Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(6); 2013 > Article
-
Original Article
Diagnostic Accuracy of Cerebrospinal Fluid (CSF) Cytology in Metastatic Tumors: An Analysis of Consecutive CSF Samples - Yoon Sung Bae1, June-Won Cheong2, Won Seok Chang3,4, Sewha Kim1, Eun Ji Oh1, Se Hoon Kim1
-
Korean Journal of Pathology 2013;47(6):563-568.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.6.563
Published online: December 24, 2013
1Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
2Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
3Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea.
4Brain Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- Corresponding Author: Se Hoon Kim, M.D. Department of Pathology, Yonsei University College of Medicine, 50 Yonsei-ro, Seodaemun-gu, Seoul 120-752, Korea. Tel: +82-2-2228-1769, Fax: +82-2-362-0860, paxco@yuhs.ac
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Analytical validation of the Belay Vantage™ assay for evaluation of MGMT promoter methylation using enzymatically converted tumorDNA from cerebrospinal fluid
Kala F Schilter, Qian Nie, Jennifer N Adams, Rakshitha Jagadish, Anthony Acevedo, Alexandra Larson, Samantha A Vo, Brett A Domagala, Kyle M Hernandez, Christopher Douville, Yuxuan Wang, Brian Coe, Chetan Bettegowda, Honey V Reddi
Cancer Genetics.2025; 294-295: 94. CrossRef - Analytical Validation and Clinical Sensitivity of the Belay Summit Assay for the Detection of DNA Variants in Cerebrospinal Fluid of Primary and Metastatic Central Nervous System Cancer
Qian Nie, Kala F. Schilter, Kyle M. Hernandez, Jennifer N. Adams, Rakshitha Jagadish, Anthony Acevedo, Alexandra Larson, Brett A. Domagala, Samantha A. Vo, Sakshi Khurana, Kathleen Mitchell, Dean Ellis, Baymuhammet Muhammedov, Yuxuan Wang, Christopher Dou
The Journal of Molecular Diagnostics.2025; 27(7): 615. CrossRef - Demonstrating the clinical utility of genomic profiling using cerebrospinal fluid to inform management of central nervous system tumors – a meta analysis of the literature
Sakshi Khurana, Qian Nie, Kala F. Schilter, Honey V. Reddi
The Journal of Liquid Biopsy.2025; 9: 100317. CrossRef - Application of the International System for Serous Fluid Cytopathology in Cerebrospinal Fluid Cytology
Ioannina Vidali, Konstantinos Christofidis, Georgia Bairaktari, Maria Sevastiadou, Alexandros Pergaris, Aglaia Dimitrakopoulou, Panagiota Keramari, Panagiota Mikou
Cytopathology.2025; 36(6): 589. CrossRef - The Spectrum of Malignant Diagnoses in Cerebrospinal Fluid (CSF) Cytology in Both Pediatric and Adult Populations: A Single‐Institutional Retrospective Review
