Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(2); 2013 > Article
-
Original Article
Prognostic Significance of BCL9 Expression in Hepatocellular Carcinoma - Jiyeon Hyeon, Soomin Ahn, Jae Jun Lee, Dae Hyun Song, Cheol-Keun Park
-
Korean Journal of Pathology 2013;47(2):130-136.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.2.130
Published online: April 24, 2013
Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- Corresponding Author: Cheol-Keun Park, M.D. Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea. Tel: +82-2-3410-2766, Fax: +82-2-3410-6396, ckpark@skku.edu
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- The Wnt-dependent and Wnt-independent functions of BCL9 in development, tumorigenesis, and immunity: Implications in therapeutic opportunities
Minjie Wu, Heng Dong, Chao Xu, Mengqing Sun, Haojin Gao, Fangtian Bu, Jianxiang Chen
Genes & Diseases.2024; 11(2): 701. CrossRef - The role of BCL9 genetic variation as a biomarker for hepatitis C-related hepatocellular carcinoma in Egyptian patients
Eman Abd El Razek Abbas, Ahmed Barakat Barakat, Mohamed Hassany, Samar Samir Youssef
Journal of Genetic Engineering and Biotechnology.2022; 20(1): 4. CrossRef - Molecular Targets and Signaling Pathways of microRNA-122 in Hepatocellular Carcinoma
Kwang-Hoon Chun
Pharmaceutics.2022; 14(7): 1380. CrossRef - Wnt/β-Catenin Signalling and Its Cofactor BCL9L Have an Oncogenic Effect in Bladder Cancer Cells
Roland Kotolloshi, Mieczyslaw Gajda, Marc-Oliver Grimm, Daniel Steinbach
International Journal of Molecular Sciences.2022; 23(10): 5319. CrossRef - Bcl9 Depletion Modulates Endothelial Cell in Tumor Immune Microenvironment in Colorectal Cancer Tumor
Zhuang Wei, Mei Feng, Zhongen Wu, Shuru Shen, Di Zhu
Frontiers in Oncology.2021;[Epub] CrossRef - Wnt Signaling Pathway Is among the Drivers of Liver Metastasis
Ivana Samaržija
Livers.2021; 1(4): 180. CrossRef - Nuclear Expression of Pygo2 Correlates with Poorly Differentiated State Involving c-Myc, PCNA and Bcl9 in Myanmar Hepatocellular Carcinoma
Myo Win Htun, Yasuaki Shibata, Kyaw Soe, Takehiko Koji
ACTA HISTOCHEMICA ET CYTOCHEMICA.2021; 54(6): 195. CrossRef - Wnt status-dependent oncogenic role of BCL9 and BCL9L in hepatocellular carcinoma
Nicole Huge, Maria Sandbothe, Anna K. Schröder, Amelie Stalke, Marlies Eilers, Vera Schäffer, Brigitte Schlegelberger, Thomas Illig, Beate Vajen, Britta Skawran
Hepatology International.2020; 14(3): 373. CrossRef - Structure and function of Pygo in organ development dependent and independent Wnt signalling
Yan Shi, Xiushan Wu, Shuoji Zhu, Huanlei Huang, Jian Zhuang, Haiyun Yuan, Wuzhou Yuan, Ping Zhu
Biochemical Society Transactions.2020; 48(4): 1781. CrossRef - BCL9/BCL9L in hepatocellular carcinoma: will it or Wnt it be the next therapeutic target?
