Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(4); 2012 > Article
-
Case Report
Endoscopic Ultrasound-Guided Fine Needle Aspiration Cytology Diagnosis of Solid Pseudopapillary Neoplasm: Three Case Reports with Review of Literature - Joon Seon Song1,2, Chong Woo Yoo1, Youngmee Kwon1, Eun Kyung Hong1
-
Korean Journal of Pathology 2012;46(4):399-406.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.4.399
Published online: August 23, 2012
1Department of Pathology, Center for Liver Cancer, National Cancer Center, Goyang, Korea.
2Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- Corresponding Author: Eun Kyung Hong, M.D. Department of Pathology, Center for Liver Cancer, National Cancer Center, 323 Ilsan-ro, Ilsandong-gu, Goyang 410-769, Korea. Tel: +82-31-920-1739, Fax: +82-31-920-1369, hongek@ncc.re.kr
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Spleen Preservation in Solid Pseudopapillary Neoplasms of the Distal Pancreas: A Single-Center Experience
Tiffany M. Yue, Beatrice J. Sun, Daniel J. Delitto, Monica M. Dua, Jeffrey A. Norton, George A. Poultsides, Brendan C. Visser, Byrne Lee
Journal of Surgical Research.2025; 310: 17. CrossRef - Laparoscopic parenchyma-sparing resections for solid pseudopapillary tumors located in the head of pancreas
Zhengdong Zou, Lu Feng, Bing Peng, Jianhua Liu, Yunqiang Cai
BMC Surgery.2023;[Epub] CrossRef - Solid pancreatic masses in children: A review of current evidence and clinical challenges
Kelli N. Patterson, Andrew T. Trout, Archana Shenoy, Maisam Abu-El-Haija, Jaimie D. Nathan
Frontiers in Pediatrics.2022;[Epub] CrossRef - Pancreatic solid pseudopapillary neoplasm in male patients: systematic review with three new cases
Anna Caterina Milanetto, Anna-Lea Gais Zürcher, Lorenzo Macchi, Alina David, Claudio Pasquali
Updates in Surgery.2021; 73(4): 1285. CrossRef - Silencing c-Myc Enhances the Antitumor Activity of Bufalin by Suppressing the HIF-1α/SDF-1/CXCR4 Pathway in Pancreatic Cancer Cells
