurioma-like area of the mediastinal tumor was diffusely and strongly positive for EMA and claudin-1, but negative for S-100 protein (Fig. 2B, C). However, the tumor cells were evenly and strongly immunoreactive for S-100 protein in the schwannoma-looking area, but did not stain for EMA or claudin-1 (Fig. 2E, F).
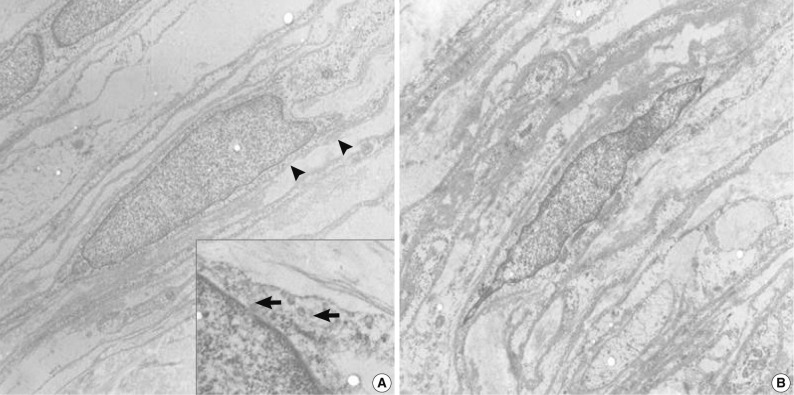
- Specimens were sampled separately from the two representative areas of the formalin-fixed paraffin-embedded block for ultrastructural studies. The cells of the perineurioma-like area showed slender and long-tapered processes containing pinocytic vesicles and discontinuous external lamina (Fig. 3A). In contrast, the schwannoma-looking area exhibited tumor cells with attenuated cell processes invested by continuous basal lamina (Fig. 3B). The pathological diagnosis was a hybrid perineurioma and schwannoma.
CASE REPORTS
- PNSTs include common schwannomas and neurofibromas, and, rare perineuriomas.
- Schwannomas are composed of Schwann cells and have characteristic cellular features, including Antoni A and B areas, nuclear palisadings (Verocay bodies), and hyalinized vessels with perivascular hemosiderin depositions. Neurofibromas consist of an admixture of Schwann cells, fibroblasts, perineurial cells, and scattered axons.1
- Perineuriomas are exclusively composed of differentiated perineurial cells. Perineuriomas can be divided into four categories, rare intraneural perineuriomas, more common soft tissue (extraneural) perineuriomas, and sclerosing and reticular variants.2,6
- Intraneural perineuriomas, formerly known as 'localized hypertrophic neuropathy' usually occur in the extremities of young individuals.1 They microscopically show concentric proliferation of perineurial cells around axons and Schwann cells, forming "onion bulbs" that stain positive for EMA.1 Sclerosing perineuriomas occur exclusively in the acral areas of young adults and are characterized by cords of bland epithelioid cells embedded in sclerotic collagen.1 Reticular perineuriomas are a recently recognized variant of perineuriomas, which are characterized by a predominantly lace-like or reticular growth pattern composed of fusiform cells with bipolar cytoplasmic processes.5
- Soft tissue perineuriomas primarily affect adults and involve the superficial soft tissues of the extremities and trunk; however, approximately 30% can develop in deep soft tissue and, rarely, visceral locations.2 Soft tissue perineuriomas show microscopic proliferation of spindle cells with wavy nuclei and elongated bipolar cytoplasmic processes arranged in a storiform or short fascicular pattern.1
- Perineurioimas are usually positive for EMA and can also express claudin-1, GLUT-1, and CD34.7 Electron microscopy findings are most useful for diagnosing perineuromas, which are characterized by showing perineurial cells with slender bipolar cytoplasmic processes containing pinocytic vesicles and discontinuous external lamina.1 They generally behave in a benign fashion, and surgical removal is curative.1
- Although the tumors are not associated with neurofibromatosis type 1 or 2, a case of soft tissue perineurioma in a patient with neurofibromatosis type 2 has been reported.3 Additionally, some studies have described that fluorescence in situ hybridization and molecular analysis demonstrate deletions or point mutations on the chromosome 22q11 in the region of the NF2 gene and chromosome 10. These findings suggested that there might be diverse genetic mechanisms underlying the development of perineuriomas.6
- These benign PNSTs are currently distinct entities. However, small numbers of the hybrid form of PNST have been recently reported. These include hybrid schwannoma-neurofibromas, hybrid schwannoma-perineuriomas, and hybrid neurofibroma-perineuriomas.4,5,7
- Feany et al.4 and Hornick et al.5 reported nine cases of schwannomas and neurofibromas hybrids and 42 cases of hybrid schwannomas and perineuriomas. They reported that hybrid tumors have well-documented histological differences with immunohistochemical and electron microscopic features and suggested that the pathogenesis of the diverse differentiation remains unknown, but could result from incomplete differentiation or genetic alteration of primitive tumor cells, or in response to a localized microenvironmental change.4,5,7
- Hybrid tumors usually arise in the dermis and subcutis over a wide range of age and anatomical distribution and seem to pursue a benign course.5 Nuclear atypia is relatively common in the hybrid tumors, which seems degenerative in nature, including smudged chromatin and occasional nuclear pseudoinclusions. The differential diagnoses of the hybrid tumors may include other benign nerve sheath tumors and low grade malignant peripheral nerve sheath tumors (MPNST). MPNST should be considered, if nuclear atypia is prominent.5
- As information and clinical follow-up data on hybrid tumors are limited, additional studies are needed to understand the pathogenesis and clinicopathological significance of hybrid tumors.