Akshata Moghe, Satdarshan P. Monga
Hepatology International.2020; 14(4): 460. CrossRef - Loss of BCL9/9l suppresses Wnt driven tumourigenesis in models that recapitulate human cancer
David M. Gay, Rachel A. Ridgway, Miryam Müller, Michael C. Hodder, Ann Hedley, William Clark, Joshua D. Leach, Rene Jackstadt, Colin Nixon, David J. Huels, Andrew D. Campbell, Thomas G. Bird, Owen J. Sansom
Nature Communications.2019;[Epub] CrossRef - Immunohistochemical Mapping of Bcl9 Using Two Antibodies that Recognize Different Epitopes Is Useful to Characterize Juvenile Development of Hepatocellular Carcinoma in Myanmar
Myat Thu Soe, Yasuaki Shibata, Myo Win Htun, Kuniko Abe, Kyaw Soe, Nay Win Than, Thann Lwin, Myat Phone Kyaw, Takehiko Koji
ACTA HISTOCHEMICA ET CYTOCHEMICA.2019; 52(1): 9. CrossRef - Low BCL9 expression inhibited ovarian epithelial malignant tumor progression by decreasing proliferation, migration, and increasing apoptosis to cancer cells
Jing Wang, Mingjun Zheng, Liancheng Zhu, Lu Deng, Xiao Li, Linging Gao, Caixia Wang, Huimin Wang, Juanjuan Liu, Bei Lin
Cancer Cell International.2019;[Epub] CrossRef - SOX7 Suppresses Wnt Signaling by Disrupting β-Catenin/BCL9 Interaction
Rong Fan, HaiYan He, Wang Yao, YanFeng Zhu, XunJie Zhou, MingTai Gui, Jing Lu, Hao Xi, ZhongLong Deng, Min Fan
DNA and Cell Biology.2018; 37(2): 126. CrossRef - Hypoxia activates Wnt/β-catenin signaling by regulating the expression of BCL9 in human hepatocellular carcinoma
Wei Xu, Wang Zhou, Mo Cheng, Jing Wang, Zhian Liu, Shaohui He, Xiangji Luo, Wending Huang, Tianrui Chen, Wangjun Yan, Jianru Xiao
Scientific Reports.2017;[Epub] CrossRef - Hepatocellular carcinoma biology predicts survival outcome after liver transplantation in the USA
Mohamed Abd El-Fattah
Indian Journal of Gastroenterology.2017; 36(2): 117. CrossRef - miR-30a acts as a tumor suppressor by double-targeting COX-2 and BCL9 in H. pylori gastric cancer models
Xuan Liu, Qing Ji, Chengcheng Zhang, Xiaowei Liu, Yanna Liu, Ningning Liu, Hua Sui, Lihong Zhou, Songpo Wang, Qi Li
Scientific Reports.2017;[Epub] CrossRef - BCL9, a coactivator for Wnt/β-catenin transcription, is targeted by miR-30c and is associated with prostate cancer progression
XIAO-HUI LING, ZHI-YUN CHEN, HONG-WEI LUO, ZE-ZHEN LIU, YING-KE LIANG, GUAN-XING CHEN, FU-NENG JIANG, WEI-DE ZHONG
Oncology Letters.2016; 11(3): 2001. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles


