Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(3); 2012 > Article
-
Case Report
Gastric Adenocarcinoma of Fundic Gland Type: Report of Three Cases - Eun Su Park, Young Eun Kim1, Cheol Keun Park1, Takashi Yao2, Ryoji Kushima3, Kyoung-Mee Kim1
-
Korean Journal of Pathology 2012;46(3):287-291.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.3.287
Published online: June 22, 2012
Department of Hospital Pathology, Incheon St. Mary's Hospital, The Catholic University of Korea School of Medicine, Korea.
1Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
2Department of Human Pathology, Juntendo University, Tokyo, Japan.
3Clinical Laboratory Division, National Cancer Center Hospital, Tokyo, Japan.
- Corresponding Author: Kyoung Mee Kim, M.D. Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea. Tel: +82-2-3410-2800, Fax: +82-2-3410-0025, km7353.kim@samsung.com
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Endoscopic Submucosal Dissection of Early Gastric Adenocarcinoma of Fundic Gland Type: A Case Report
Ming Zhong, Wei Wei, Huang Zhong, Hang Gong, Tingyu Wang
Revista Española de Enfermedades Digestivas.2025;[Epub] CrossRef - Oxyntic Gland Neoplasms - From Adenoma to Advanced Gastric Cancer: A Review of 29 Cases
Gi Hwan Kim, Jun Su Lee, Jeong Hoon Lee, Young Soo Park
Journal of Gastric Cancer.2024; 24(4): 378. CrossRef - Transcriptome analysis reveals the essential role of NK2 homeobox 1/thyroid transcription factor 1 (NKX2-1/TTF-1) in gastric adenocarcinoma of fundic-gland type