Nida Babar, Asif Loya, Sajid Mushtaq, Maryam Hameed, Usman Hassan, Mudassar Hussain
Diagnostic Cytopathology.2025; 53(12): 620. CrossRef - Molecular Analysis of Cerebrospinal Fluid Tumor-Derived DNA to Aid in the Diagnosis and Targeted Treatment of Breast Cancer Brain Metastasis
Michael Youssef, Alexandra Larson, Vindhya Udhane, Viriya Keo, Kala F. Schilter, Qian Nie, Honey V. Reddi
Diseases.2025; 13(10): 336. CrossRef - Numb cheek syndrome in breast cancer: a case report
Zhibin Tan, Si Ying Tan
Frontiers in Oncology.2024;[Epub] CrossRef - Utility and performance of cell blocks in cerebrospinal fluid cytology: Experience at two teaching hospitals
Hyeji Yoon, Constance V. Chen, Vimal Krishnan, Jill Grochowski, Gioia Iezza, Poonam Vohra, Ronald Balassanian, Nancy Y. Greenland
Cancer Cytopathology.2024; 132(10): 621. CrossRef - Liquid biopsy for evaluating mutations and chromosomal aberrations in cerebrospinal fluid from patients with primary or metastatic CNS tumors
Ahmad Charifa, Sally Agersborg, Arash Mohtashamian, Andrew Ip, Andre Goy, Maher Albitar
The Journal of Liquid Biopsy.2024; 6: 100281. CrossRef - Body fluids
Shyam H. Nemade, Meherbano M. Kamal
Indian Journal of Pathology and Microbiology.2023; 66(1): 75. CrossRef - Standardizing a volume benchmark for cerebrospinal fluids for optimal diagnostic accuracy
David Kim, Susan A. Alperstein, Momin T. Siddiqui
Diagnostic Cytopathology.2021; 49(2): 258. CrossRef - Evaluating Infectious, Neoplastic, Immunological, and Degenerative Diseases of the Central Nervous System with Cerebrospinal Fluid-Based Next-Generation Sequencing
Konstantinos I. Tsamis, Hercules Sakkas, Alexandros Giannakis, Han Suk Ryu, Constantina Gartzonika, Ilias P. Nikas
Molecular Diagnosis & Therapy.2021; 25(2): 207. CrossRef - Imaging of Intraspinal Tumors
Luke N. Ledbetter, John D. Leever
Radiologic Clinics of North America.2019; 57(2): 341. CrossRef - Isolated leptomeningeal carcinomatosis and possible fungal meningitis as late sequelae of oesophageal adenocarcinoma
Richard Dumbill, Sanja Thompson, Heiko Peschl, GDH Turner, Charles Woodrow
BMJ Case Reports.2019; 12(11): e230117. CrossRef - Cytomorphological and immunocytochemical examinations of cerebrospinal fluid in primary and metastatic brain lesions
M. V. Savostikova, L. Ya. Fomina, E. S. Fedoseeva, E. Yu. Furminskaya
Onkologiya. Zhurnal imeni P.A.Gertsena.2018; 7(1): 28. CrossRef - Metastatic Breast Carcinoma in Cerebrospinal Fluid: A Cytopathological Review of 15 Cases
Rema Rao, Syed A. Hoda, Alan Marcus, Rana S. Hoda
The Breast Journal.2017; 23(4): 456. CrossRef - Clinicocytological analysis of cases with positive cerebrospinal fluid in our hospital
