Search
- Page Path
- HOME > Search
- Mucocele of the rectal stump: mucinous cystic neoplasm with low-grade dysplasia simulating low-grade appendiceal mucinous neoplasm
- Hasan Basri Aydin, Maria Faraz, A. David Chismark, Haiyan Qiu, Hwajeong Lee
- J Pathol Transl Med. 2025;59(2):139-146. Published online February 26, 2025
- DOI: https://doi.org/10.4132/jptm.2024.12.27
- 2,762 View
- 170 Download
-
 Abstract
Abstract
 PDF
PDF - Mucoceles, commonly observed in the appendix, are mucin-filled, dilated structures arising from a range of etiologies. Cases associated with dysplastic or neoplastic epithelium can rupture and disseminate within the abdominopelvic cavity. Similar lesions in other parts of the colon are exceedingly rare, with only 16 colonic mucoceles having been reported. The first case of a colonic mucinous neoplasm with dysplasia resembling a low-grade appendiceal mucinous neoplasm involving rectal stump was described in 2016. Here, we present the second such case arising in the rectal stump, identified in a 44-year-old male with extensive surgical history. Microscopic examination revealed low-grade dysplastic epithelium lining the cyst and mucin dissecting into the stroma, without evidence of rupture or extramural mucin. The patient was followed for 16 months without recurrence or peritoneal disease. The exact etiology and outcome of these rare lesions remain unknown, requiring close follow-up.
- Tubular adenoma arising in tubular colonic duplication: a case report
- Heonwoo Lee, Hyeong Rok An, Chan Wook Kim, Young Soo Park
- J Pathol Transl Med. 2024;58(4):198-200. Published online July 3, 2024
- DOI: https://doi.org/10.4132/jptm.2024.06.04
- 4,056 View
- 220 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - Colonic duplication constitutes a rare congenital anomaly, characterized by the presence of hollow cystic or tubular structures exhibiting an epithelial-lined intestinal wall. Diagnostic challenges persist due to its low incidence and manifestation of nonspecific symptoms such as abdominal pain or constipation, resulting in a reluctance to pursue surgical resection. As associated malignancies in colonic duplication are rare, the inherent malignant potential of these anomalies remains undetermined. Additionally, despite reported instances of associated malignancies in colonic duplication, there is an absence of reports in the literature detailing tubular adenoma within these cases. The histologic features of the presented case are particularly noteworthy, situated at the precancerous stage, intimating potential progression towards adenocarcinoma within colonic duplication.
-
Citations
Citations to this article as recorded by- Low-grade mucinous neoplasm originating from intestinal duplication: a case report and review of the literature
Huihui Yin, Jie Yu, Yunzhao Chen
World Journal of Surgical Oncology.2025;[Epub] CrossRef
- Low-grade mucinous neoplasm originating from intestinal duplication: a case report and review of the literature
- Evolving pathologic concepts of serrated lesions of the colorectum
- Jung Ho Kim, Gyeong Hoon Kang
- J Pathol Transl Med. 2020;54(4):276-289. Published online June 26, 2020
- DOI: https://doi.org/10.4132/jptm.2020.04.15
- 18,122 View
- 841 Download
- 36 Web of Science
- 34 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Here, we provide an up-to-date review of the histopathology and molecular pathology of serrated colorectal lesions. First, we introduce the updated contents of the 2019 World Health Organization classification for serrated lesions. The sessile serrated lesion (SSL) is a new diagnostic terminology that replaces sessile serrated adenoma and sessile serrated polyp. The diagnostic criteria for SSL were revised to require only one unequivocal distorted serrated crypt, which is sufficient for diagnosis. Unclassified serrated adenomas have been included as a new category of serrated lesions. Second, we review ongoing issues concerning the morphology of serrated lesions. Minor morphologic variants with distinct molecular features were recently defined, including serrated tubulovillous adenoma, mucin-rich variant of traditional serrated adenoma (TSA), and superficially serrated adenoma. In addition to intestinal dysplasia and serrated dysplasia, minimal deviation dysplasia and not otherwise specified dysplasia were newly suggested as dysplasia subtypes of SSLs. Third, we summarize the molecular features of serrated lesions. The critical determinant of CpG island methylation development in SSLs is patient age. Interestingly, there may be ethnic differences in BRAF/KRAS mutation frequencies in SSLs. The molecular pathogenesis of TSAs is divided into KRAS and BRAF mutation pathways. SSLs with MLH1 methylation can progress into favorable prognostic microsatellite instability-positive (MSI+)/CpG island methylator phenotype-positive (CIMP+) carcinomas, whereas MLH1-unmethylated SSLs and BRAF-mutated TSAs can be precursors of poor-prognostic MSI−/CIMP+ carcinomas. Finally, based on our recent data, we propose an algorithm for stratifying risk subgroups of non-dysplastic SSLs.