Xia Liu, Yayun Zhou, Jiamin Peng, Bei Xie, Qiyang Shou, Jianchao Wang
Frontiers in Pharmacology.2020;[Epub] CrossRef - Simultaneous colorectal and parenchymal-sparing liver resection for advanced colorectal carcinoma with synchronous liver metastases: Between conventional and mini-invasive approaches
Emilio De Raffele, Mariateresa Mirarchi, Dajana Cuicchi, Ferdinando Lecce, Riccardo Casadei, Claudio Ricci, Saverio Selva, Francesco Minni
World Journal of Gastroenterology.2020; 26(42): 6529. CrossRef - Solid pseudopapillary neoplasm of the pancreas showing marked distal atrophy
Masanori Tsujie, Tomoko Wakasa, Shigeto Mizuno, Hajime Ishikawa, Hironobu Manabe, Taichi Koyama, Kotaro Kitani, Shumpei Satoi, Keisuke Inoue, Shuichi Fukuda, Toshihiko Kawasaki, Masao Yukawa, Yoshio Ohta, Masatoshi Inoue
International Journal of Surgery Case Reports.2019; 55(C): 136. CrossRef - Solid pseudopapillary neoplasm of the pancreas in pediatric patients: A case report and institutional case series
Justin B. Mahida, Rajan K. Thakkar, Jon Walker, Rulong Shen, Brian D. Kenney, Vinay Prasad, Jennifer H. Aldrink
Journal of Pediatric Surgery Case Reports.2015; 3(4): 149. CrossRef - Onsite cytopathology evaluation and ancillary studies beneficial in EUS‐FNA of pancreatic, mediastinal, intra‐abdominal, and submucosal lesions
Shafqat Mehmood, Amna Jahan, Asif Loya, Muhammed Aasim Yusuf
Diagnostic Cytopathology.2015; 43(4): 278. CrossRef - Multicentric solid pseudopapillary neoplasms of the pancreas diagnosed by endoscopic ultrasound-guided fine needle aspiration: a case report
Megumi Yamaguchi, Toshikatsu Fukuda, Masahiro Nakahara, Mio Amano, Daisuke Takei, Masumi Kawashima, Yusuke Sumi, Hironobu Amano, Shuji Yonehara, Keiji Hanada, Toshio Noriyuki
Surgical Case Reports.2015;[Epub] CrossRef - A solid pseudopapillary neoplasm without cysts that occurred in a patient diagnosed by endoscopic ultrasound-guided fine-needle aspiration: a case report
