Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(6); 2012 > Article
-
Original Article
Methylation and Immunoexpression ofp16INK4a Tumor Suppressor Gene in Primary Breast Cancer Tissue and Their Quantitativep16INK4a Hypermethylation in Plasma by Real-Time PCR - Jae Jun Lee, Eunkyung Ko1, Junhun Cho, Ha Young Park, Jeong Eon Lee1, Seok Jin Nam1, Duk-Hwan Kim2, Eun Yoon Cho
-
Korean Journal of Pathology 2012;46(6):554-561.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.6.554
Published online: December 26, 2012
Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
2Department of Genome Research, Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Seoul, Korea.
- Corresponding Author: Eun Yoon Cho, M.D. Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea. Tel: +82-2-3410-2800, Fax: +82-2-3410-0025, eunyoon.cho@samsung.com
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Progress in epigenetic research of breast cancer: a bibliometric analysis since the 2000s
Hua Yang, Yu Fang, Haijuan Wang, Ting Lu, Qihua Chen, Hui Liu
Frontiers in Oncology.2025;[Epub] CrossRef - Epigenetic Silencing of p16INK4a gene in Sporadic Breast Cancer
Satya P. Singh, Mallika Tewari, Alok K. Singh, Raghvendra R. Mishra, Hari S. Shukla
Indian Journal of Surgical Oncology.2023; 14(4): 822. CrossRef - Pathogenesis and Potential Therapeutic Targets for Triple-Negative Breast Cancer
Chia-Jung Li, Yen-Dun Tony Tzeng, Yi-Han Chiu, Hung-Yu Lin, Ming-Feng Hou, Pei-Yi Chu