-
Citations
Citations to this article as recorded by- Predominant Serrated Molecular Signature in Postcolonoscopy Colorectal Cancer: A Systematic Review and Meta-Analysis
Jen-Hao Yeh, Sin-Hua Moi, Chia-Chi Chen, Chao-Wen Hsu, Wen-Shuo Yeh, Tzu-Ning Tseng, Chuan-Pin Lin, Yu-Peng Liu, Jaw-Yuan Wang
American Journal of Gastroenterology.2026; 121(1): 122. CrossRef - Clinical and endoscopic characteristics of colorectal traditional serrated adenomas with dysplasia/adenocarcinoma in a Korean population
Ki-Hyun Kim, Eun Myung, Hyung Hoon Oh, Chan-Muk Im, Young-Eun Seo, Je-Seong Kim, Chae-June Lim, Ga-Ram You, Sung-Bum Cho, Wan-Sik Lee, Myung-Giun Noh, Kyung-Hwa Lee, Young-Eun Joo
World Journal of Gastrointestinal Oncology.2025;[Epub] CrossRef - MicroRNA: role in macrophage polarisation and colorectal cancer pathogenesis
Haihong Lin, Jun Zhou, Ying He, Yifan Zhu, Puwen Chen, Hongwei Yan, Junyun Huang, Ersheng Gong, Xiaoling Wang
Frontiers in Cell and Developmental Biology.2025;[Epub] CrossRef - Submucosal fibrosis in large colorectal serrated lesions in cases receiving endoscopic submucosal dissection
Erik Manriquez-Alegria, Naohisa Yoshida, Reo Kobayashi, Naoto Iwai, Ken Inoue, Osamu Dohi, Lucas Cardoso, Hideyuki Konishi
Therapeutic Advances in Gastroenterology.2025;[Epub] CrossRef - Navigating the Colorectal Cancer Maze: Unveiling Pathways To Diagnosis, Management, Pathophysiology and Prevention
Khalid Ali Mohammed Al Kamzari, Constantina Constantinou
Current Oncology Reports.2025; 27(10): 1115. CrossRef - Fosl1 is a transcriptional effector of BRAFV600E-driven intestinal tumorigenesis
Zakia Alam, Rebecca Nightingale, Analia Lesmana, Cheng Liu, Laura J. Jenkins, Mark F. Richardson, Lawrence Croft, Ian Y. Luk, Camilla M. Reehorst, Fiona Chionh, Natalia Vukelic, Faiza Basheer, Eugene Tulchinsky, Joshua Badshah, Troy Dumenil, Latifa Bakiri
iScience.2025; 28(11): 113875. CrossRef - Sessile Serrated Lesions in Inflammatory Bowel Disease: Hidden Players in Colitis-Associated Colorectal Cancer?
Roberto de Sire, Diletta De Deo, Miriana Mercurio, Gianluca Franchellucci, Giulio Calabrese, Livio Bonacci, Mauro Sollai Pinna, Cristina Bezzio, Alessandro Armuzzi, Cesare Hassan, Alessandro Repici, Fabiana Castiglione, Sandro Ardizzone, Roberta Maselli
Journal of Clinical Medicine.2025; 14(22): 8042. CrossRef - Histologic Reappraisal and Evaluation of MLH1 Protein Expression in Sessile Serrated Lesions of the Proximal Colon
Priscilla de Sene Portel Oliveira, Miriam Aparecida da Silva Trevisan, Rita Barbosa de Carvalho, Rita de Cássia Perina Martins, João José Fagundes, Claudio Saddy Rodrigues Coy, Ashwini Esnakula
Gastroenterology Research and Practice.2025;[Epub] CrossRef - Impact of AI-aided colonoscopy in clinical practice: a prospective randomised controlled trial
Johanna Schöler, Marko Alavanja, Thomas de Lange, Shunsuke Yamamoto, Per Hedenström, Jonas Varkey
BMJ Open Gastroenterology.2024; 11(1): e001247. CrossRef - The histologic features, molecular features, detection and management of serrated polyps: a review
Jin-Dong Wang, Guo-Shuai Xu, Xin-Long Hu, Wen-Qiang Li, Nan Yao, Fu-Zhou Han, Yin Zhang, Jun Qu
Frontiers in Oncology.2024;[Epub] CrossRef - Serrated polyps <10 mm cannot reliably be characterized by i-Scan without magnification at routine colonoscopy
Sabrina G.G. TESTONI, Chiara NOTARISTEFANO, Giuliano F. BONURA, Maria NAPOLITANO, Dario ESPOSITO, Edi VIALE, Lorella FANTI, Francesco AZZOLINI, Giulia M. CAVESTRO, PierAlberto TESTONI
Minerva Gastroenterology.2024;[Epub] CrossRef - Interobserver variability in the histopathological classification and grading of dysplasia in elevated colon lesions in the city of Lima
Guido Gallegos-Serruto, Aldo Gutiérrez, César Chian García, Isthvan Torres Perez
Revista de Gastroenterología del Perú.2024; 44(3): 239. CrossRef - Comparison of adenoma detection rate and proximal serrated polyp detection rate and their effect on post-colonoscopy colorectal cancer mortality in screening patients
Jasmin Zessner-Spitzenberg, Elisabeth Waldmann, Lena Jiricka, Lisa-Maria Rockenbauer, Anna Hinterberger, Jeremy Cook, Arno Asaturi, Aleksandra Szymanska, Barbara Majcher, Michael Trauner, Monika Ferlitsch