Kazushi Fukagawa, Yu Takahashi, Nobutake Yamamichi, Natsuko Kageyama-Yahara, Yoshiki Sakaguchi, Miho Obata, Rina Cho, Nobuyuki Sakuma, Sayaka Nagao, Yuko Miura, Naoki Tamura, Daisuke Ohki, Hiroya Mizutani, Seiichi Yakabi, Chihiro Minatsuki, Keiko Niimi, Y
Gastric Cancer.2023; 26(1): 44. CrossRef - Clinicopathological Features and the Prevalence of Oxyntic Gland Neoplasm: A Single-center Retrospective Study
Hikari Asahara, Toshitatsu Takao, Yumiko Asahara, Masakyo Asahara, Douglas Motomura, Hiroya Sakaguchi, Tetsuya Yoshizaki, Nobuaki Ikezawa, Madoka Takao, Yoshinori Morita, Takashi Toyonaga, Masato Komatsu, Ryoji Kushima, Yuzo Kodama
Internal Medicine.2023; 62(19): 2763. CrossRef - Clinicopathological features of gastric adenocarcinoma of fundic gland type
Bao-Zhen Guo, Zhen-Zhen Liu, Gao-Fei Shen, Fei Zhu, Hui-Fen Lian, Xin Li, Jun-Yi Zheng, Jin-Peng Li, Shui-Miao Deng, Rui Huang
World Chinese Journal of Digestology.2023; 31(6): 244. CrossRef - Endoscopic Resection for Gastric Adenocarcinoma of the Fundic Gland Type: A Case Series
Hwa Jin Lee, Gwang Ha Kim, Dong Chan Joo, Moon Won Lee, Bong Eun Lee, Kyungbin Kim
The Korean Journal of Gastroenterology.2023; 81(6): 259. CrossRef - Gastric adenocarcinoma of the fundic gland type: A review of the literature
Zhiyong Zhai, Wei Hu, Zhaoyu Huang, Zemin Chen, Sicun Lu, Wei Gong
JGH Open.2023; 7(12): 812. CrossRef - Clinicopathological features of early stage gastric adenocarcinoma of fundic gland type
Huan Zhang, Shuyan Wang, Yongping Zhang, Fusang Ye, Chunnian Wang
Medicine.2022; 101(2): e28469. CrossRef - Gastric Adenocarcinoma of Fundic Gland Type Treated by Endoscopic Submucosal Dissection