Nozomi IWAMOTO, Mitsuaki ISHIDA, Akiko KAGOTANI, Nozomi KASUGA, Muneo IWAI, Yuji HAYASHI, Namie ARITA, Yoshimitsu MIYAHIRA, Ryoji KUSHIMA
The Journal of the Japanese Society of Clinical Cytology.2016; 55(5): 291. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


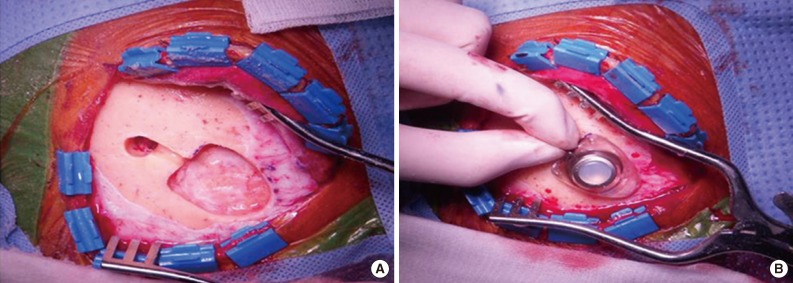
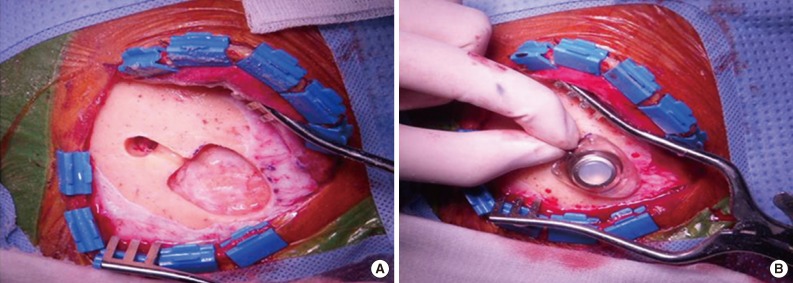
Fig. 1
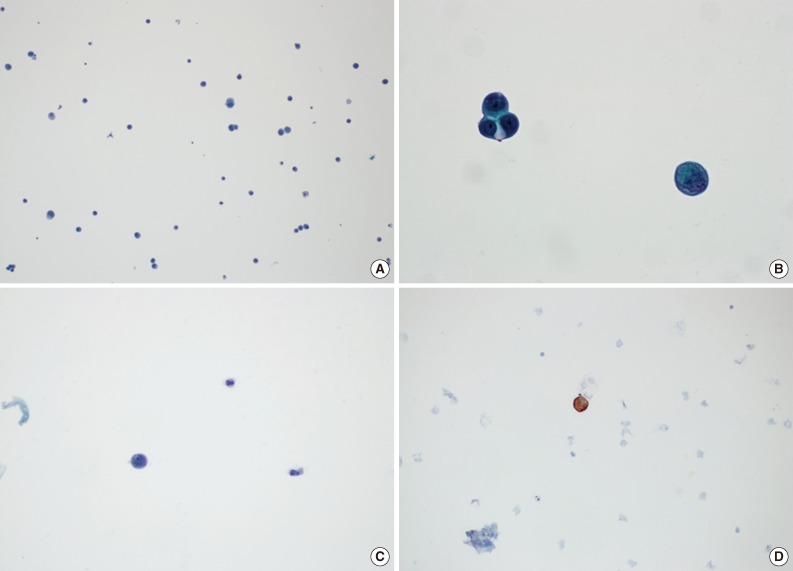
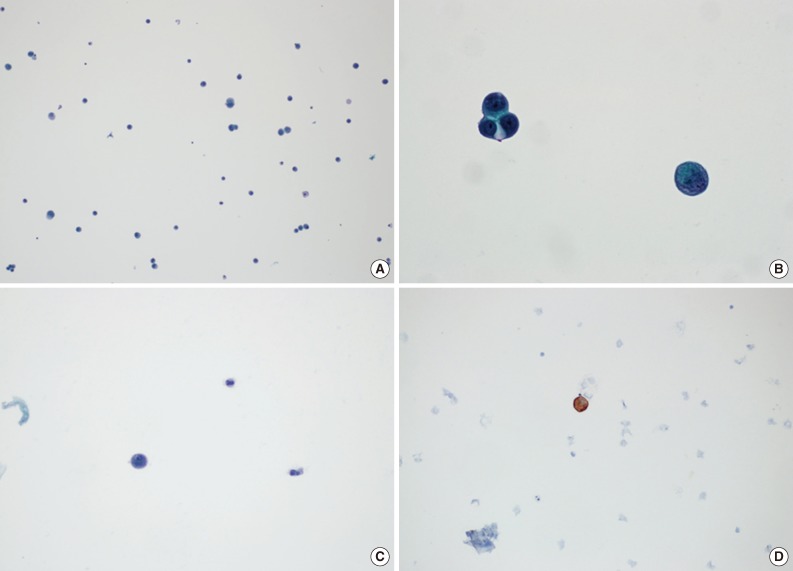
Fig. 2
| Case No. | Sex | Age (yr) | Primary site | Histologic type | Period (mo) | Total | Negative rate | Positive rate |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 48 | Lung | Adenocarcinoma | 1 | 6 | 3 (50.0) | 3 (50.0) |
| 2 | M | 63 | Lung | Adenocarcinoma | 1 | 4 | 2 (50.0) | 2 (50.0) |
| 3 | F | 60 | Ovary | Serous papillary carcinoma | 8 | 13 | 10 (76.9) | 3 (23.1) |
| 4 | M | 64 | Lung | Adenocarcinoma | 2 | 13 | 7 (53.8) | 6 (46.2) |
| 5 | M | 56 | Rectum | Adenocarcinoma | 7 | 12 | 12 (100.0) | 0 (0) |
| 6 | F | 56 | Breast | Invasive ductal carcinoma | 5 | 11 | 10 (90.9) | 1 (9.1) |
| 7 | F | 43 | Lung | Adenocarcinoma | 3 | 13 | 8 (61.5) | 5 (38.5) |
| 8 | M | 58 | Lung | Adenocarcinoma | 11 | 22 | 19 (86.0) | 3 (14.0) |
| 9 | M | 52 | Kidney | Renal cell carcinoma | 5 | 9 | 9 (100.0) | 0 (0.0) |
| 10 | F | 66 | Lung | Adenocarcinoma | 22 | 22 | 21 (95.5) | 1 (4.5) |
| 11 | M | 66 | Lung | Adenocarcinoma | 1 | 2 | 0 (0.0) | 2 (100.0) |
| 12 | M | 58 | Lung | Adenocarcinoma | 2 | 15 | 5 (33.3) | 10 (66.7) |
| 13 | M | 62 | Lung | Adenocarcinoma | 21 | 34 | 18 (52.9) | 16 (47.1) |
| 14 | M | 65 | Stomach | Adenocarcinoma | 1 | 7 | 2 (28.6) | 5 (71.4) |
| 15 | M | 66 | Lung | Adenocarcinoma | 6 | 15 | 12 (80.0) | 3 (20.0) |
| 16 | F | 58 | Lung | Adenocarcinoma | 4 | 17 | 11 (64.7) | 6 (35.3) |
| 17 | M | 29 | Stomach | Signet ring cell carcinoma | 3 | 9 | 4 (44.4) | 5 (55.6) |
| 18 | M | 51 | Lung | Adenocarcinoma | 1 | 3 | 0 (0.0) | 3 (100.0) |
| 19 | F | 71 | Lung | Adenocarcinoma | 1 | 4 | 1 (25.0) | 3 (75.0) |
| 20 | F | 74 | Ovary | Serous papillary carcinoma | 3 | 8 | 0 (0.0) | 8 (100.0) |
| 21 | M | 64 | Stomach | Adenocarcinoma | 1 | 4 | 0 (0.0) | 4 (100.0) |
| 22 | M | 52 | Lung | Adenocarcinoma | 1 | 8 | 1 (12.5) | 7 (87.5) |
| 23 | F | 58 | Stomach | Adenocarcinoma | 1 | 3 | 0 (0.0) | 3 (100.0) |
| 24 | M | 65 | Lung | Adenocarcinoma | 9 | 14 | 12 (85.7) | 2 (14.3) |
| 25 | F | 47 | Breast | Invasive ductal carcinoma | 1 | 2 | 0 (0.0) | 2 (100.0) |
| 26 | F | 40 | Breast | Invasive ductal carcinoma | 11 | 7 | 2 (28.6) | 5 (71.4) |
| 27 | F | 56 | Breast | Invasive ductal carcinoma | 1 | 2 | 0 (0.0) | 2 (100.0) |