Endoscopy.2023; 55(05): 434. CrossRef - The yield of dysplasia and serrated lesions in a single-centre tertiary inflammatory bowel disease cohort
Fiona Yeaman, Lena Thin
Therapeutic Advances in Gastroenterology.2023;[Epub] CrossRef -
The BEETS (JACCRO CC-18) Trial: An Observational and Translational Study of
BRAF
-Mutated Metastatic Colorectal Cancer
Chiaki Inagaki, Ryo Matoba, Satoshi Yuki, Manabu Shiozawa, Akihito Tsuji, Eisuke Inoue, Kei Muro, Wataru Ichikawa, Masashi Fujii, Yu Sunakawa
Future Oncology.2023; 19(17): 1165. CrossRef - A retrospective analysis of the histology of resected polyps and colonoscopy quality parameters in Belgium
E Macken, S Van Dongen, G Van Hal
Acta Gastro Enterologica Belgica.2023; 86(2): 277. CrossRef - Prognostic Biomarkers of Cell Proliferation in Colorectal Cancer (CRC): From Immunohistochemistry to Molecular Biology Techniques
Aldona Kasprzak
Cancers.2023; 15(18): 4570. CrossRef - Assimilating Epigenetics and Transcriptomics for the Identification
of Prognostic Novel Biomarkers and Imminent Targets in
Colorectal Carcinoma with Therapeutic Potential
Suman Kumar Ray, Sukhes Mukherjee
Current Molecular Medicine.2023; 23(8): 784. CrossRef - Multitarget Stool RNA Test for Colorectal Cancer Screening
Erica K. Barnell, Elizabeth M. Wurtzler, Julie La Rocca, Thomas Fitzgerald, Jessica Petrone, Yansheng Hao, Yiming Kang, Faith L. Holmes, David A. Lieberman
JAMA.2023; 330(18): 1760. CrossRef - Microbiome in Colonic Carcinogenesis
Jun Sun, Yinglin Xia
Comprehensive Physiology.2023; 13(3): 4685. CrossRef - Impact of comprehensive optical diagnosis training using Workgroup serrAted polypS and Polyposis classification on detection of adenoma and sessile serrated lesion
Jooyoung Lee, Jung Ho Bae, Su Jin Chung, Hae Yeon Kang, Seung Joo Kang, Min‐Sun Kwak, Ji Yeon Seo, Ji Hyun Song, Sun Young Yang, Jong In Yang, Seon Hee Lim, Jeong Yoon Yim, Joo Hyun Lim, Goh Eun Chung, Eun Hyo Jin, Ji Min Choi, Yoo Min Han, Joo Sung Kim
Digestive Endoscopy.2022; 34(1): 180. CrossRef - Clinicopathological and molecular analyses of hyperplastic lesions including microvesicular variant and goblet cell rich variant hyperplastic polyps and hyperplastic nodules—Hyperplastic nodule is an independent histological entity
Noriyuki Uesugi, Yoichi Ajioka, Tomio Arai, Yoshihito Tanaka, Tamotsu Sugai
Pathology International.2022; 72(2): 128. CrossRef - Comprehensive clinicopathologic, molecular, and immunologic characterization of colorectal carcinomas with loss of three intestinal markers, CDX2, SATB2, and KRT20
Ji Ae Lee, Mi-Kyoung Seo, Seung-Yeon Yoo, Nam-Yun Cho, Yoonjin Kwak, Kyoungbun Lee, Jung Ho Kim, Gyeong Hoon Kang
Virchows Archiv.2022; 480(3): 543. CrossRef - Serrated Colorectal Lesions: An Up-to-Date Review from Histological Pattern to Molecular Pathogenesis
Martino Mezzapesa, Giuseppe Losurdo, Francesca Celiberto, Salvatore Rizzi, Antonio d’Amati, Domenico Piscitelli, Enzo Ierardi, Alfredo Di Leo
International Journal of Molecular Sciences.2022; 23(8): 4461. CrossRef - Arterial stiffness is associated with high-risk colorectal adenomas and serrated lesions: A cross-sectional study in a Taiwanese population
Hung-Yu Chen, Wen-Huang Lee, Hung-Lung Hsu, Yu-Tsung Chou, Fei-Lin Su, I-Hsuan Wu, Ting-Hsing Chao
Journal of Cardiology.2022; 80(2): 139. CrossRef - Morphological and molecular characterization of colorectal sessile serrated lesions with dysplasia
Filippo Cappello, Valentina Angerilli, Luca Dal Santo, Giada Munari, Marianna Sabbadin, Marcello Lo Mele, Gianmaria Pennelli, Claudio Luchini, Paola Parente, Stefano Lazzi, Matteo Fassan
Pathology - Research and Practice.2022; 240: 154214. CrossRef - Serrated polyposis: an overview
Jonathan Fawkes
Gastrointestinal Nursing.2022; 20(9): 24. CrossRef - Sessile serrated lesion presenting as large pedunculated polyp in the rectum: A case report
Shin Ju Oh, Jung-Wook Kim, Chi Hyuk Oh
Medicine.2022; 101(51): e32287. CrossRef - WHICH LESIONS ARE AT HIGHER RISK OF DEVELOPING COLORECTAL CARCINOMAS: SUPERFICIALLY ELEVATED SERRATED LESIONS OR DEPRESSED LESIONS?
