Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(3); 2012 > Article
-
Original Article
The Expression of Pigment Epithelium-Derived Factor in Bladder Transitional Cell Carcinoma - Tae Jung Jang, Sung Woo Kim1, Kyung Seop Lee2
-
Korean Journal of Pathology 2012;46(3):261-265.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.3.261
Published online: June 22, 2012
Department of Pathology, Dongguk University College of Medicine, Gyeongju, Korea.
1Department of Radiology, Dongguk University College of Medicine, Gyeongju, Korea.
2Department of Urology, Dongguk University College of Medicine, Gyeongju, Korea.
- Corresponding Author: Tae Jung Jang, M.D. Department of Pathology, Dongguk University College of Medicine, 123 Dongdae-ro, Gyeongju 780-714, Korea. Tel: +82-54-770-2410, Fax: +82-54-770-2431, taejung@mail.dongguk.ac.kr
• Received: March 30, 2012 • Revised: June 5, 2012 • Accepted: June 5, 2012
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations
Citations to this article as recorded by 

- Association of pigment epithelium derived factor expression with cancer progression and prognosis: a meta-analysis study
Guo Cheng, Crystal Song
Discover Oncology.2021;[Epub] CrossRef - Level of mitoses in non-muscle invasive papillary urothelial carcinomas (pTa and pT1) at initial bladder biopsy is a simple and powerful predictor of clinical outcome: a multi-center study in South Korea
Ji Eun Kwon, Nam Hoon Cho, Yeong-Jin Choi, So Dug Lim, Yong Mee Cho, Sun Young Jun, Sanghui Park, Young A. Kim, Sung-Sun Kim, Mi Sun Choe, Jung-dong Lee, Dae Yong Kang, Jae Y. Ro, Hyun-Jung Kim
Diagnostic Pathology.2017;[Epub] CrossRef - Endogenous Gastric-Resident Mesenchymal Stem Cells Contribute to Formation of Cancer Stroma and Progression of Gastric Cancer
Eun-Kyung Kim, Hye-Jung Kim, Young-Il Yang, Jong Tae Kim, Min-Young Choi, Chang Soo Choi, Kwang-Hee Kim, Jeong-Han Lee, Won-Hee Jang, Soon-Ho Cheong
Korean Journal of Pathology.2013; 47(6): 507. CrossRef
The Expression of Pigment Epithelium-Derived Factor in Bladder Transitional Cell Carcinoma


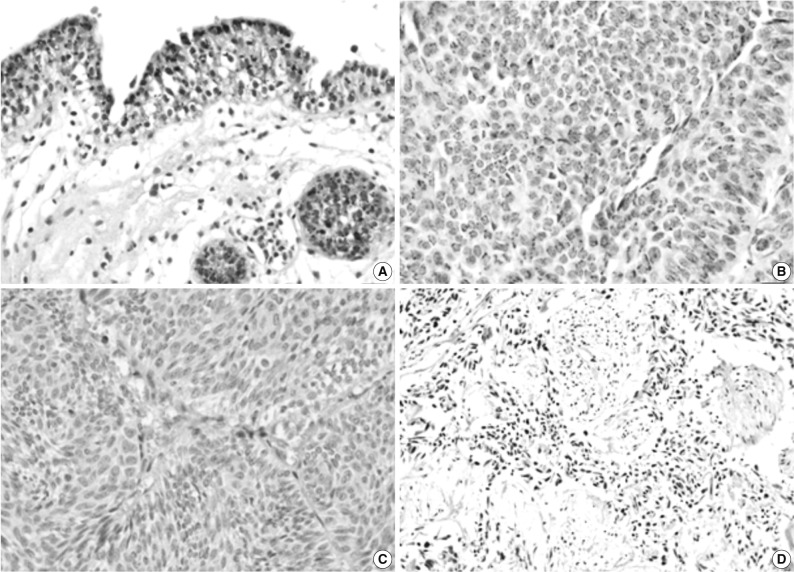
Fig. 1 Immunohistochemical staining of pigment epithelium-derived factor (PEDF) in normal urothelium (A) and bladder transitional cell carcinoma (TCC) (B-D). The expression of PEDF has a granular pattern in the cytoplasm of normal and neoplastic urothelial cells (B), and some TCCs do not show PEDF expression (C, D).
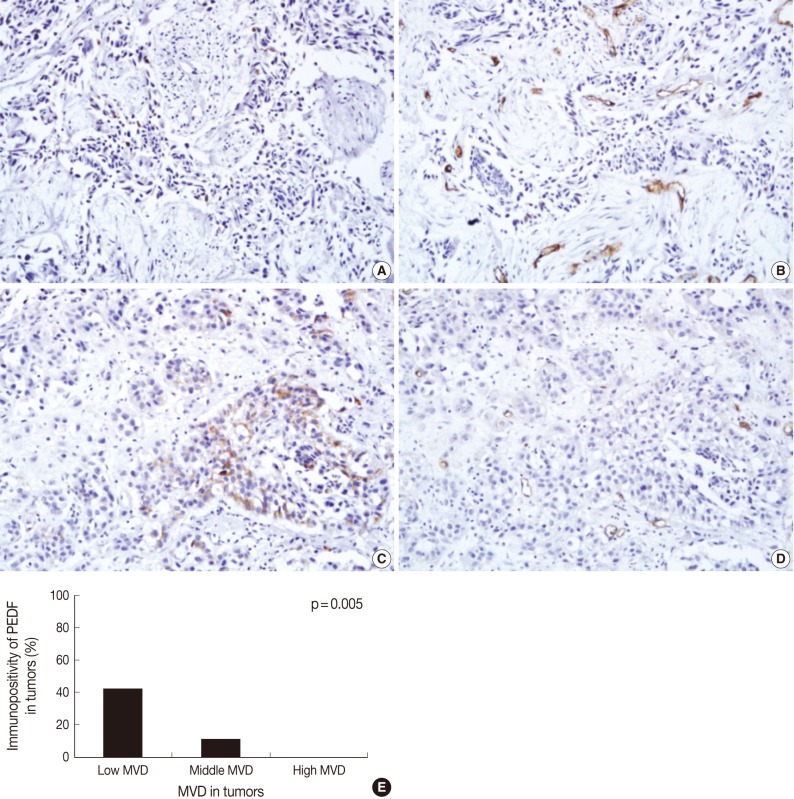
Fig. 2 Immunohistochemical staining of pigment epithelium-derived factor (PEDF) (A, C) and CD34 (B, D) in bladder transitional cell carcinoma (TCC), and the relationship between PEDF expression and microvessel density (MVD) (E). The degree of MVD is significantly higher in TCCs without PEDF expression (A, B) than TCC with PEDF expression (C, D). Bladder TCC shows a significant reciprocal correlation between PEDF expression and MVD (p=0.005) (E).
Fig. 1
Fig. 2
The Expression of Pigment Epithelium-Derived Factor in Bladder Transitional Cell Carcinoma

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article