Cancers.2021; 13(12): 2978. CrossRef - Mechanisms of resistance to estrogen receptor modulators in ER+/HER2− advanced breast cancer
Jin Zhang, Qianying Wang, Qing Wang, Jiangran Cao, Jiafu Sun, Zhengmao Zhu
Cellular and Molecular Life Sciences.2020; 77(4): 559. CrossRef - Aberrantly Methylated cfDNA in Body Fluids as a Promising Diagnostic Tool for Early Detection of Breast Cancer
Igor Stastny, Pavol Zubor, Karol Kajo, Peter Kubatka, Olga Golubnitschaja, Zuzana Dankova
Clinical Breast Cancer.2020; 20(6): e711. CrossRef - Epigenetic modulation of BRCA‐1 and MGMT genes, and histones H4 and H3 are associated with breast tumors
Parisa Paydar, Gholamreza Asadikaram, Hamid Zeynali Nejad, Hamed Akbari, Moslem Abolhassani, Vahid Moazed, Mohammad Hadi Nematollahi, Ghasem Ebrahimi, Hossein Fallah
Journal of Cellular Biochemistry.2019; 120(8): 13726. CrossRef - The 9p21 locus: A potential therapeutic target and prognostic marker in breast cancer
Mahdi Rivandi, Mohammad‐Sadegh Khorrami, Hamid Fiuji, Soodabeh Shahidsales, Malihe Hasanzadeh, Mir Hadi Jazayeri, Seyed Mahdi Hassanian, Gordon A. Ferns, Nafiseh Saghafi, Amir Avan
Journal of Cellular Physiology.2018; 233(7): 5170. CrossRef - p16INK4a overexpression as a predictor of survival in ocular surface squamous neoplasia
Sheetal Chauhan, Seema Sen, Anjana Sharma, Seema Kashyap, Radhika Tandon, Mandeep S Bajaj, Neelam Pushker, Murugesan Vanathi, Shyam S Chauhan
British Journal of Ophthalmology.2018; 102(6): 840. CrossRef - Liquid biopsy prediction of axillary lymph node metastasis, cancer recurrence, and patient survival in breast cancer