| 28 | M | 65 | Lung | Small cell carcinoma | 4 | 4 | 3 (75.0) | 1 (25.0) |
| 29 | M | 51 | Skin | Malignant melanoma | 1 | 2 | 0 (0.0) | 2 (100.0) |
| 30 | F | 46 | Breast | Invasive ductal carcinoma | 5 | 18 | 17 (94.4) | 1 (5.6) |
| 31 | M | 55 | Urinary bladder | Urothelial carcinoma | 1 | 2 | 1 (50.0) | 1 (50.0) |
| 32 | M | 41 | Lung | Adenocarcinoma | 2 | 11 | 3 (27.3) | 8 (72.7) |
| 33 | M | 53 | Lung | Adenocarcinoma | 2 | 8 | 7 (87.5) | 1 (12.5) |
| 34 | M | 55 | Lung | Adenocarcinoma | 8 | 7 | 1 (14.3) | 6 (85.7) |
| 35 | F | 46 | Breast | Invasive ductal carcinoma | 5 | 3 | 3 (100.0) | 0 (0.0) |
| 36 | M | 39 | Pancreas | Adenocarcinoma | 6 | 7 | 5 (71.4) | 2 (28.6) |
| 37 | F | 65 | Breast | Invasive ductal carcinoma | 5 | 13 | 6 (46.2) | 7 (53.8) |
| 38 | M | 31 | Skin | Malignant melanoma | 2 | 4 | 3 (75.0) | 1 (25.0) |
| 39 | F | 46 | Breast | Invasive ductal carcinoma | 1 | 4 | 2 (50.0) | 2 (50.0) |
| 40 | F | 49 | Lung | Adenocarcinoma | 10 | 8 | 3 (37.5) | 5 (62.5) |
| 41 | M | 49 | Lung | Adenocarcinoma | 1 | 9 | 0 (0.0) | 9 (100.0) |
| 42 | F | 77 | Lung | Adenocarcinoma | 1 | 6 | 3 (50.0) | 3 (50.0) |
| Mean | 5 | 385 | 226 (58.7) | 159 (41.3) |
| Case No. 4 | Cytological diagnosis | Date | Case No. 12 | Cytological diagnosis | Date |
|---|---|---|---|---|---|
| 1 | Negative for malignancy | Oct 20, 2010 | 1 | Positive for malignancy | Feb 5, 2013 |
| 2 | Positive for malignancy | Oct 23, 2010 | 2 | Positive for malignancy | Feb 15, 2013 |
| 3 | Negative for malignancy | Oct 30, 2010 | 3 | Negative for malignancy | Feb 25, 2013 |
| 4 | Positive for malignancy | Nov 2, 2010 | 4 | Positive for malignancy | Feb 27, 2013 |
| 5 | Positive for malignancy | Nov 8, 2010 | 5 | Positive for malignancy | Mar 5, 2013 |
| 6 | Negative for malignancy | Nov 10, 2010 | 6 | Positive for malignancy | Mar 6, 2013 |
| 7 | Suspicious for malignancy | Nov 23, 2010 | 7 | Negative for malignancy | Mar 12, 2013 |
| 8 | Positive for malignancy | Nov 24, 2010 | 8 | Positive for malignancy | Mar 14, 2013 |
| 9 | Negative for malignancy | Nov 24, 2010 | 9 | Positive for malignancy | Mar 18, 2013 |
| 10 | Negative for malignancy | Dec 1, 2010 | 10 | Negative for malignancy | Mar 21, 2013 |
| 11 | Suspicious for malignancy | Dec 3, 2010 | 11 | Positive for malignancy | Mar 26, 2013 |
| 12 | Negative for malignancy | Dec 9, 2010 | 12 | Negative for malignancy | Mar 27, 2013 |
| 13 | Negative for malignancy | Dec 16, 2010 | 13 | Positive for malignancy | Mar 29, 2013 |
| 14 | Negative for malignancy | Apr 2, 2013 | |||
| 15 | Positive for malignancy | Apr 3, 2013 |
Values are presented as number (%). M, male; F, female.

 E-submission
E-submission