Yong Bo Park, Gwang Ha Kim, Kyungbin Kim, Tae Kyoung Ha, Guk Bin Park, Young Min Kwak
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2021; 21(1): 82. CrossRef - Gastric epithelial neoplasm of fundic-gland mucosa lineage: proposal for a new classification in association with gastric adenocarcinoma of fundic-gland type
Hiroya Ueyama, Takashi Yao, Yoichi Akazawa, Takuo Hayashi, Koichi Kurahara, Yumi Oshiro, Masayoshi Yamada, Ichiro Oda, Shin Fujioka, Chiaki Kusumoto, Masayoshi Fukuda, Kunihisa Uchita, Tomohiro Kadota, Yasuhiro Oono, Kazuhisa Okamoto, Kazunari Murakami, Y
Journal of Gastroenterology.2021; 56(9): 814. CrossRef - Endoscopic resection is a suitable initial treatment strategy for oxyntic gland adenoma or gastric adenocarcinoma of the fundic gland type
Masaya Iwamuro, Chiaki Kusumoto, Masahiro Nakagawa, Sayo Kobayashi, Masao Yoshioka, Tomoki Inaba, Tatsuya Toyokawa, Shinichiro Hori, Shouichi Tanaka, Kazuhiro Matsueda, Takehiro Tanaka, Hiroyuki Okada
Scientific Reports.2021;[Epub] CrossRef - A series of five patients with oxyntic gland adenoma: Deciphering the clinical and histological features of these rare gastric polyps
Jerry C. Nagaputra, Tracy Jie Zhen Loh, Sangeeta Mantoo, Rafay Azhar, Vikneswaran Namasivayam, Wei Qiang Leow
Human Pathology Reports.2021; 26: 300566. CrossRef - Gastric adenocarcinoma of the fundic gland: A review of clinicopathological characteristics, treatment and prognosis
Xiang-yu Meng, Guang Yang, Cheng-ji Dong, Ru-yi Zheng