- In conclusion, we described two interesting cases of a pure form of soft tissue perineurioma, which stained positively for EMA and claudin-1, and a hybrid perineurioma and schwannoma documented by immunohistochemical and ultrastructural features. To our knowledge, this is the first report of a hybrid peripheral nerve sheath tumor in Korea.
DISCUSSION
- 1. Skovronsky DM, Oberholtzer JC. Pathologic classification of peripheral nerve tumors. Neurosurg Clin N Am 2004; 15: 157-166. ArticlePubMed
- 2. Kim JM, Choi JH. Soft tissue perineurioma: a case report. Korean J Pathol 2009; 43: 266-270. Article
- 3. Pitchford CW, Schwartz HS, Atkinson JB, Cates JM. Soft tissue perineurioma in a patient with neurofibromatosis type 2: a tumor not previously associated with the NF2 syndrome. Am J Surg Pathol 2006; 30: 1624-1629. ArticlePubMed
- 4. Feany MB, Anthony DC, Fletcher CD. Nerve sheath tumours with hybrid features of neurofibroma and schwannoma: a conceptual challenge. Histopathology 1998; 32: 405-410. ArticlePubMedPDF
- 5. Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma/perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol 2009; 33: 1554-1561. PubMed
- 6. Brock JE, Perez-Atayde AR, Kozakewich HP, Richkind KE, Fletcher JA, Vargas SO. Cytogenetic aberrations in perineurioma: variation with subtype. Am J Surg Pathol 2005; 29: 1164-1169. PubMed
- 7. Kazakov DV, Magro G, Yu Orlov A, et al. Benign schwannoma with perineurioma-like areas: a clinicopathologic study of 11 cases. Int J Surg Pathol 2006; 14: 320-325. ArticlePubMedPDF
REFERENCES
Fig. 1A soft tissue perineurioma arising in the skin. Micrographs (A, B) show proliferation of spindle cells with wavy nuclei and delicate elongated bipolar cytoplasmic processes. Immunohistochemical staining reveals proliferating spindle cells, diffusely and strongly positive for epithelial membrane antigen (C) and focally positive for the S-100 protein (D).
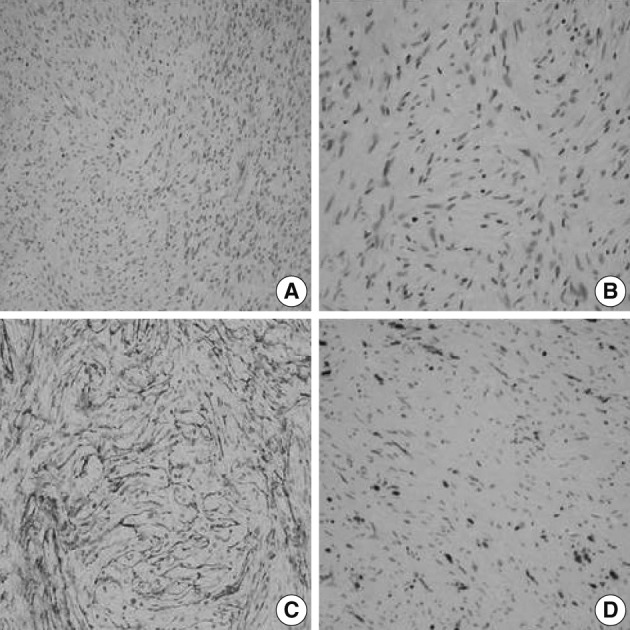

Fig. 2A hybrid perineurioma and schwannoma in the posterior mediastinum. Central circle shows two distinct representative areas of the hybrid tumor. The perineurioma-looking area (A) is composed of spindle cells with wavy nuclei and elongated bipolar cytoplasmic processes. The perineurioma area is also diffusely and strongly positive for epithelial membrane antigen (EMA) (B) but, negative for S-100 protein (C). Another spindle area manifests as a typical schwannoma (D), including Antoni A and B areas and nuclear palisading. In the schwannoma-looking area, the tumor cells are evenly and strongly immunoreactive for S-100 protein (F), but do not stain for EMA (E). H&E, hamatoxylin and eosin.