Artur Adolfo PARADA, Filadelfio Euclydes VENCO, Miguel Reynaldo VARCA-NETO, Roberto EL IBRAHIM, Paula Bechara POLETTI, Helcio Pedrosa BRITO, Heloisa de Fátima SARE, Osvaldo MALAFAIA
ABCD. Arquivos Brasileiros de Cirurgia Digestiva (São Paulo).2022;[Epub] CrossRef - WNT5a in Colorectal Cancer: Research Progress and Challenges
Guangshun Sun, Liangliang Wu, Guoqiang Sun, Xuesong Shi, Hongyong Cao, Weiwei Tang
Cancer Management and Research.2021; Volume 13: 2483. CrossRef - Endoscopic diagnosis for colorectal sessile serrated lesions
Toshihiro Nishizawa, Shuntaro Yoshida, Akira Toyoshima, Tomoharu Yamada, Yoshiki Sakaguchi, Taiga Irako, Hirotoshi Ebinuma, Takanori Kanai, Kazuhiko Koike, Osamu Toyoshima
World Journal of Gastroenterology.2021; 27(13): 1321. CrossRef - NTRK oncogenic fusions are exclusively associated with the serrated neoplasia pathway in the colorectum and begin to occur in sessile serrated lesions
Jung Ho Kim, Jeong Hoon Hong, Yoon‐La Choi, Ji Ae Lee, Mi‐kyoung Seo, Mi‐Sook Lee, Sung Bin An, Min Jung Sung, Nam‐Yun Cho, Sung‐Su Kim, Young Kee Shin, Sangwoo Kim, Gyeong Hoon Kang
The Journal of Pathology.2021; 255(4): 399. CrossRef - Differential pre-malignant programs and microenvironment chart distinct paths to malignancy in human colorectal polyps
Bob Chen, Cherie’ R. Scurrah, Eliot T. McKinley, Alan J. Simmons, Marisol A. Ramirez-Solano, Xiangzhu Zhu, Nicholas O. Markham, Cody N. Heiser, Paige N. Vega, Andrea Rolong, Hyeyon Kim, Quanhu Sheng, Julia L. Drewes, Yuan Zhou, Austin N. Southard-Smith, Y
Cell.2021; 184(26): 6262. CrossRef - Molecular Insights Into Colorectal Carcinoma
Domenika Ortiz Requena, Monica Garcia-Buitrago
Archives of Medical Research.2020; 51(8): 839. CrossRef
- Predominant Serrated Molecular Signature in Postcolonoscopy Colorectal Cancer: A Systematic Review and Meta-Analysis
- Rectal Invasion by Prostatic Adenocarcinoma That Was Initially Diagnosed in a Rectal Polyp on Colonoscopy
- Ghilsuk Yoon, Man-Hoon Han, An Na Seo
- J Pathol Transl Med. 2019;53(4):266-269. Published online April 11, 2019
- DOI: https://doi.org/10.4132/jptm.2019.03.25
- 9,236 View
- 129 Download
- 9 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF - Despite anatomical proximity, prostatic adenocarcinoma with rectal invasion is extremely rare. We present a case of rectal invasion by prostatic adenocarcinoma that was initially diagnosed from a rectal polyp biopsied on colonoscopy in a 69-year-old Korean man. He presented with dull anal pain and voiding discomfort for several days. Computed tomography revealed either prostatic adenocarcinoma with rectal invasion or rectal adenocarcinoma with prostatic invasion. His tumor marker profile showed normal prostate specific antigen (PSA) level and significantly elevated carcinoembryonic antigen level. Colonoscopy was performed, and a specimen was obtained from a round, 1.5 cm, sessile polyp that was 1.5 cm above the anal verge. Microscopically, glandular tumor structures infiltrated into the rectal mucosa and submucosa. Immunohistochemically, the tumor cells showed alpha-methylacyl-CoA-racemase positivity, PSA positivity, and caudal-related homeobox 2 negativity. The final diagnosis of the rectal polyp was consistent with prostatic adenocarcinoma. Here, we present a rare case that could have been misdiagnosed as rectal adenocarcinoma.
-
Citations
Citations to this article as recorded by- An Extremely Rare Metastatic Prostate Tumor From Rectal Cancer With Characteristic MRI Findings Due to Necrosis
Sohei Iwagami, Shoji Oura, Haruka Miyai, Naoki Kataoka, Masaya Nishihata
Cureus.2025;[Epub] CrossRef - Prostate cancer invading rectal serosa and anal sphincter treated with definitive radiation therapy: Case report and review of the literature
Mi-Jo Lee
Journal of Cancer Research and Therapeutics.2024; 20(3): 1081. CrossRef - Metastatic Adenocarcinoma of the Prostate Masquerading as a Splenic Flexure Colonic Polyp: A Diagnostic Conundrum
Zakaria W Shkoukani, Alaa Chamsin, Mohamed I Abdulmajed
Cureus.2024;[Epub] CrossRef - An Interesting Case of Prostate Cancer Presenting With Colonic Metastasis
Shawn Keating, Ayesha Imtiaz, Kenneth Nahum, Ankita Prasad, Pramil Cheriyath
Cureus.2023;[Epub] CrossRef - Metastase d’un adenocarcinome prostatique au sein d’un polype colique. À propos d’un cas et revue de la littérature
Guillaume Abitbol, Clémence Barthomeuf, Olivier Varennes, Marine Clement, Sami Hakim, Denis Chatelain