Ju-Han Lee, Hoiseon Jeong, Jung-Woo Choi, Hwa Eun Oh, Young-Sik Kim
Medicine.2018; 97(42): e12862. CrossRef - EZH2 inhibition sensitizes tamoxifen‑resistant breast cancer cells through cell cycle regulation
Si Chen, Fan Yao, Qinghuan Xiao, Qiannan Liu, Yikun Yang, Xuejuan Li, Guanglie Jiang, Takayoshi Kuno, Yue Fang
Molecular Medicine Reports.2017;[Epub] CrossRef - Aberrant promoter methylation of cancer-related genes in human breast cancer
Liang Wu, Ye Shen, Xianzhen Peng, Simin Zhang, Ming Wang, Guisheng Xu, Xianzhi Zheng, Jianming Wang, Cheng Lu
Oncology Letters.2016; 12(6): 5145. CrossRef - Centrosome aberrations in human mammary epithelial cells driven by cooperative interactions between p16INK4a deficiency and telomere-dependent genotoxic stress
Daniel Domínguez, Purificación Feijoo, Aina Bernal, Amaia Ercilla, Neus Agell, Anna Genescà, Laura Tusell
Oncotarget.2015; 6(29): 28238. CrossRef - Relationships Betweenp16Gene Promoter Methylation and Clinicopathologic Features of Colorectal Cancer: A Meta-Analysis of 27 Cohort Studies
Yan-Zhi Chen, Dan Liu, Yu-Xia Zhao, He-Tong Wang, Ya Gao, Ying Chen
DNA and Cell Biology.2014; 33(10): 729. CrossRef - Endocrine disruption of the epigenome: a breast cancer link
Kevin C Knower, Sarah Q To, Yuet-Kin Leung, Shuk-Mei Ho, Colin D Clyne
Endocrine-Related Cancer.2014; 21(2): T33. CrossRef - Cyclin-dependent kinase inhibitors, p16 and p27, demonstrate different expression patterns in thymoma and thymic carcinoma