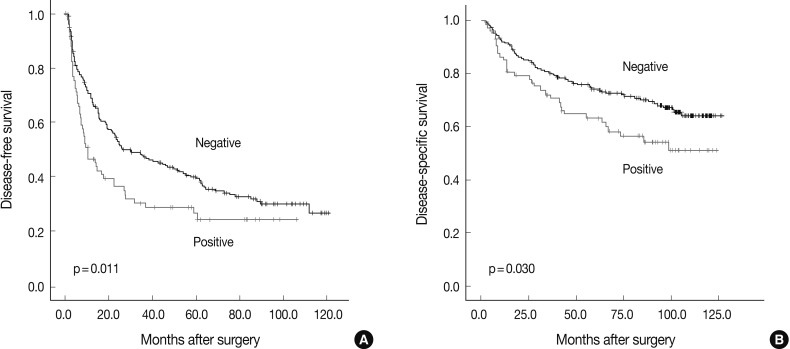
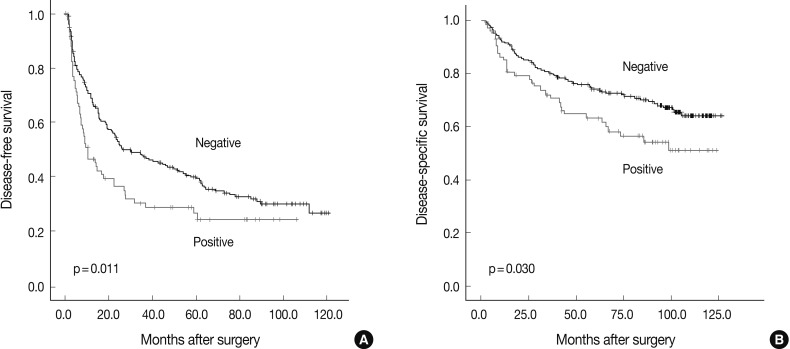
Fig. 1
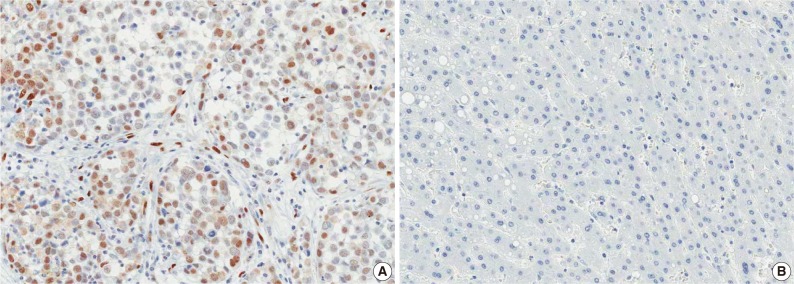
Fig. 2
| Variable | n | Tumor recurrence | p-value | BCL9 expression | p-value |
|---|---|---|---|---|---|
| Age (yr) | 0.151 | 0.038 | |||
| ≤ 55 | 165 | 114 (69.1) | 50 (30.3) | ||
| > 55 | 123 | 75 (61.0) | 24 (19.5) | ||
| Gender | 0.619 | 0.457 | |||
| Male | 237 | 154 (65.0) | 63 (26.6) | ||
| Female | 51 | 35 (68.6) | 11 (21.6) | ||
| Tumor size (cm) | 0.011 | 0.816 | |||
| ≤ 5.0 | 190 | 115 (60.5) | 48 (25.3) | ||
| > 5.0 | 98 | 74 (75.5) | 26 (26.5) | ||
| Edmondson grade | 0.029 | 0.001 | |||
| I | 30 | 20 (66.7) | 3 (10.0) | ||
| II | 195 | 119 (61.0) | 44 (22.6) | ||
| III | 63 | 50 (79.4) | 27 (42.9) | ||
| Microvascular invasion | 0.001 | 0.013 | |||
| (-) | 129 | 71 (55.0) | 24 (18.6) | ||
| (+) | 159 | 118 (74.2) | 50 (31.4) | ||
| Major portal vein invasion | 0.038 | 0.084 | |||
| (-) | 275 | 177 (64.4) | 68 (24.7) | ||
| (+) | 13 | 12 (92.3) | 6 (46.2) | ||
| Intrahepatic metastasis | < 0.001 | 0.017 | |||
| (-) | 220 | 127 (57.7) | 49 (22.3) | ||
| (+) | 68 | 62 (91.2) | 24 (36.8) | ||
| Multicentric occurrence | 0.815 | 0.419 | |||
| (-) | 269 | 177 (65.8) | 71 (26.4) | ||
| (+) | 19 | 12 (63.2) | 3 (15.8) | ||
| AJCC T stage | < 0.001 | 0.098 | |||
| 1 | 121 | 67 (55.4) | 23 (19.0) | ||
| 2 | 117 | 76 (65.0) | 34 (29.1) | ||
| 3 | 44 | 41 (93.2) | 14 (31.8) | ||
| 4 | 6 | 5 (83.3) | 3 (50.0) | ||
| BCLC stage | 0.004 | 0.378 | |||
| 0-A | 164 | 95 (57.9) | 39 (23.8) | ||
| B | 109 | 81 (74.3) | 29 (26.6) | ||
| C | 15 | 13 (86.7) | 6 (40.0) | ||
| Albumin level (g/dL) | 0.179 | 0.312 | |||
| > 3.5 | 258 | 166 (64.3) | 64 (24.8) | ||
| ≤ 3.5 | 30 | 23 (76.7) | 10 (33.3) | ||
| AFP level (ng/mL) | 0.002 | 0.091 | |||
| ≤ 200 | 173 | 102 (59.0) | 39 (22.5) | ||
| > 200 | 104 | 80 (76.9) | 33 (31.7) | ||
| Etiology | 0.004 | 0.391 | |||
| Non-viral | 40 | 17 (42.5) | 7 (17.5) | ||
| HBV | 218 | 150 (68.8) | 60 (27.5) | ||
| HCV | 30 | 22 (73.3) | 7 (25.7) | ||
| Liver cirrhosis | 0.009 | 0.590 | |||
| (-) | 144 | 84 (58.3) | 35 (24.3) | ||
| (+) | 144 | 105 (72.9) | 39 (27.1) |
| Variable | n | Disease-free survival |
Disease-specific survival |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |||
| Tumor size (cm) | ≤ 5.0 | 190 | < 0.001 | < 0.001 | ||
| > 5.0 | 98 | 1.737 (1.296-2.329) | 2.952 (1.987-4.386) | |||