Masakuni Fujii, Masao Yoshioka, Takefumi Niguma, Hiroaki Saito, Toru Kojima, Soichiro Nose, Junji Shiode
Journal of Medical Case Reports.2014;[Epub] CrossRef - Surgical Management and Long-Term Follow-Up of Solid Pseudopapillary Tumor of Pancreas: A Large Series from a Single Institution
Yunqiang Cai, Xun Ran, Siming Xie, Xin Wang, Bing Peng, Gang Mai, Xubao Liu
Journal of Gastrointestinal Surgery.2014; 18(5): 935. CrossRef - A case of solid-pseudopapillary neoplasm in a middle-aged male preoperatively diagnosed by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA)
Shin KATO, Takuya HONBU, Moriya ZAKIMI, Kenji CHINEN, Tomiaki KUBOTA, Masayuki ARASHIRO, Kaoru KIKUCHI, Takahiro MURAKAMI, Fumihito KUNISHIMA
Suizo.2014; 29(2): 263. CrossRef - Endoscopic ultrasound-guided fine needle aspiration improves the pre-operative diagnostic yield of solid-pseudopapillary neoplasm of the pancreas: an international multicenter case series (with video)
Joanna K. Law, Alina Stoita, Wallia Weaver, Ferga C. Gleeson, Andrew M. Dries, Amanda Blackford, Vandhana Kiswani, Eun Ji Shin, Mouen A. Khashab, Marcia Irene Canto, Vikesh K. Singh, Anne Marie Lennon
Surgical Endoscopy.2014; 28(9): 2592. CrossRef - Multiple Diagnostic Imaging of a Patient with Solid Pseudopapillary Tumour of the Pancreas: EUS, CT and FDG PET/CT
Ari Chong, Jung-Min Ha, Seong Young Kwon
Nuclear Medicine and Molecular Imaging.2014; 48(1): 82. CrossRef




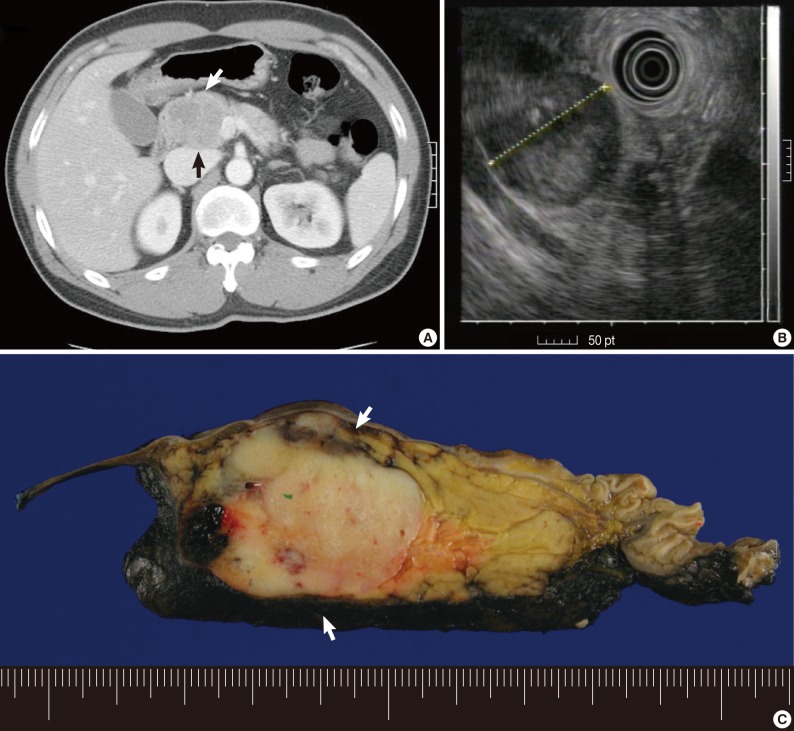
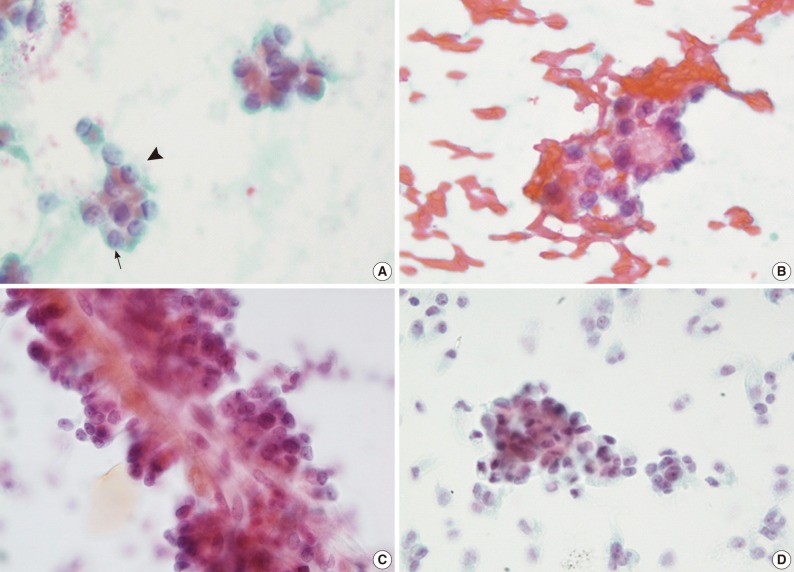
Fig. 1
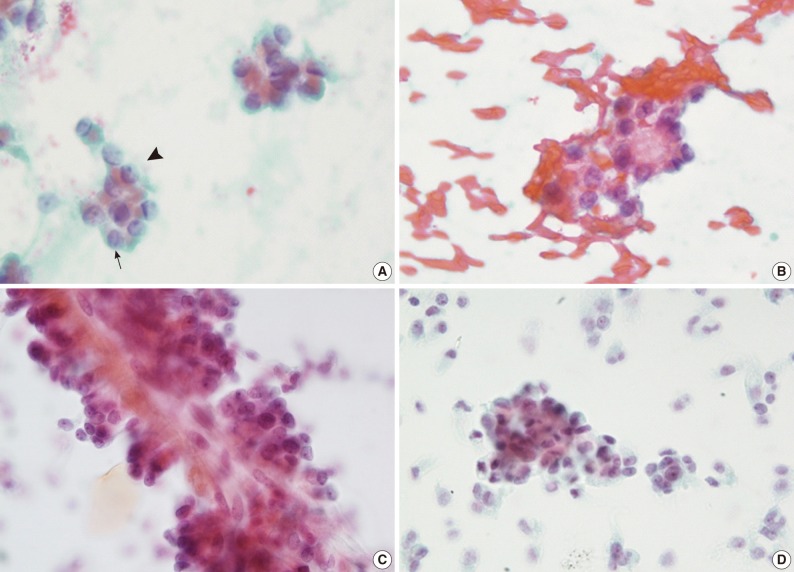
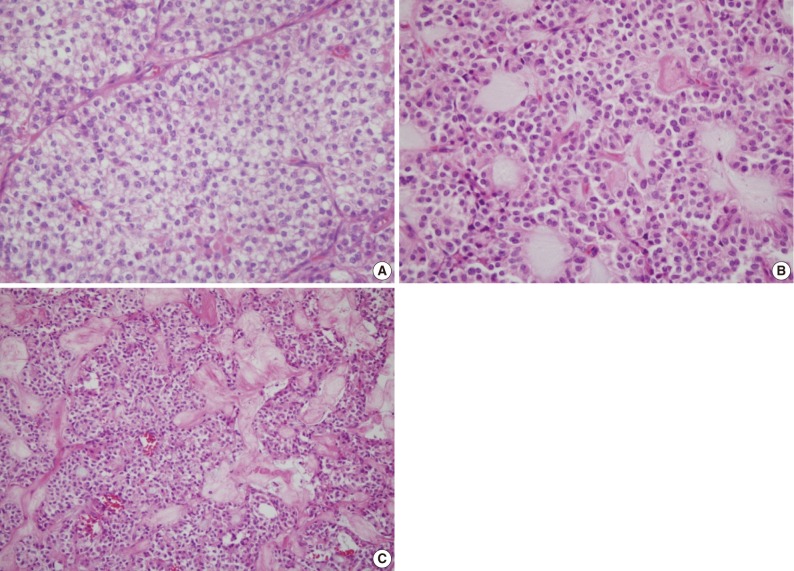
Fig. 2
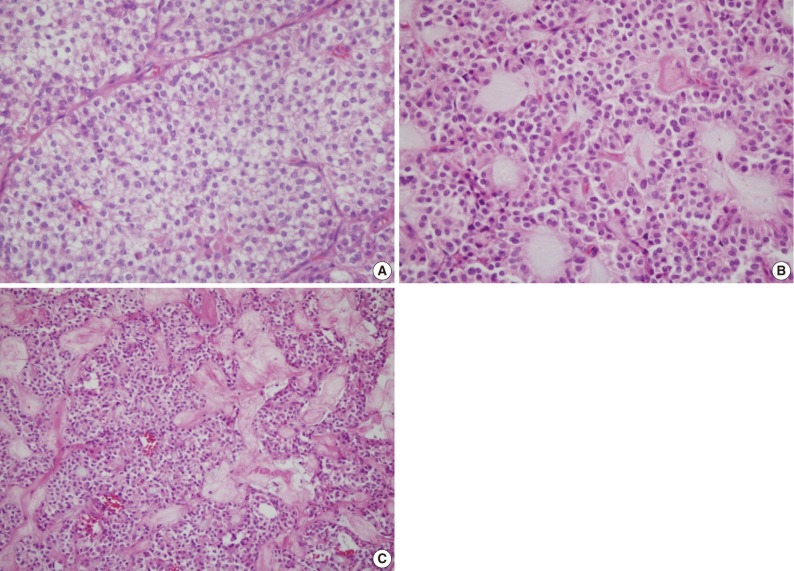
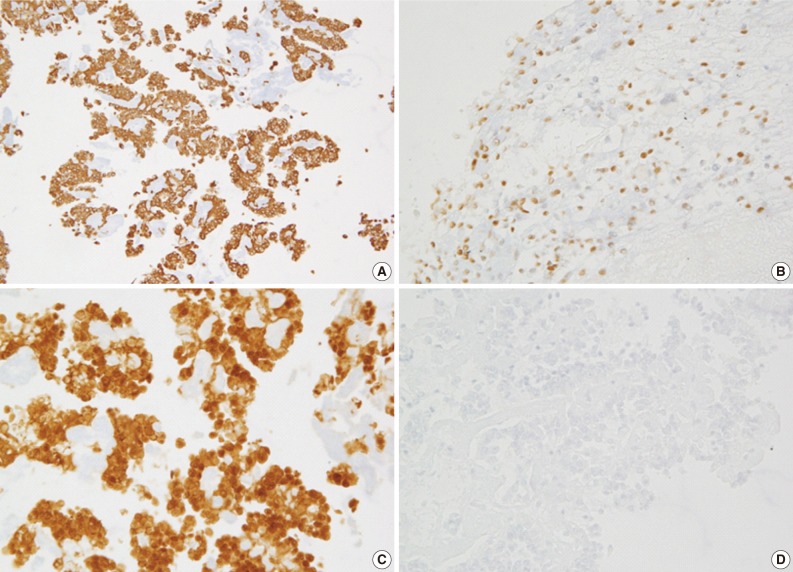
Fig. 3
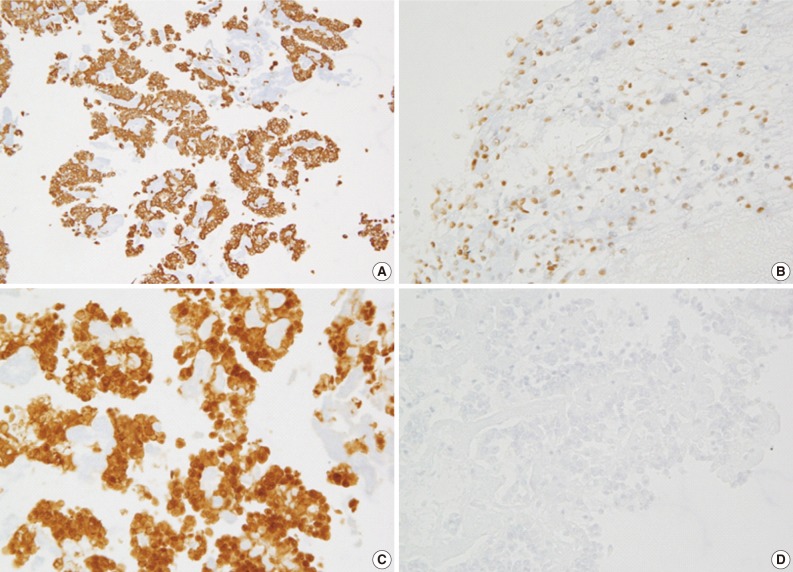
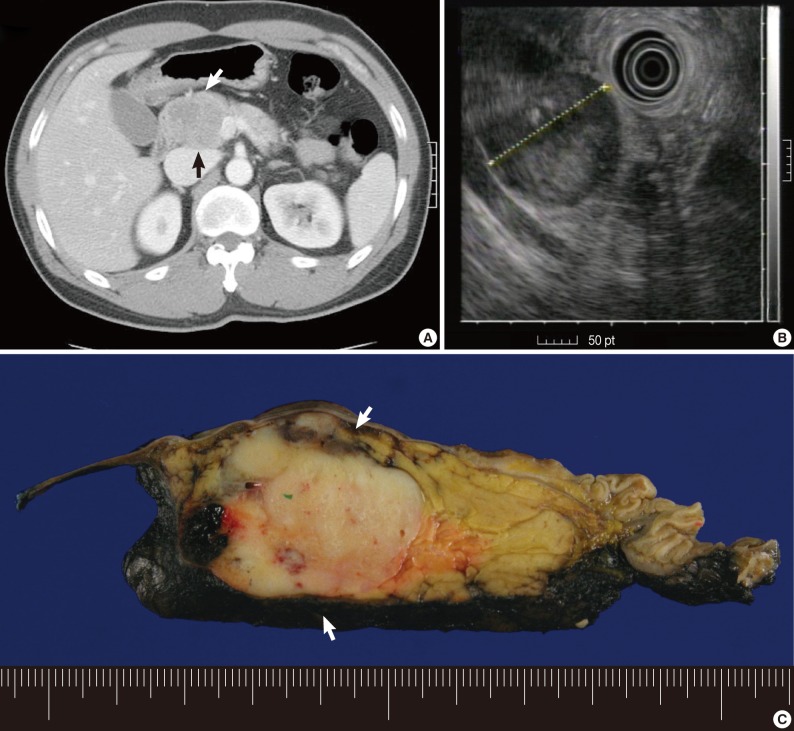
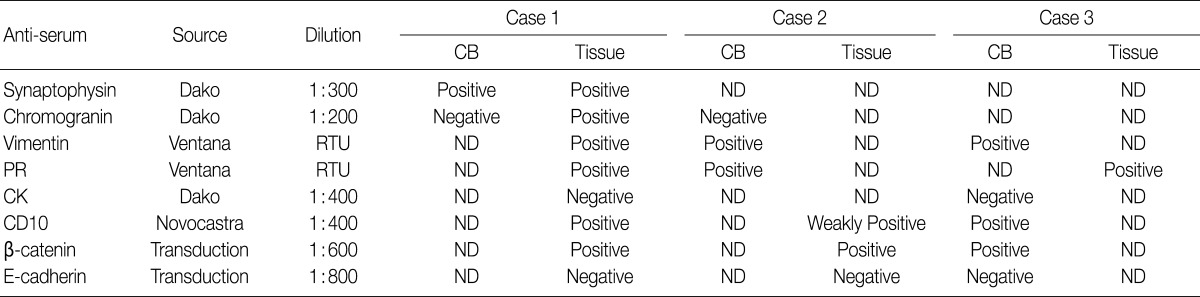
Fig. 4
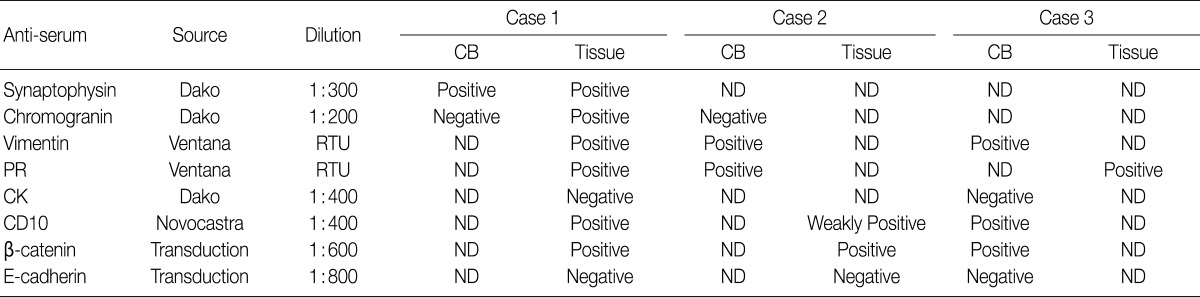
CB, cell block; ND, not done; RTU, ready to use; PR, progesterone receptor; CK, cytokeratin.