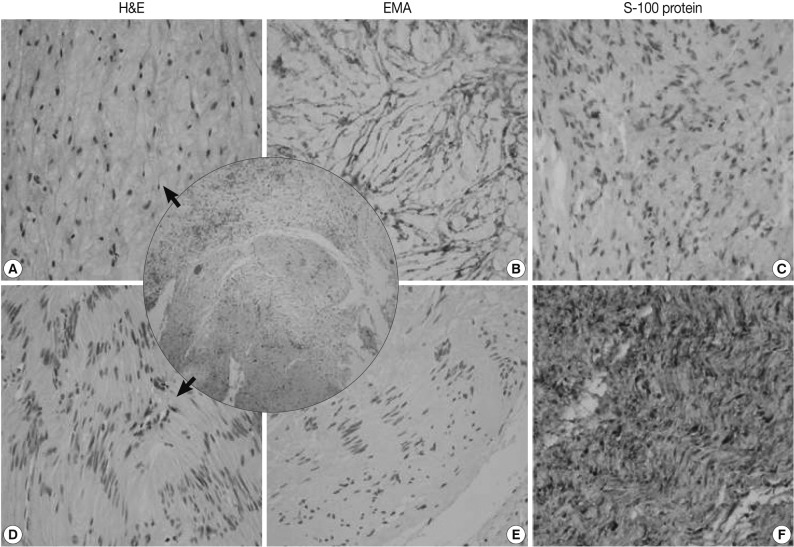

Fig. 3Transmission electron micrographs of the hybrid perineurioma and schwannoma. In the perineurioma area (A), the tumor cells show slender cytoplasmic processes containing pinocytic vesicles (indicated by arrows in the inset) and discontinuous external lamina (arrowheads). In contrast, the schwannoma area (B) exhibits attenuated cell processes invested by continuous basal lamina (×4,000).
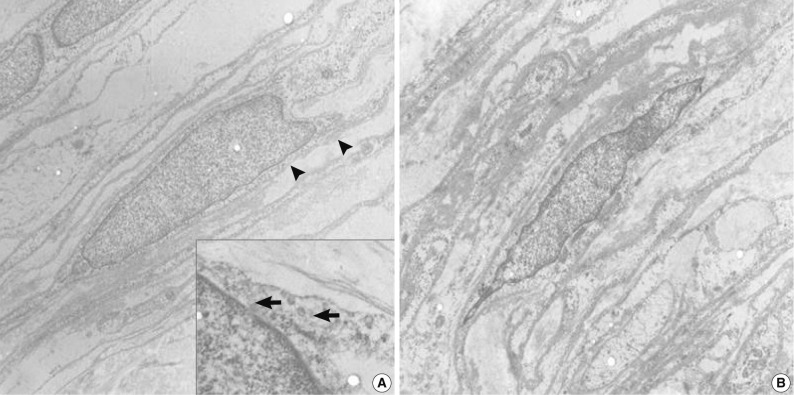

Figure & Data
References
Citations
Citations to this article as recorded by 

- Plexiform Fibromyxoma of the Gastric Body: A Rare Benign Mesenchymal Tumor in an Unconventional Location
Anan Bseiso, Dalia Ibrahim
International Journal of Surgical Pathology.2025;[Epub] CrossRef - Neurogenic tumours of the posterior mediastinum and differential diagnosis considerations
Michael A den Bakker, Annikka Weissferdt
Histopathology.2024; 84(1): 238. CrossRef - Hybrid tumors with perineurioma components: a systematic review of the literature and illustrative case
Karina A. Lenartowicz, Dileep D. Monie, Kimberly K. Amrami, Christopher J. Klein, Caterina Giannini, Robert J. Spinner
Acta Neurochirurgica.2022; 165(4): 935. CrossRef - Hybrid Schwannoma/Perineurioma: Morphologic Variations and Genetic Profiles
Takanori Hirose, Anna Kobayashi, Sumihito Nobusawa, Naoe Jimbo
Applied Immunohistochemistry & Molecular Morphology.2021; 29(6): 433. CrossRef - Mesenchymal Tumors of the Mediastinum: An Update on Diagnostic Approach
Joon Hyuk Choi, Jae Y. Ro
Advances in Anatomic Pathology.2021; 28(5): 351. CrossRef - Neurogenic Tumors of the Mediastinum
Erika F. Rodriguez, Robert Jones, Daniel Miller, Fausto J. Rodriguez
Seminars in Diagnostic Pathology.2020; 37(4): 179. CrossRef - A Rare Perineurioma/Granular Cell Tumor Hybrid Peripheral Nerve Sheath Tumor
Koorosh Haghayeghi, Gladys Telang, Sonja Chen, Jack Bevivino, Shamlal Mangray, Yiang Hui, Leslie Robinson-Bostom
The American Journal of Dermatopathology.2020; 42(10): 762. CrossRef - Hybrid peripheral nerve sheath tumors
Emine KILIÇ BAĞIR, Arbil AÇIKALIN, Gülfiliz GÖNLÜŞEN, Suzan ZORLUDEMİR, Mehmet Ali DEVECİ
Cukurova Medical Journal.2019; 44(3): 804. CrossRef - Primary intraosseous hybrid epithelioid schwannoma/perineurioma in the proximal tibia: a case report of benign hybrid neoplasm with local hypercellularity
Yuejiao Lang, Dawei Liu, Pei Xiang, Jilin Wang, Yang Li
Diagnostic Pathology.2019;[Epub] CrossRef - Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature
Nasir Ud Din, Zubair Ahmad, Jamshid Abdul-Ghafar, Rashida Ahmed
BMC Cancer.2017;[Epub] CrossRef - Primary intraosseous hybrid nerve sheath tumor of femur: A hitherto undescribed occurrence in bone with secondary aneurysmal bone cyst formation resulting in pathological fracture