Mutsuko Omatsu, Toshiaki Kunimura, Tetsuya Mikogami, Akira Shiokawa, Atsuko Masunaga, Tomoko Nagai, Akihiko Kitami, Takashi Suzuki, Mitsutaka Kadokura
General Thoracic and Cardiovascular Surgery.2014; 62(11): 678. CrossRef - Limoniastrum guyonianum aqueous gall extract induces apoptosis in human cervical cancer cells involving p16INK4A re-expression related to UHRF1 and DNMT1 down-regulation
Mounira Krifa, Mahmoud Alhosin, Christian D Muller, Jean-Pierre Gies, Leila Chekir-Ghedira, Kamel Ghedira, Yves Mély, Christian Bronner, Marc Mousli
Journal of Experimental & Clinical Cancer Research.2013;[Epub] CrossRef - Diagnostic and prognostic value of circulating tumor-related DNA in cancer patients
Diego M Marzese, Hajime Hirose, Dave S B Hoon
Expert Review of Molecular Diagnostics.2013; 13(8): 827. CrossRef - Epigallocatechin-3-gallate and trichostatin A synergistically inhibit human lymphoma cell proliferation through epigenetic modification of p16INK4a
DAN-SEN WU, JIAN-ZHEN SHEN, AI-FANG YU, HAI-YING FU, HUA-RONG ZHOU, SONG-FEI SHEN
Oncology Reports.2013; 30(6): 2969. CrossRef



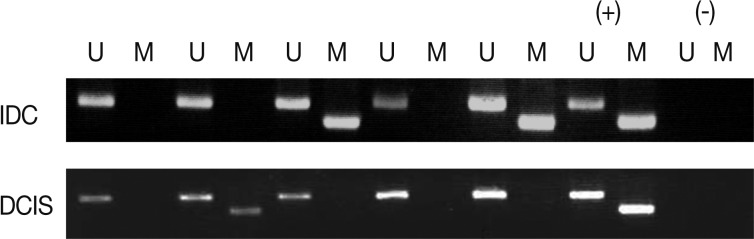
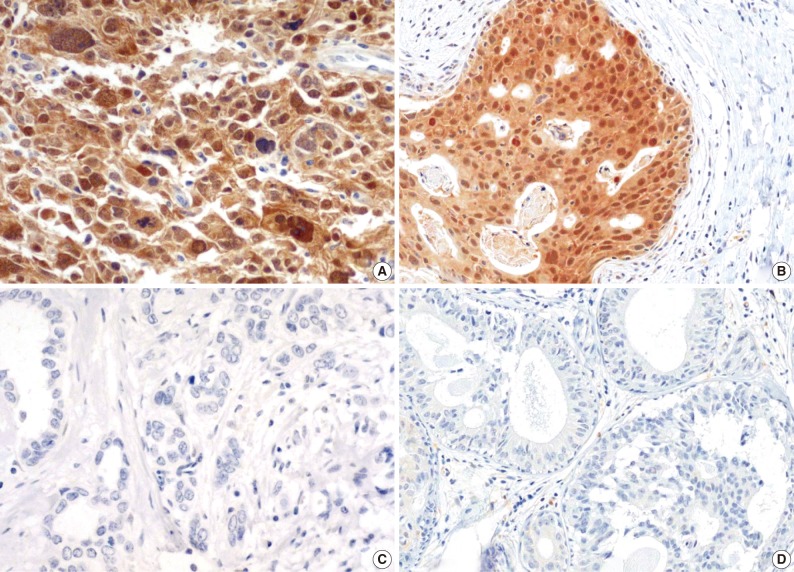
Fig. 1
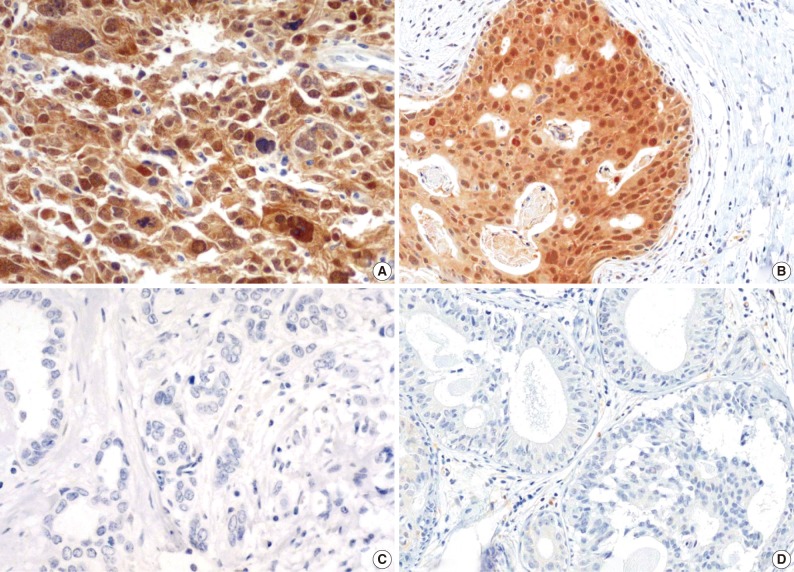
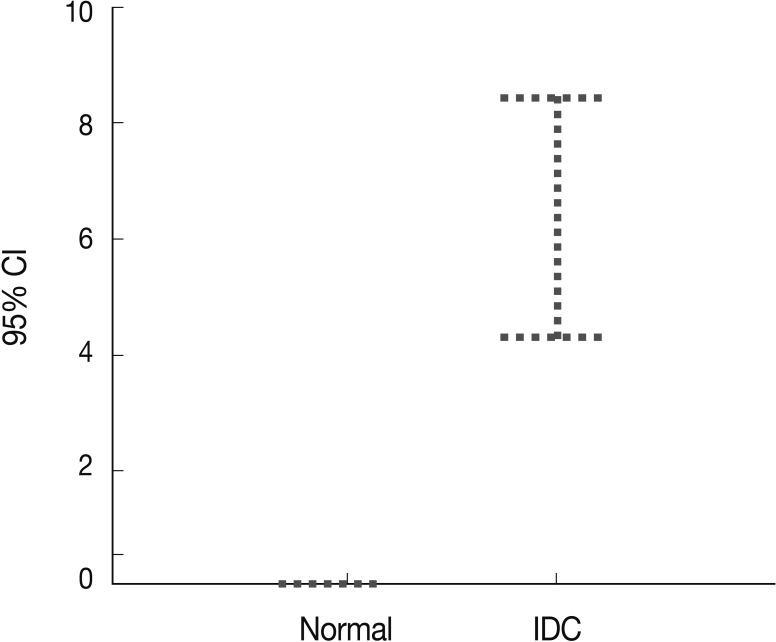
Fig. 2
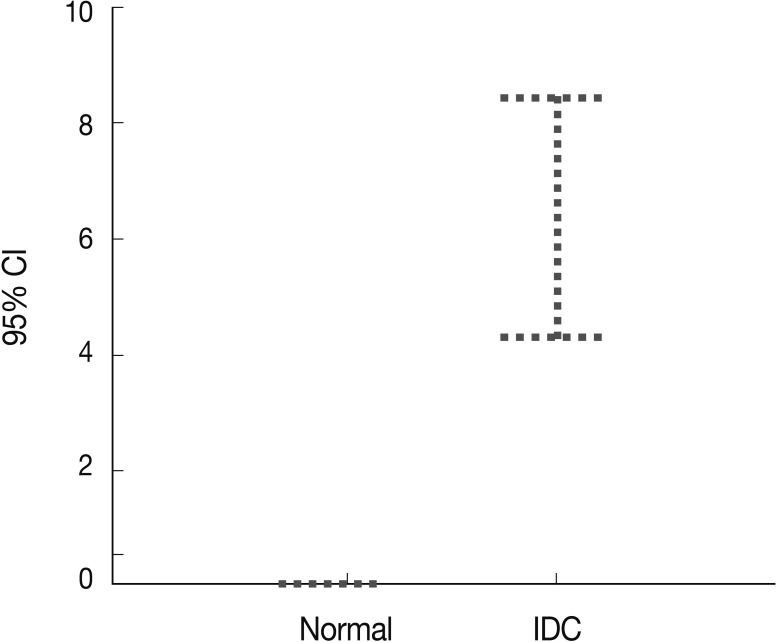
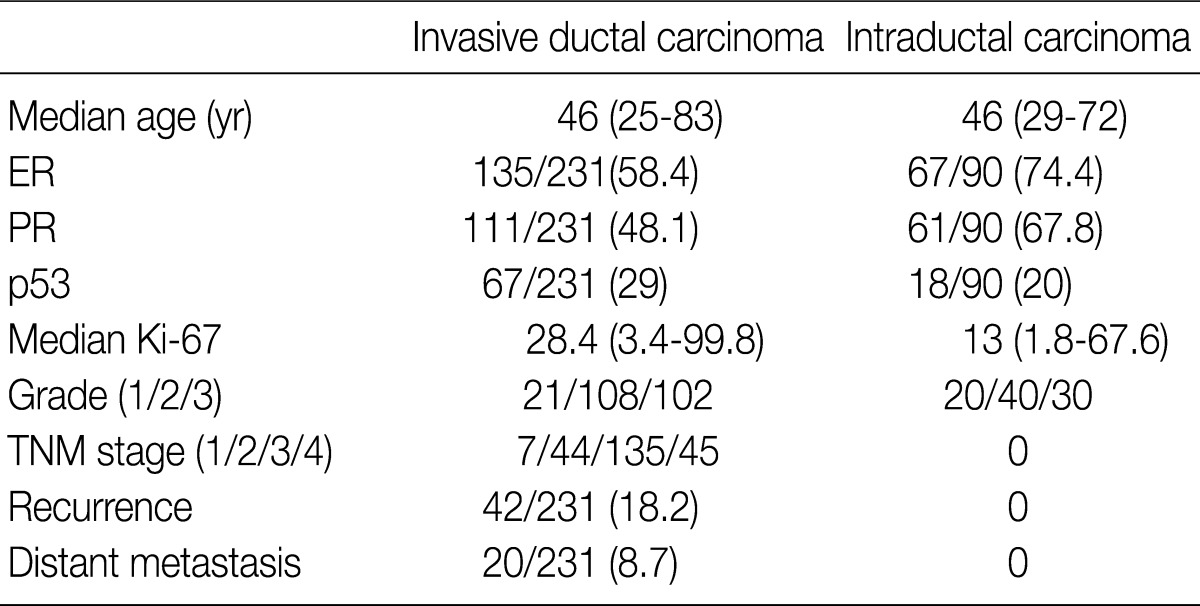
Fig. 3
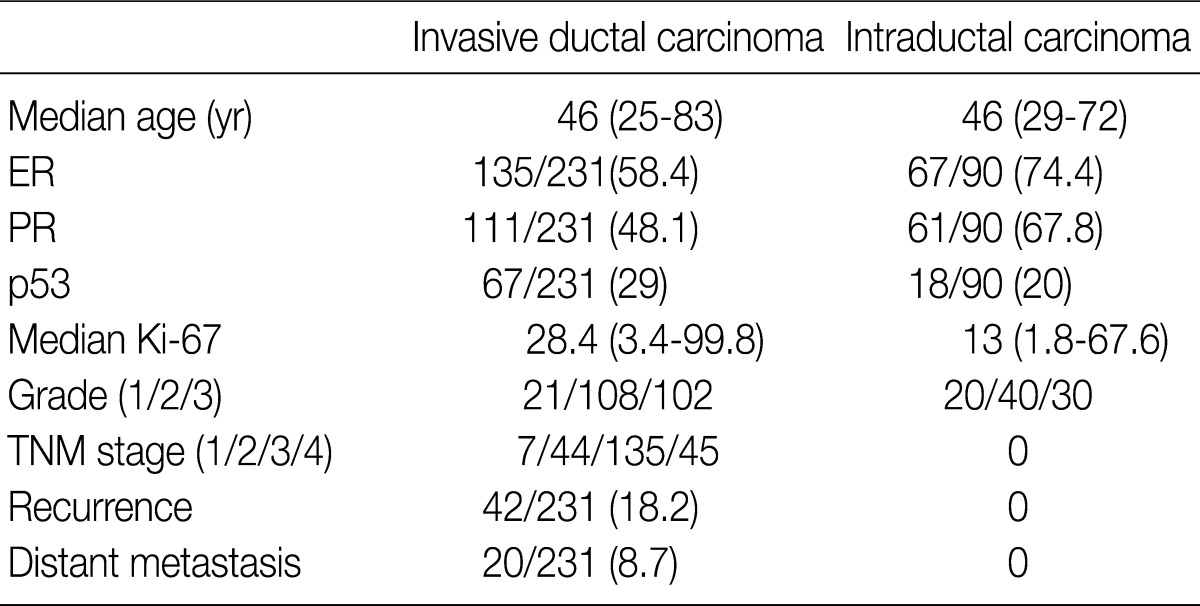
Values are presented as number (% or range). ER, estrogen receptor; PR, progesterone receptor; TNM, tumor-node-metastasis.