Annales de Pathologie.2023; 43(4): 342. CrossRef - Isolated Rectal Metastases from Locally Advanced Carcinoma Prostate Detected by 18F-PSMA-1007 PET/CT
Shashank Shekhar Singh, Rani Kunti Randhir Singh, Narvesh Kumar, Harshvardhan Atrey
World Journal of Nuclear Medicine.2022; 21(03): 248. CrossRef - Rectal Invasion by Metastatic Prostate Adenocarcinoma
Anshu Wadehra, Samer Alkassis, Aliza Rizwan, Omid Yazdanpanah
Cureus.2021;[Epub] CrossRef - Metastatic Prostate Cancer Presenting as a Rectal Polyp: A Rare Occurrence
Ese Uwagbale, Ifeanyichukwu Onukogu, Vimal Bodiwala, Solomon Agbroko, Niket Sonpal
Cureus.2021;[Epub] CrossRef - Local Staging of Prostate Cancer with Multiparametric MRI
Nandan Keshav, Mark D. Ehrhart, Steven C. Eberhardt, Martha F. Terrazas
Seminars in Roentgenology.2021; 56(4): 366. CrossRef
- An Extremely Rare Metastatic Prostate Tumor From Rectal Cancer With Characteristic MRI Findings Due to Necrosis
- The Smad4/PTEN Expression Pattern Predicts Clinical Outcomes in Colorectal Adenocarcinoma
- Yumin Chung, Young Chan Wi, Yeseul Kim, Seong Sik Bang, Jung-Ho Yang, Kiseok Jang, Kyueng-Whan Min, Seung Sam Paik
- J Pathol Transl Med. 2018;52(1):37-44. Published online October 23, 2017
- DOI: https://doi.org/10.4132/jptm.2017.10.20
- 12,763 View
- 234 Download
- 13 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background
Smad4 and PTEN are prognostic indicators for various tumor types. Smad4 regulates tumor suppression, whereas PTEN inhibits cell proliferation. We analyzed and compared the performance of Smad4 and PTEN for predicting the prognosis of patients with colorectal adenocarcinoma.
Methods
Combined expression patterns based on Smad4+/– and PTEN+/– status were evaluated by immunostaining using a tissue microarray of colorectal adenocarcinoma. The relationships between the protein expression and clinicopathological variables were analyzed.
Results
Smad4–/PTEN– status was most frequently observed in metastatic adenocarcinoma, followed by primary adenocarcinoma and tubular adenoma (p<.001). When Smad4–/PTEN– and Smad4+/PTEN+ groups were compared, Smad4–/PTEN– status was associated with high N stage (p=.018) and defective mismatch repair proteins (p=.006). Significant differences in diseasefree survival and overall survival were observed among the three groups (Smad4+/PTEN+, Smad4–/PTEN+ or Smad4+/PTEN–, and Smad4–/PTEN–) (all p<.05).
Conclusions
Concurrent loss of Smad4 and PTEN may lead to more aggressive disease and poor prognosis in patients with colorectal adenocarcinoma compared to the loss of Smad4 or PTEN alone. -
Citations
Citations to this article as recorded by- Precision oncolytic viral therapy in colorectal cancer: Genetic targeting and immune modulation for personalized treatment (Review)
Muhammad Haris Sultan, Qi Zhan, Yigang Wang, Yulong Xia, Xiaoyuan Jia
International Journal of Molecular Medicine.2025; 56(1): 1. CrossRef - Focal Adhesion Kinase Intersects With the BRD4‐MYC Axis and YAP1 to Drive Tumor Cell Growth, Phenotypic Plasticity, Stemness, and Metastatic Potential in Colorectal Cancer
Rongbo Han, Junfeng Shi, Kai Cheng, Zian Wang, Yecang Chen, Orion Spellecy, Abu Saleh Mosa Faisal, Isha Aryal, Jinfei Chen, Rolf Craven, Olivier Thibault, Lauren Baldwin, Lawrence D. Brewer, Sonia Erfani, Chi Wang, Zhenheng Guo, Eric Chen, Burton Yang, Fr
Cancer Medicine.2025;[Epub] CrossRef - Association between the expression of epithelial–mesenchymal transition (EMT)-related markers and oncologic outcomes of colorectal cancer
Mona Hany Emile, Sameh Hany Emile, Amr Awad El-Karef, Mohamed Awad Ebrahim, Ibrahim Eldosoky Mohammed, Dina Abdallah Ibrahim
Updates in Surgery.2024; 76(6): 2181. CrossRef - The Potential Role of Genomic Signature in Stage II Relapsed Colorectal Cancer (CRC) Patients: A Mono-Institutional Study
Michela Roberto, Giulia Arrivi, Emanuela Pilozzi, Andrea Montori, Genoveffa Balducci, Paolo Mercantini, Andrea Laghi, Debora Ierinò, Martina Panebianco, Daniele Marinelli, Silverio Tomao, Paolo Marchetti, Federica Mazzuca
Cancer Management and Research.2022; Volume 14: 1353. CrossRef - Alterations of PTEN and SMAD4 methylation in diagnosis of breast cancer: implications of methyl II PCR assay
Menha Swellam, Entsar A. Saad, Shimaa Sabry, Adel Denewer, Camelia Abdel Malak, Amr Abouzid
Journal of Genetic Engineering and Biotechnology.2021; 19(1): 54. CrossRef - E3 ubiquitin ligase HECW1 promotes the metastasis of non-small cell lung cancer cells through mediating the ubiquitination of Smad4