Rare Tumors.2021;[Epub] CrossRef - Gastric adenocarcinoma of the fundic gland type: clinicopathological features of eight patients treated with endoscopic submucosal dissection
Chengfang Li, Xinglong Wu, Shuang Yang, Xiaorong Yang, Jin Yao, Hong Zheng
Diagnostic Pathology.2020;[Epub] CrossRef - Multiple gastric adenocarcinoma of fundic gland type: A case report
Ou Chen, Ze-Yong Shao, Xiong Qiu, Guang-Ping Zhang
World Journal of Clinical Cases.2019; 7(18): 2871. CrossRef - Gastric Adenocarcinoma of the Fundic Gland Type
Mark A Benedict, Gregory Y Lauwers, Dhanpat Jain
American Journal of Clinical Pathology.2018; 149(6): 461. CrossRef - Oxyntic Gland Adenoma Treated by Endoscopic Mucosal Resection
In Ji Song, Jin Woo Joo, Jun Chul Park, Sung Kwan Shin, Yong Chan Lee, Sang Kil Lee
The Korean Journal of Helicobacter and Upper Gastrointestinal Research.2017; 17(2): 94. CrossRef - Chief cell‐predominant gastric polyps: a series of 12 cases with literature review
Karen Chan, Ian S Brown, Trevor Kyle, Gregory Y Lauwers, Marian Priyanthi Kumarasinghe
Histopathology.2016; 68(6): 825. CrossRef - Twelve-year natural history of a gastric adenocarcinoma of fundic gland type
Yoshinori Sato, Takashi Fujino, Akira Kasagawa, Ryo Morita, Shun-ichiro Ozawa, Yasumasa Matsuo, Tadateru Maehata, Hiroshi Yasuda, Masayuki Takagi, Fumio Itoh
Clinical Journal of Gastroenterology.2016; 9(6): 345. CrossRef - Clinicopathological features of gastric adenocarcinoma of the fundic gland (chief cell predominant type) by retrospective and prospective analyses of endoscopic findings
Takashi Chiba, Katsuaki Kato, Takayuki Masuda, Shuichi Ohara, Noriyuki Iwama, Takenobu Shimada, Daisuke Shibuya