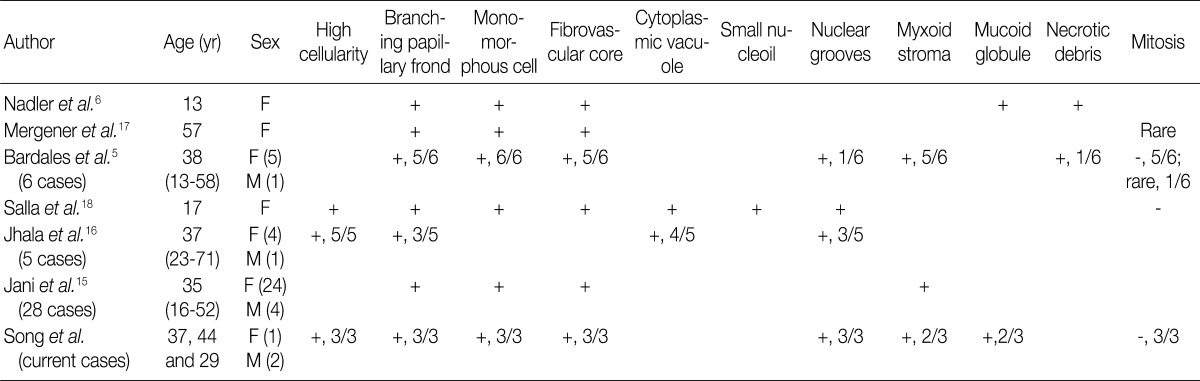
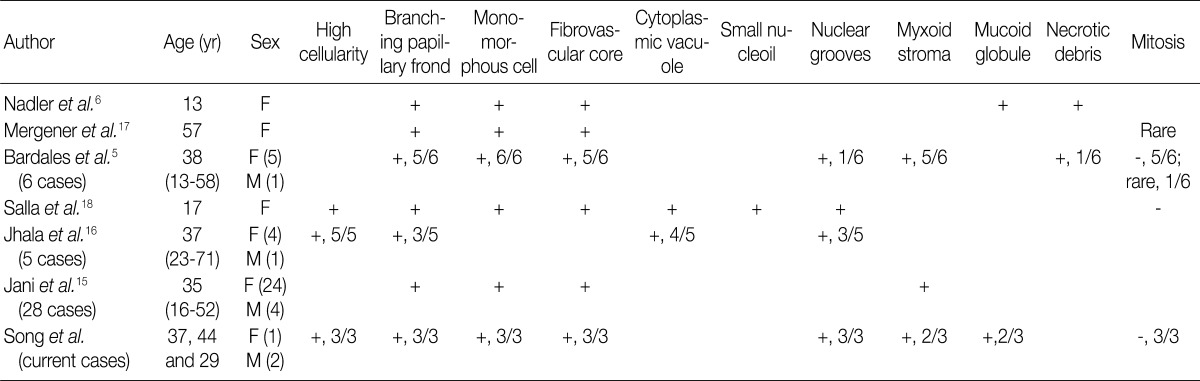
EUS-FNA, endoscopic ultrasound-guided fine-needle aspiration; SPN, solid pseudopapillary neoplasm of the pancreas; F, female; M, male.
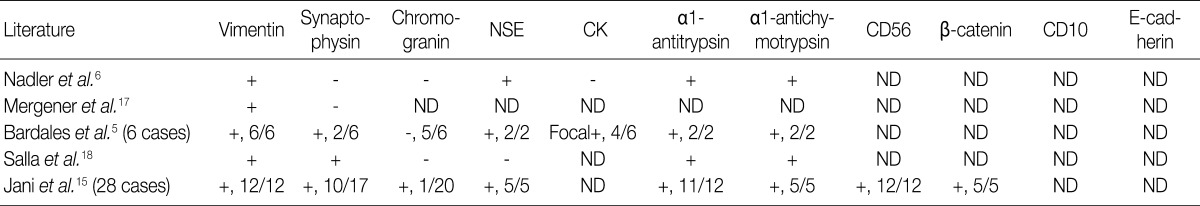
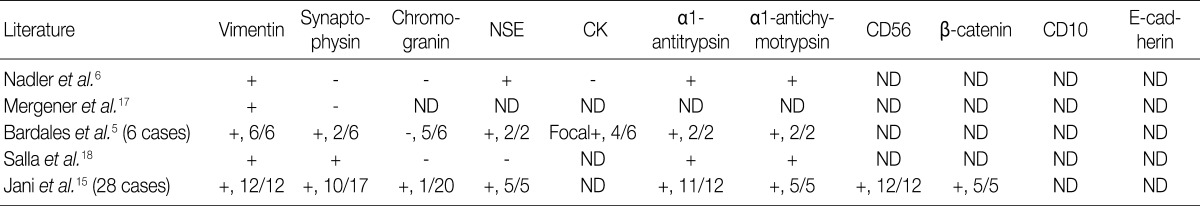
NSE, neuron specific enolase; CK, cytokeratin; ND, not done.

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article