Chen Lu, Guangyao Ning, Panpan Si, Chunsheng Zhang, Wenjian Liu, Wei Ge, Kai Cui, Renquan Zhang, Shenglin Ge
Biochemistry and Cell Biology.2021; 99(5): 675. CrossRef - Computational quantification of global effects induced by mutations and drugs in signaling networks of colorectal cancer cells
Sara Sommariva, Giacomo Caviglia, Silvia Ravera, Francesco Frassoni, Federico Benvenuto, Lorenzo Tortolina, Nicoletta Castagnino, Silvio Parodi, Michele Piana
Scientific Reports.2021;[Epub] CrossRef - Clinicopathological characterization of SMAD4-mutated intestinal adenocarcinomas: A case-control study
Xiaoyan Liao, Yansheng Hao, Xiaofei Zhang, Stephen Ward, Jane Houldsworth, Alexandros D. Polydorides, Noam Harpaz, Aldo Scarpa
PLOS ONE.2019; 14(2): e0212142. CrossRef - Clinicopathological Characterization and Prognostic Implication of SMAD4 Expression in Colorectal Carcinoma
Seung-Yeon Yoo, Ji-Ae Lee, Yunjoo Shin, Nam-Yun Cho, Jeong Mo Bae, Gyeong Hoon Kang
Journal of Pathology and Translational Medicine.2019; 53(5): 289. CrossRef - Dissecting the therapeutic implications of the complex SMAD4 regulatory network in metastatic colorectal cancer
Ion Cristóbal, Blanca Torrejón, Andrea Santos, Melani Luque, Marta Sanz-Alvarez, Federico Rojo, Jesús García-Foncillas
European Journal of Surgical Oncology.2018; 44(8): 1283. CrossRef - Reply to: Dissecting the therapeutic implications of the complex SMAD4 regulatory network in metastatic colorectal cancer
Jordan M. Cloyd, Takashi Mizuno, Jean-Nicolas Vauthey
European Journal of Surgical Oncology.2018; 44(8): 1285. CrossRef
- Precision oncolytic viral therapy in colorectal cancer: Genetic targeting and immune modulation for personalized treatment (Review)
- A Case of Giant Colonic Muco-submucosal Elongated Polyps Associated with Intussusception
- Joo Heon Kim, Seung Yun Lee, Je Ho Jang, Hyun Young Han, Dong Wook Kang
- J Pathol Transl Med. 2016;50(6):474-478. Published online May 23, 2016
- DOI: https://doi.org/10.4132/jptm.2016.04.27
- 11,497 View
- 133 Download
- 6 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF - Colonic muco-submucosal elongated polyp (CMSEP), a newly categorized non-neoplastic colorectal polyp, is a pedunculated and elongated polyp composed of normal mucosal and submucosal layers without any proper muscle layer. We herein report a giant variant of CMSEP associated with intussusception in the rectosigmoid colon, with a review of the literature. A 48-year-old woman underwent a laparoscopic low anterior resection due to multiple large submucosal polypoid masses associated with intussusception. Grossly, the colonic masses were multiple pedunculated polyps with a long stalk and branches ranging in size from a few millimeters to 14.0 cm in length. Microscopically, there was no evidence of hyperplasia, atypia, or active inflammation in the mucosa. The submucosal layers were composed of edematous and fibrotic stroma with fat tissue, dilated vessels, and lymphoid follicles.
-
Citations
Citations to this article as recorded by- Unusually rapid growth of a duodenal muco-submucosal elongated polyp: A case report
Yi Yang, Ding-Fu Zhong
World Journal of Gastrointestinal Surgery.2025;[Epub] CrossRef - Multiple enteric muco-submucosal elongated polyps causing intussusception
Atsuki Taniguchi, Izuru Endo, Takeyoshi Nishiyama, Nobuyuki Watanabe, Osamu Yoshida, Hiroaki Asano, Masatoshi Kubo, Tetsunobu Udaka
Clinical Journal of Gastroenterology.2024; 17(1): 41. CrossRef - Intussusception due to a Muco-submucosal Elongated Polyp in the Small Intestine—A Case Report—
Hiroki ISHIGE, Ken IMAIZUMI, Takumu FUKASAWA, Keiichiro ITO, Hiroyuki KASAJIMA, Satoru MUNAKATA, Norihiko SHIMOYAMA, Kazuaki NAKANISHI
Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association).2024; 85(6): 744. CrossRef - Jejunal Intussusception Caused by Enteric Muco-submucosal Elongated Polyp: A Case Report
Young Min Jo
Soonchunhyang Medical Science.2024; 30(2): 60. CrossRef - Jejunal intussusception and perforation due to enteric muco-submucosal elongated polyp: a case report and literature review
Ryosuke Kikuchi, Shigenobu Emoto, Hiroaki Nozawa, Kazuhito Sasaki, Koji Murono, Shinya Abe, Hirofumi Sonoda, Aya Shinozaki-Ushiku, Soichiro Ishihara
Surgical Case Reports.2023;[Epub] CrossRef - A stalk with no polyp—A muco‐submucosal elongated polyp in the duodenum
Neil O’Morain, Ciaran McCloskey, Sinead Flanagan, Glen Doherty
United European Gastroenterology Journal.2023; 11(4): 392. CrossRef - Duodenal Worm-Like Polyp