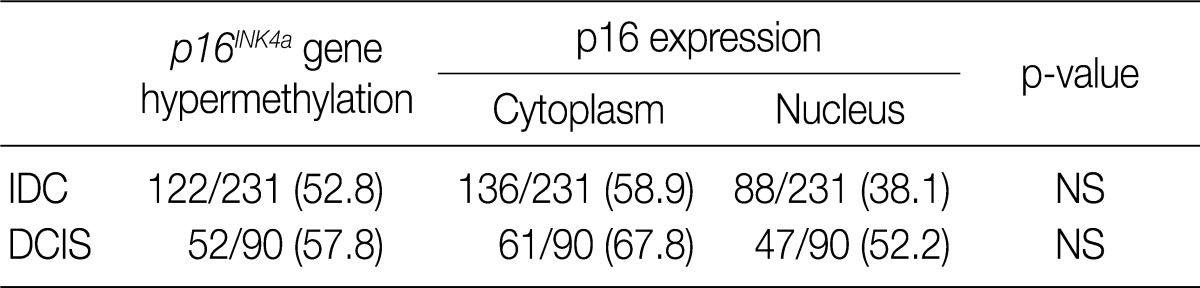
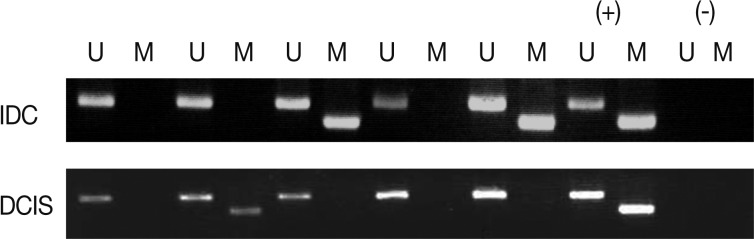
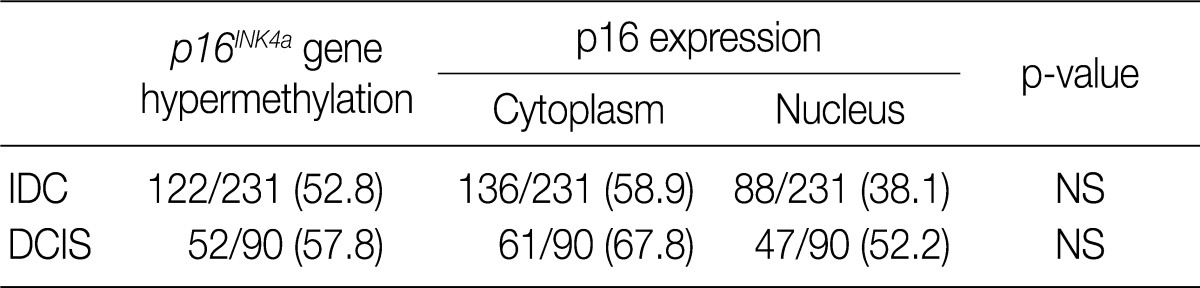
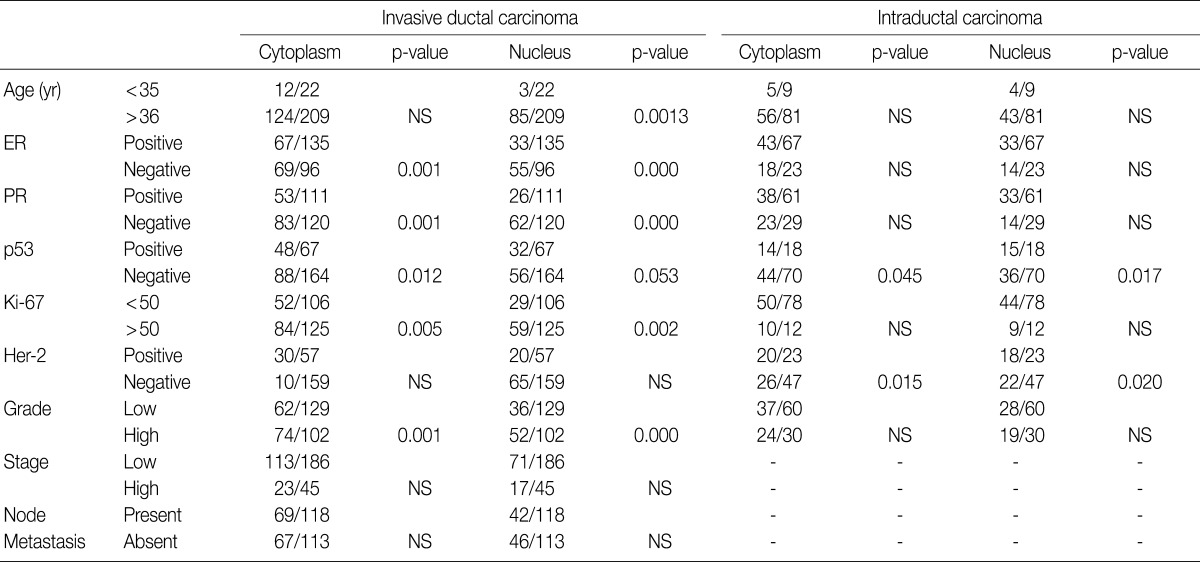
Values are presented as number (%). IDC, invasive ductal carcinoma; NS; not significant; DCIS, ductal carcinoma
