Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(5); 2012 > Article
-
Case Report
Cytologic Features of Giant Cell Ependymoma: A Case Report and Review of the Literature - Myoung Ju Koh, Sun Och Yoon,, Hyae Min Jeon, Hyeon Joo Jeong, Soon Won Hong, Se Hoon Kim,
-
Korean Journal of Pathology 2012;46(5):507-513.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.5.507
Published online: October 25, 2012
Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- Corresponding Author: Se Hoon Kim, M.D. Department of Pathology, Yonsei University College of Medicine, 250 Seongsan-ro, Seodaemun-gu, Seoul 120-752, Korea. Tel: +82-2-2228-1769, Fax: +82-2-362-0860, paxco@yuhs.ac, Sun Och Yoon, M.D. Department of Pathology, Yonsei University College of Medicine, 211 Eonju-ro, Gangnam-gu, Seoul 135-720, Korea. Tel: +82-2-2019-2791, Fax: +82-2-362-0860, soyoon@yuhs.ac
- *Sun Och Yoon and Se Hoon Kim contributed equally to this work.
*Dr. Yoon consulted this case to Dr. Kim.
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- A case of myxopapillary ependymoma with predominant giant cell morphology: A rare entity with comprehensive genomic profiling and review of literature
Bryan Morales‐Vargas, Hassan Saad, Daniel Refai, Matthew Schniederjan, Zied Abdullaev, Kenneth Aldape, Malak Abedalthagafi
Neuropathology.2025; 45(1): 13. CrossRef - Report of a case of giant cell ependymoma with unusual clinical and pathological presentation
Mónica B. Mezmezian, Victor Del Caño, Liliana G. Olvi
Neuropathology.2019; 39(4): 313. CrossRef - Giant Cell Ependymoma of Cervicomedullary Junction: A Case Report of a Long-Term Survivor and Literature Review
Martina Cappelletti, Andrea G. Ruggeri, Giorgia Iacopino, Roberto Delfini