Pan Pan, Guoshan Zhang, Xiao Cui, Liang Liu
Digestive Diseases and Sciences.2023; 68(12): 4275. CrossRef - Colonic Mucosubmucosal Elongated Polyp in the Sigmoid Colon on Surveillance Colonoscopy
Xiaowen Fan, Melissa Hershman, Gabriel Levi, Ilan Weisberg
ACG Case Reports Journal.2019; 6(6): e00110. CrossRef
- Unusually rapid growth of a duodenal muco-submucosal elongated polyp: A case report
- Follicular Dendritic Cell Sarcoma of the Inflammatory Pseudotumor-like Variant Presenting as a Colonic Polyp
- Shien-Tung Pan, Chih-Yuan Cheng, Nie-Sue Lee, Peir-In Liang, Shih-Sung Chuang
- Korean J Pathol. 2014;48(2):140-145. Published online April 28, 2014
- DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.2.140
- 11,506 View
- 105 Download
- 34 Crossref
-
 Abstract
Abstract
 PDF
PDF Follicular dendritic cell (FDC) sarcoma is rare and is classified either as conventional type or inflammatory pseudotumor (IPT)-like variant. Extranodal presentation is uncommon and nearly all gastrointestinal FDC tumors are of the conventional type. IPT-like variant tumors occur almost exclusively in the liver and spleen and are consistently associated with Epstein-Barr virus (EBV). Here we report the case of a 78-year-old woman with an IPT-like FDC sarcoma presenting as a pedunculated colonic polyp. Histologically, scanty atypical ovoid to spindle cells were mixed with a background of florid lymphoplasmacytic infiltrate, which led to an initial misdiagnosis of pseudolymphoma. These atypical cells expressed CD21, CD23, CD35, and D2-40, and were positive for EBV by
in situ hybridization, confirming the diagnosis. The patient was free of disease five months after polypectomy without adjuvant therapy. Although extremely rare, the differential diagnosis for colonic polyp should include FDC sarcoma to avoid an erroneous diagnosis. A review of the 24 cases of IPT-like FDC sarcoma reported in the literature reveal that this tumor occurs predominantly in females with a predilection for liver and spleen, and has a strong association with EBV.-
Citations
Citations to this article as recorded by- The fifth edition of the WHO classification of mature T cell, NK cell and stroma-derived neoplasms
Ayoma D Attygalle, Kennosuke Karube, Yoon Kyung Jeon, Wah Cheuk, Govind Bhagat, John K C Chan, Kikkeri N Naresh
Journal of Clinical Pathology.2025; 78(4): 217. CrossRef - Genomic and Transcriptomic Landscape of Epstein-Barr Virus-Positive Inflammatory Follicular Dendritic Cell Sarcoma: A Multicenter Study
Yan Li, Ze-Lin Weng, Han-Xiao Fei, Hai-Feng Li, Yi-Na Liu, Le-Le Zhang, Qiong Zhang, Xin Weng, Yuan-Yuan Wang, Wen-Yong Huang, Zhi-Xing Cao, Kai-Yan Yang, Xi-Liang Chen, Jie Gao, Wen-Sheng Yang, Fang Liu, Juan-Juan Yong, Jing-Ping Yun, Hua Zhang, Yu-Hua H
Modern Pathology.2025; 38(10): 100864. CrossRef - What is new in the 5th edition of the World Health Organization classification of mature B and T/NK cell tumors and stromal neoplasms?
Ayoma D. Attygalle, John K. C. Chan, Sarah E. Coupland, Ming-Qing Du, Judith A. Ferry, Daphne de Jong, Dita Gratzinger, Megan S. Lim, Alina Nicolae, German Ott, Andreas Rosenwald, Anna Schuh, Reiner Siebert
Journal of Hematopathology.2024; 17(2): 71. CrossRef - Pathologic characteristics of histiocytic and dendritic cell neoplasms
Sun Och Yoon
Blood Research.2024;[Epub] CrossRef - Epstein-barr virus (EBV)-positive inflammatory pseudotumor-like follicular dendritic cell sarcoma (IPT-like FDCS) presenting as thrombocytopenia: A case report and literature review
Jiawei Jin, Xiaolong Zhu, Yi Wan, Yang Shi
Heliyon.2024; 10(12): e32997. CrossRef - EBV-positive inflammatory follicular dendritic cell sarcoma of the colon with clonal immunoglobulin gene rearrangement: A case report and literature review
Xia Xu, Xiuzhen Li, Qun Deng, Kaihang Yu, Jinfan Li
Heliyon.2024; 10(11): e31947. CrossRef - Challenges in the Diagnosis of Epstein-Barr Virus-positive Inflammatory Follicular Dendritic Cell Sarcoma
Yan Li, Xia Yang, Lili Tao, Weimei Zeng, Min Zuo, Shuo Li, Liyan Wu, Yanshong Lin, Ziying Zhang, Jingping Yun, Yuhua Huang
American Journal of Surgical Pathology.2023; 47(4): 476. CrossRef - Epstein-Barr Virus-Positive Inflammatory Follicular Dendritic Cell Sarcoma Presenting as a Colonic Polyp: Report of a Case with a Literature Review
Jiahui Hu, Dongdong Huang, Chengfu Xu, Yi Chen, Han Ma, Zhe Shen
Medicina.2023; 59(7): 1341. CrossRef - A Clinicopathology Review and Update of Epstein–Barr Virus-Associated Mesenchymal Tumors