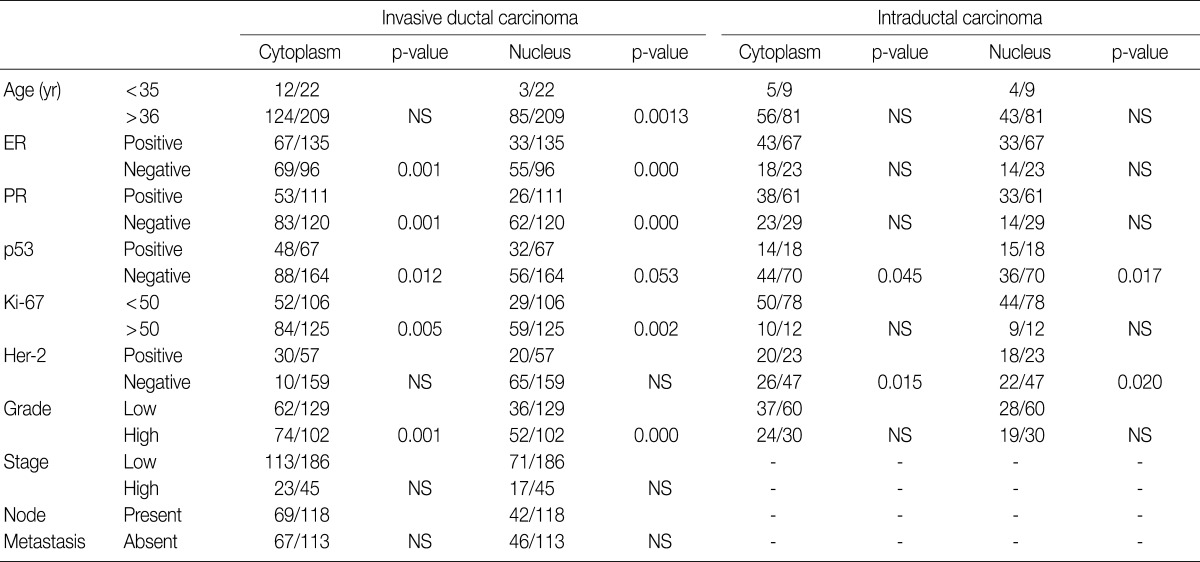
NS, not significant; ER, estrogen receptor; PR, progesterone receptor.

 E-submission
E-submission
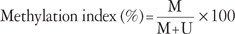
 PubReader
PubReader Cite this Article
Cite this Article