Digestive Endoscopy.2016; 28(7): 722. CrossRef - Gastric Adenocarcinoma of the Fundic Gland Type Treated by Endoscopic Mucosal Resection: A Case Report and Review of the Literature
Eleanor Lewin, Philip Daroca, Sanjay Sikka, Tong Wu, Yukihiro Nakanishi
Case Reports in Pathology.2016; 2016: 1. CrossRef - Gastric adenocarcinoma of the fundic gland (chief cell-predominant type): A review of endoscopic and clinicopathological features
Masaki Miyazawa, Mitsuru Matsuda, Masaaki Yano, Yasumasa Hara, Fumitaka Arihara, Yosuke Horita, Koichiro Matsuda, Akito Sakai, Yatsugi Noda
World Journal of Gastroenterology.2016; 22(48): 10523. CrossRef - Oxyntic gland adenoma endoscopically mimicking a gastric neuroendocrine tumor: A case report
Tae-In Lee
World Journal of Gastroenterology.2015; 21(16): 5099. CrossRef - Oxyntic gland polyp/adenoma
Rajkumar Vajpeyi, Jyoti Dekate
Diagnostic Histopathology.2014; 20(11): 446. CrossRef - Gastric adenocarcinoma of fundic gland type with unusual behavior
Tetsuya Ueo, Hirotoshi Yonemasu, Tetsuya Ishida
Digestive Endoscopy.2014; 26(2): 293. CrossRef

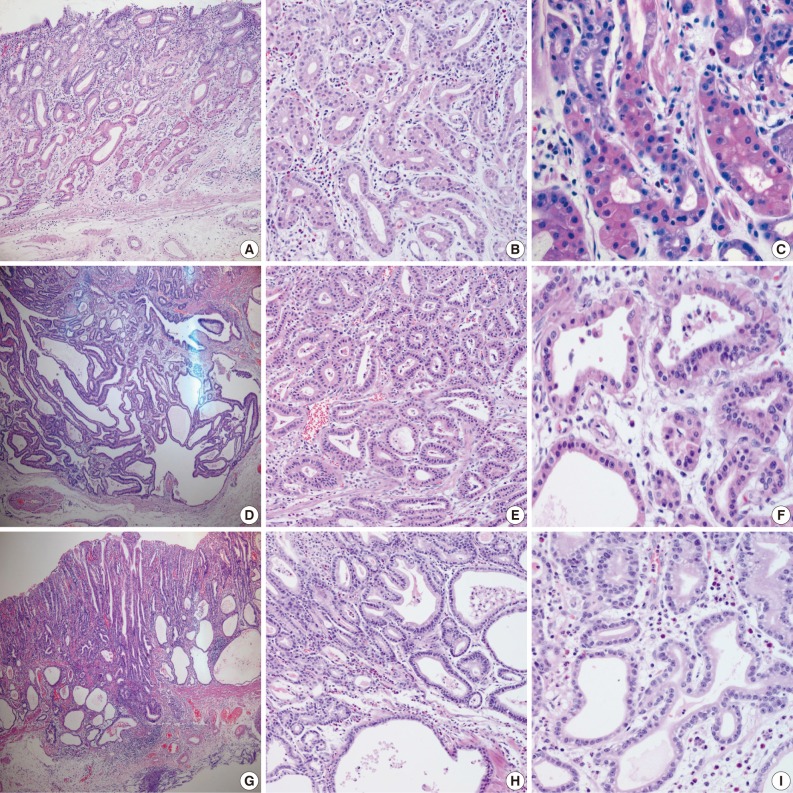
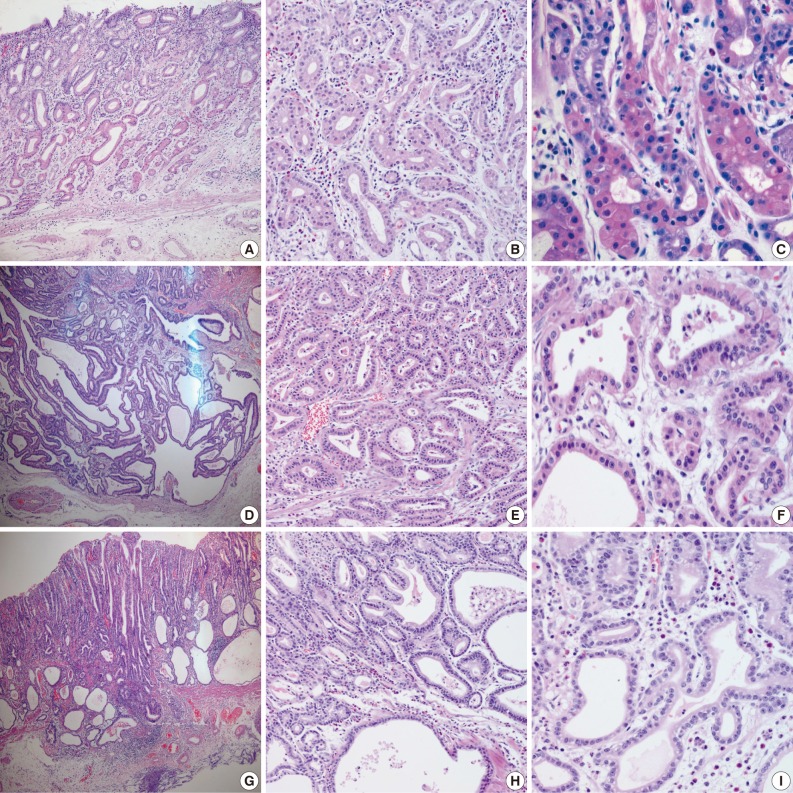
Fig. 1
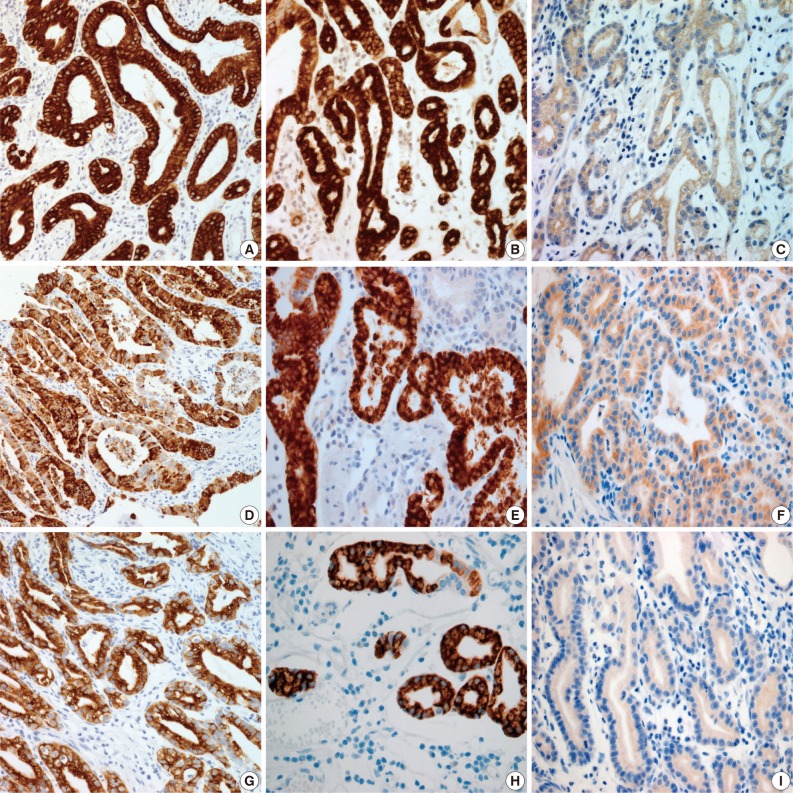
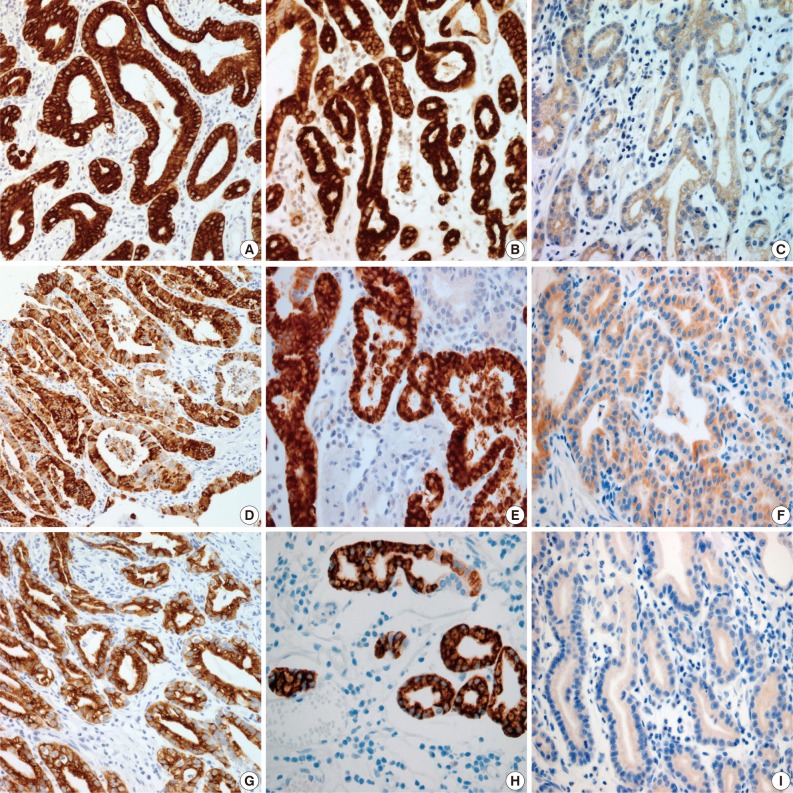
Fig. 2

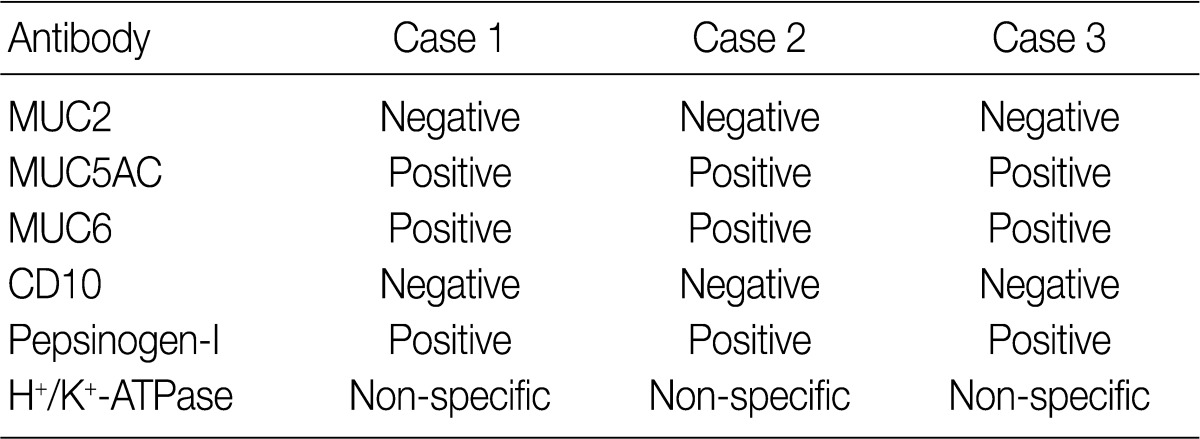
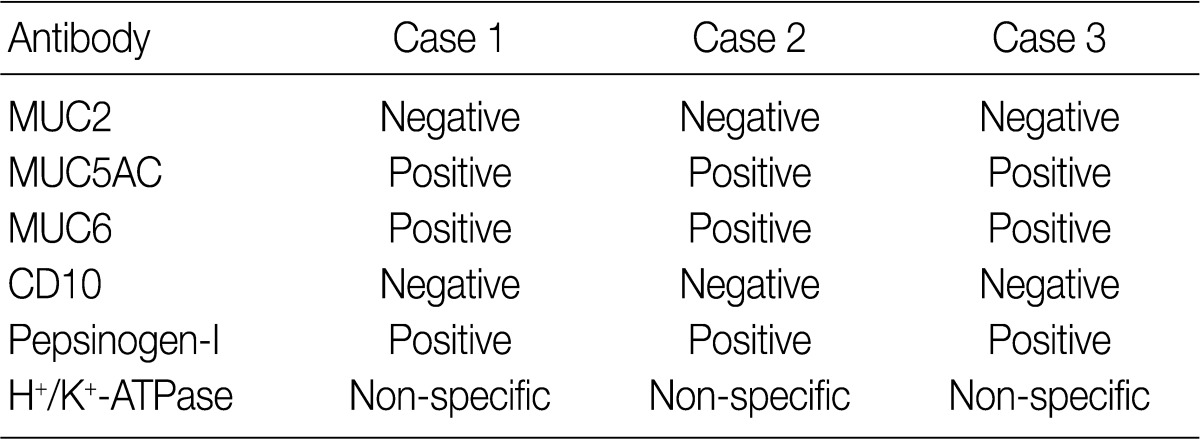
M, male; ESD, endoscopic mucosal dissection; IIa+IIc, slightly elevated and depressed; LN, lymph node.
MUC, mucin.

 E-submission
E-submission

 PubReader
PubReader Cite this Article
Cite this Article