| Edmondson grade | I+II | 225 | < 0.001 | 0.001 | ||
| III | 63 | 1.856 (1.340-2.570) | 2.047 (1.338-3.133) | |||
| Microvascular invasion | (-) | 129 | < 0.001 | < 0.001 | ||
| (+) | 159 | 2.134 (1.587-2.869) | 3.056 (1.952-4.786) | |||
| Major portal vein invasion | (-) | 275 | < 0.001 | < 0.001 | ||
| (+) | 13 | 3.905 (2.165-7.043) | 5.530 (2.855-10.711) | |||
| Intrahepatic metastasis | (-) | 220 | < 0.001 | < 0.001 | ||
| (+) | 68 | 4.624 (3.360-6.364) | 5.580 (3.729-8.350) | |||
| Multicentric occurrence | (-) | 269 | 0.328 | 0.319 | ||
| (+) | 19 | 1.340 (0.746-2.409) | 0.601 (0.221-1.635) | |||
| AJCC T stage | 1 | 121 | < 0.001 | < 0.001 | ||
| 2+3+4 | 167 | 2.173 (1.610-2.934) | 3.090 (1.949-4.898) | |||
| BCLC stage | 0+A | 164 | < 0.001 | < 0.001 | ||
| B+C | 124 | 2.141 (1.606-2.853) | 3.732 (2.460-5.661) | |||
| Albumin level (g/dL) | > 3.5 | 258 | 0.008 | 0.001 | ||
| ≤ 3.5 | 30 | 1.813 (1.170-2.809) | 2.430 (1.418-4.163) | |||
| AFP level (ng/mL) | ≤ 200 | 173 | 0.002 | 0.033 | ||
| > 200 | 104 | 1.604 (1.197-2.150) | 1.552 (1.036-2.324) | |||
| Etiology | Non-viral | 40 | 0.025 | 0.154 | ||
| viral | 248 | 1.390 (1.043-1.852) | 1.609 (0.836-3.094) | |||
| Liver cirrhosis | (-) | 144 | 0.004 | 0.554 | ||
| (+) | 144 | 2.094 (1.271-3.449) | 1.126 (0.759-1.671) | |||
| BCL9 expression | (-) | 214 | 0.012 | 0.032 | ||
| (+) | 74 | 1.508 (1.096-2.075) | 1.589 (1.042-2.423) | |||
| Variable | n | Disease-free survival |
Disease-specific survival |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |||
| Edmondson grade | I+II | 225 | 0.146 | 0.213 | ||
| III | 63 | 1.298 (0.913-1.843) | 1.335 (0.847-2.104) | |||
| BCLC stage | 0+A | 164 | < 0.001 | < 0.001 | ||
| B+C | 124 | 2.279 (1.678-3.096) | 4.034 (2.594-6.273) | |||
| Etiology | Non-viral | 40 | 0.022 | 0.241 | ||
| Viral | 248 | 1.833 (1.092-3.077) | 1.509 (0.759-3.001) | |||
| Liver cirrhosis | (-) | 144 | 0.011 | 0.068 | ||
| (+) | 144 | 1.488 (1.095-2.023) | 1.488 (0.971-2.282) | |||
| BCL9 expression | (-) | 214 | 0.078 | 0.115 | ||
| (+) | 74 | 1.351 (0.967-1.888) | 1.428 (0.917-2.225) | |||
Values are presented as number (%). AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; AFP, α-fetoprotein; HBV, hepatitis B virus; HCV, hepatitis C virus.
HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; AFP, α-fetoprotein.
HR, hazard ratio; CI, confidence interval; BCLC, Barcelona Clinic Liver Cancer.

 E-submission
E-submission