Louis Tsun Cheung Chow
Pathology - Research and Practice.2015; 211(5): 409. CrossRef - Mesenchymal tumours of the mediastinum—part II
Michael A. den Bakker, Alexander Marx, Kiyoshi Mukai, Philipp Ströbel
Virchows Archiv.2015; 467(5): 501. CrossRef - Primary pleural hybrid cellular schwannoma/perineurioma: A case report
Danny Soria-Céspedes, Carlos Robles-Vidal, Arturo Gómez-González, Rosalinda Peñaloza-Ramírez, Carlos Ortiz-Hidalgo
Respiratory Investigation.2014; 52(4): 269. CrossRef - Hybrid peripheral nerve sheath tumour with intermingled perineuriomatous and schwannomatous areas reflected in skin ultrasonography image
H. Saeki, K. Ito, Y. Nobeyama, T. Ishiji, M. Fukunaga, H. Nakagawa
Clinical and Experimental Dermatology.2014; 39(6): 747. CrossRef - Périneuriome extraneural des tissus mous localisé au nez
A. Zaouak, R. Benmously, M. Belhadj Salah, W. Koubaa, A. Debbiche, I. Mokhtar
Annales de Dermatologie et de Vénéréologie.2013; 140(8-9): 540. CrossRef
A Soft Tissue Perineurioma and a Hybrid Tumor of Perineurioma and Schwannoma



Fig. 1 A soft tissue perineurioma arising in the skin. Micrographs (A, B) show proliferation of spindle cells with wavy nuclei and delicate elongated bipolar cytoplasmic processes. Immunohistochemical staining reveals proliferating spindle cells, diffusely and strongly positive for epithelial membrane antigen (C) and focally positive for the S-100 protein (D).
Fig. 2 A hybrid perineurioma and schwannoma in the posterior mediastinum. Central circle shows two distinct representative areas of the hybrid tumor. The perineurioma-looking area (A) is composed of spindle cells with wavy nuclei and elongated bipolar cytoplasmic processes. The perineurioma area is also diffusely and strongly positive for epithelial membrane antigen (EMA) (B) but, negative for S-100 protein (C). Another spindle area manifests as a typical schwannoma (D), including Antoni A and B areas and nuclear palisading. In the schwannoma-looking area, the tumor cells are evenly and strongly immunoreactive for S-100 protein (F), but do not stain for EMA (E). H&E, hamatoxylin and eosin.
Fig. 3 Transmission electron micrographs of the hybrid perineurioma and schwannoma. In the perineurioma area (A), the tumor cells show slender cytoplasmic processes containing pinocytic vesicles (indicated by arrows in the inset) and discontinuous external lamina (arrowheads). In contrast, the schwannoma area (B) exhibits attenuated cell processes invested by continuous basal lamina (×4,000).
Fig. 1
Fig. 2
Fig. 3
A Soft Tissue Perineurioma and a Hybrid Tumor of Perineurioma and Schwannoma

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article