World Neurosurgery.2018; 116: 121. CrossRef - Immunohistochemical features of giant cell ependymoma of the filum terminale with unusual clinical and radiological presentation
Fernando Candanedo-Gonzalez, Cindy Sharon Ortiz-Arce, Samuel Rosales-Perez, Ana Lilia Remirez-Castellanos, Candelaria Cordova-Uscanga, Armando Gamboa-Dominguez
Diagnostic Pathology.2017;[Epub] CrossRef - Giant Cell Ependymoma of Lateral Ventricle: Case Report, Literature Review, and Analysis of Prognostic Factors and Genetic Profile
Hirokazu Takami, Christopher S. Graffeo, Avital Perry, Aditya Raghunathan, Robert B. Jenkins, Caterina Giannini, Terry C. Burns
World Neurosurgery.2017; 108: 997.e9. CrossRef
 PubReader
PubReader-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles
-
- Cytological features of atypical adenomatous hyperplasia and adenocarcinoma in situ of the lung: a case report
- Metastatic choroidal melanoma in the breast: a case report and review of the literature
- Hepatic carcinoma expressing inhibin: case report of a proposed novel entity and review of the literature




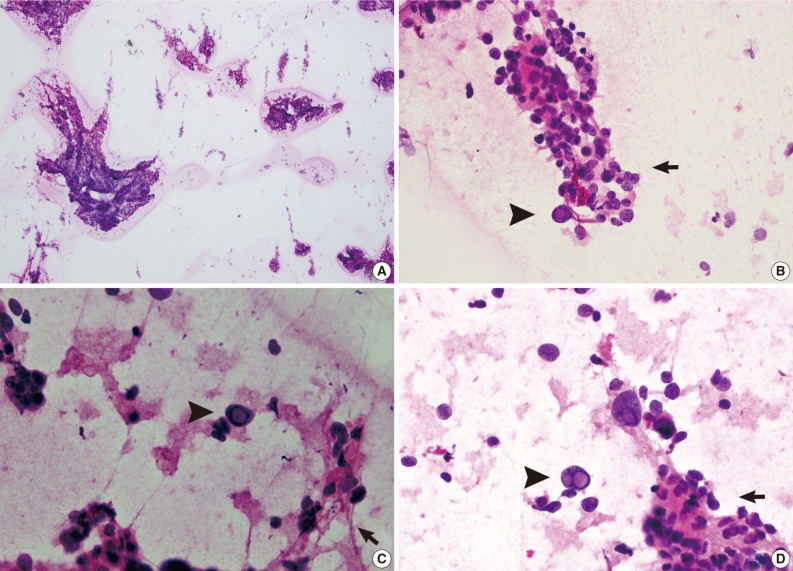
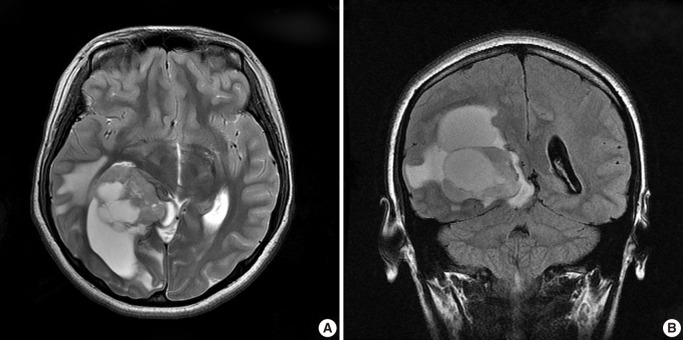
Fig. 1
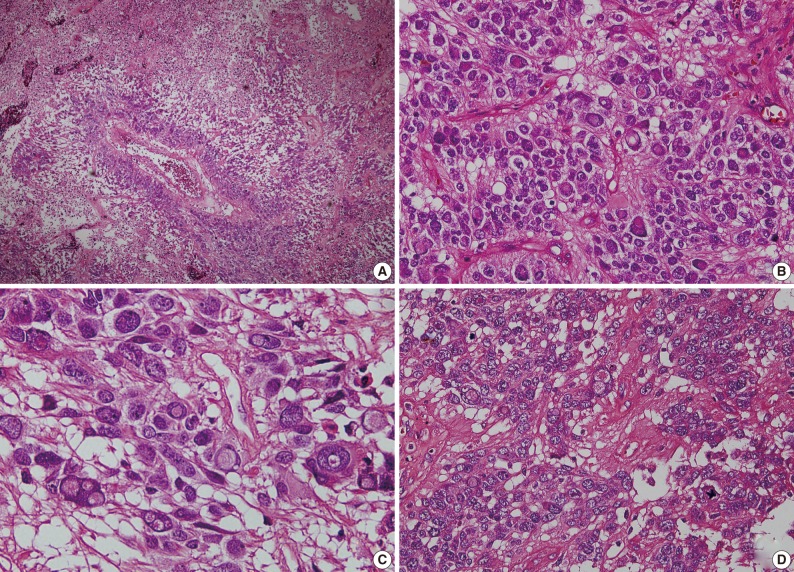
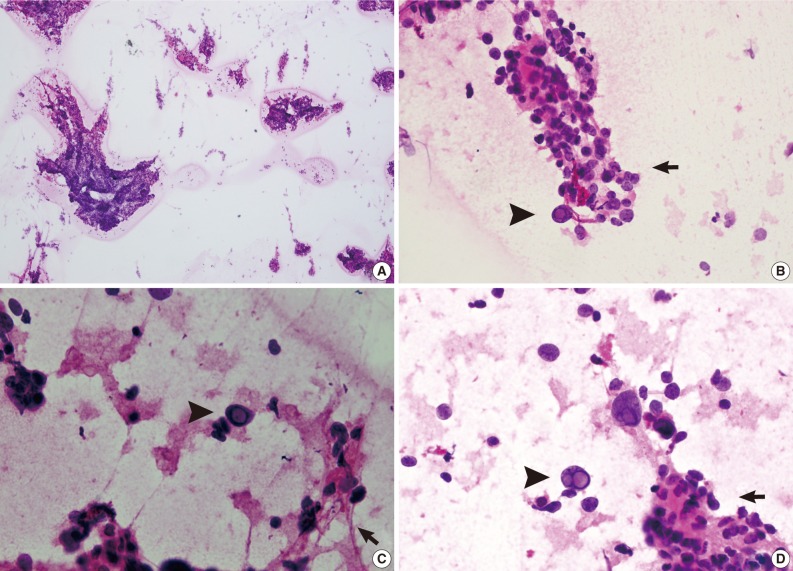
Fig. 2
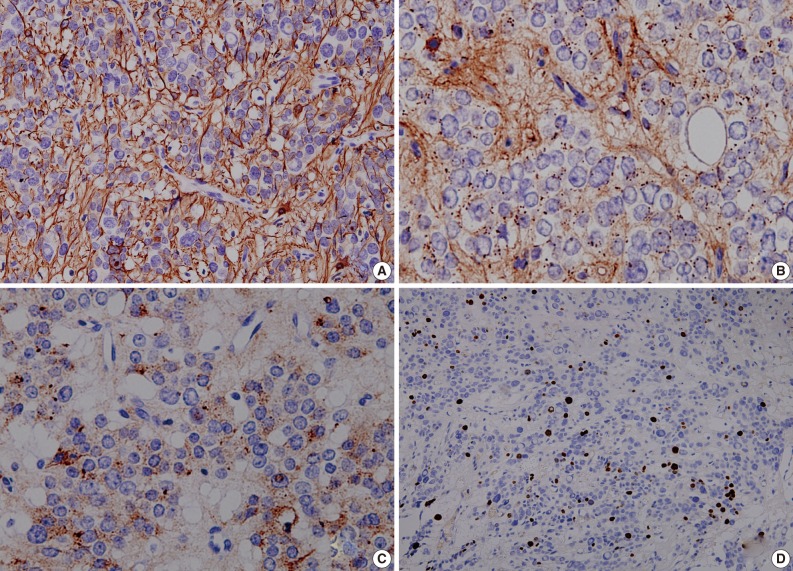
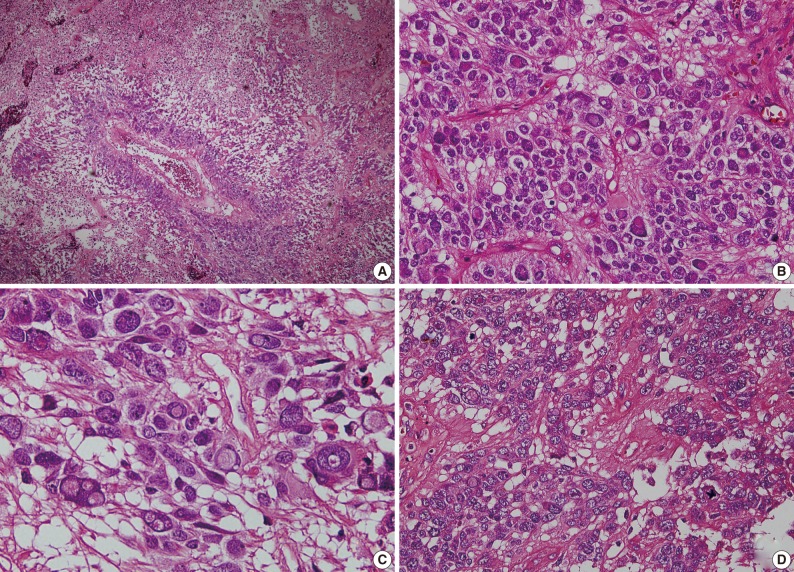
Fig. 3
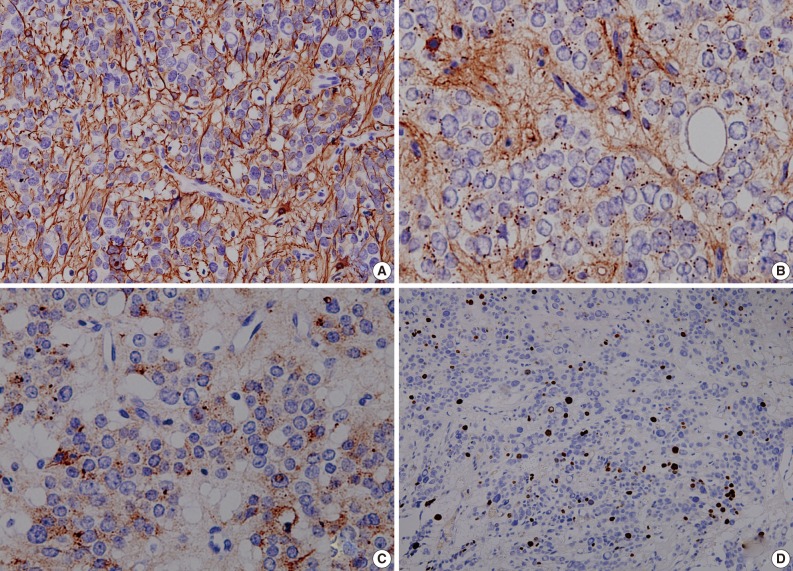
Fig. 4
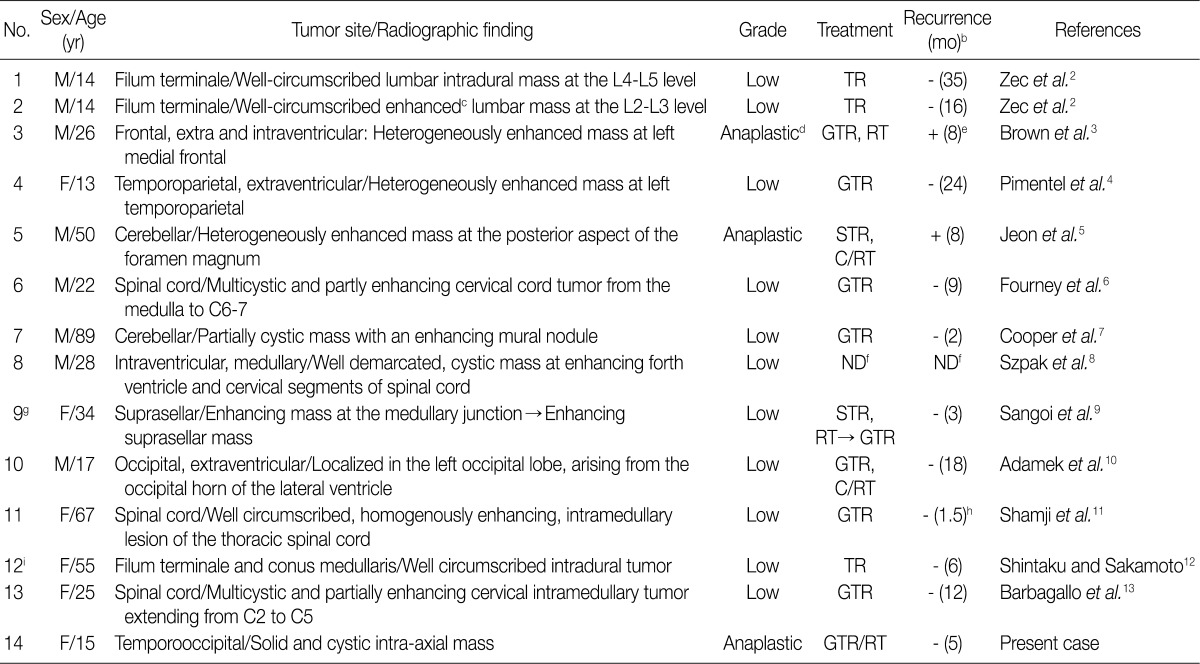
M, male; F, female;TR, total resection; GTR, grossly total resection; STR, subtotal resection; C/RT, chemotherapy/radiotherapy; ND, not described. aThe table is a modified version of Table 1 from the work of Adamek et al.
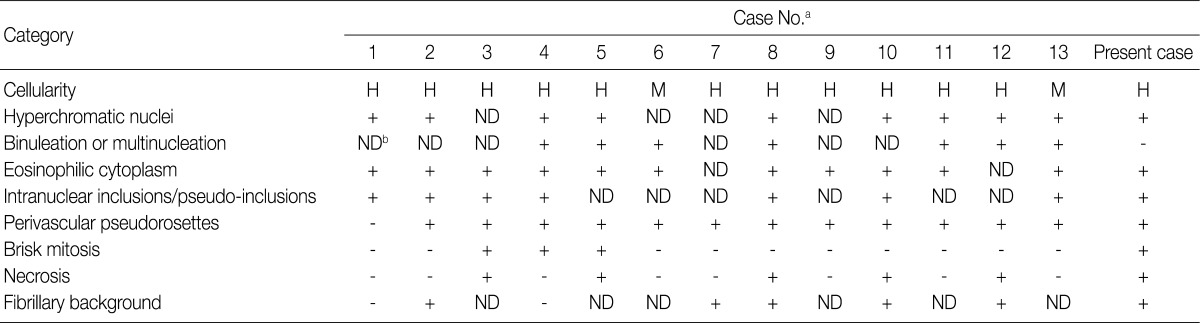
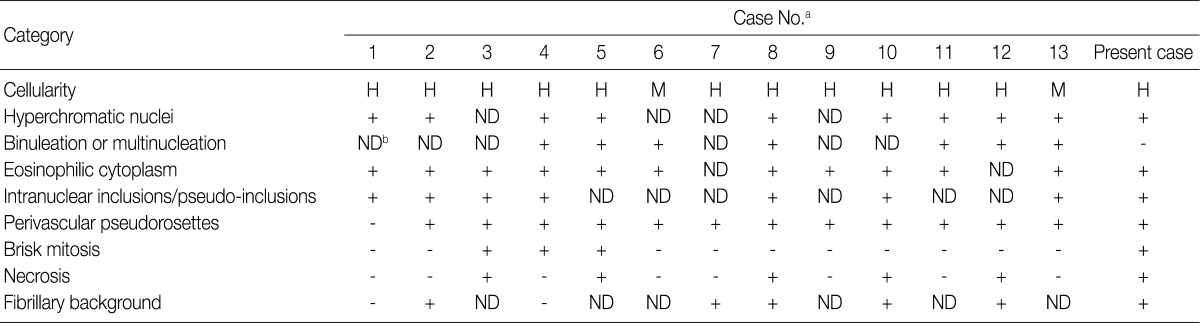
H, high; M, moderate; ND, not described. aCase No., same as the

 E-submission
E-submission