Oswald Zhao Jian Lee, Noorjehan Omar, Joshua K. Tay, Victor Kwan Min Lee
Cancers.2023; 15(23): 5563. CrossRef - Granulomatous splenic mass with necrosis revealing an EBV-positive inflammatory follicular dendritic cell sarcoma
Irena Antonia Ungureanu, Renato Micelli Lupinacci, Marie Parrens, Jean-François Emile
Journal of Surgical Case Reports.2022;[Epub] CrossRef - Case report: Hepatic inflammatory pseudotumor-like follicular dendritic cell sarcoma: A rare case and minireview of the literature
Fan Ding, Chao Wang, Chi Xu, Hui Tang
Frontiers in Medicine.2022;[Epub] CrossRef - Follicular dendritic cell sarcoma of gastrointestinal tract with two emerging distinct subtypes: a case report and systemic review
Hongxing Gui, Jigisha Chaudhari, Rifat Mannan
Diagnostic Pathology.2022;[Epub] CrossRef - Surgical treatment of liver inflammatory pseudotumor-like follicular dendritic cell sarcoma: A case report
Li-Yue Fu, Jiu-Liang Jiang, Meng Liu, Jun-Jun Li, Kai-Ping Liu, Hai-Tao Zhu
World Journal of Gastrointestinal Oncology.2022; 14(11): 2288. CrossRef - Inflammatory pseudotumor-like follicular/fibroblastic dendritic cell sarcoma: focus on immunohistochemical profile and association with Epstein-Barr virus
Francesca Pagliuca, Andrea Ronchi, Annamaria Auricchio, Eva Lieto, Renato Franco
Infectious Agents and Cancer.2022;[Epub] CrossRef - Recent Advances in Digestive Tract Tumors: Updates From the 5th Edition of the World Health Organization “Blue Book”
Raul S. Gonzalez, Anwar Raza, Robert Propst, Oyedele Adeyi, Justin Bateman, Sabrina C. Sopha, Janet Shaw, Aaron Auerbach
Archives of Pathology & Laboratory Medicine.2021; 145(5): 607. CrossRef - Hepatic inflammatory pseudotumor-like follicular dendritic cell tumor: a case report
Ana Daniela Pascariu, Andreea Ioana Neagu, Andrei Valentin Neagu, Alexandru Băjenaru, Cezar Iulian Bețianu
Journal of Medical Case Reports.2021;[Epub] CrossRef - Inflammatory pseudotumor-like follicular dendritic cell sarcoma: Literature review of 67 cases
Hao Wu, Peng Liu, Xiao-Ran Xie, Jing-Shu Chi, Huan Li, Can-Xia Xu
World Journal of Meta-Analysis.2021; 9(1): 1. CrossRef - New Clinicopathologic Scenarios of EBV+ Inflammatory Follicular Dendritic Cell Sarcoma
Xiang-Nan Jiang, Yan Zhang, Tian Xue, Jie-Yu Chen, Alex C.L. Chan, Wah Cheuk, John K.C. Chan, Xiao-Qiu Li
American Journal of Surgical Pathology.2021; 45(6): 765. CrossRef - Select Epstein-Barr Virus–Associated Digestive Tract Lesions for the Practicing Pathologist
Zainab I. Alruwaii, Elizabeth A. Montgomery
Archives of Pathology & Laboratory Medicine.2021; 145(5): 562. CrossRef - Overview of Gastrointestinal Lymphoproliferative disorders✰
Aaron Auerbach, Nadine S. Aguilera
Seminars in Diagnostic Pathology.2021; 38(4): 1. CrossRef - Follicular dendritic cell sarcoma
Fabio Facchetti, Matteo Simbeni, Luisa Lorenzi
Pathologica.2021; 113(5): 316. CrossRef - Hepatic inflammatory pseudotumor-like follicular dendritic cell tumor with hepatic lymphoma history
Jiang Li, Hai-su Tao, Dong Chen, Zhi-yong Huang, Er-lei Zhang
Medicine.2021; 100(39): e27392. CrossRef - Clinicopathological characteristics of extranodal follicular dendritic cell sarcoma: A report of two cases
Xing Zhao, Dayong Sun, Gang Zhang
Oncology Letters.2021;[Epub] CrossRef - Inflammatory pseudotumour-like follicular dendritic cell tumour of the colon with plasmacytosis mimicking EBV-positive lymphoproliferative disorder
Ying-Ren Chen, Chi-Lin Lee, Yen-Chien Lee, Kung-Chao Chang
Pathology.2020; 52(4): 484. CrossRef - Beware the inflammatory cell-rich colonic polyp: a rare case of EBV-positive inflammatory pseudotumour-like follicular dendritic cell sarcoma with increased IgG4-positive plasma cells
Lynne Goh, Nan Zun Teo, Lai Mun Wang
Pathology.2020; 52(6): 713. CrossRef - Epstein–Barr virus‐positive inflammatory follicular dendritic cell sarcoma presenting as a solitary colonic mass: two rare cases and a literature review
Xiaokang Ke, Huihua He, Qingping Zhang, Jingping Yuan, Qilin Ao
Histopathology.2020; 77(5): 832. CrossRef - Inflammatory pseudotumor-like follicular dendritic cell sarcoma: A brief report of two cases
Bi-Xi Zhang, Zhi-Hong Chen, Yu Liu, Yuan-Jun Zeng, Yan-Chun Li
World Journal of Gastrointestinal Oncology.2019; 11(12): 1231. CrossRef - Epstein-Barr virus (EBV)–associated lymphoid proliferations, a 2018 update
Sherif A. Rezk, Xiaohui Zhao, Lawrence M. Weiss
Human Pathology.2018; 79: 18. CrossRef - A Rare Case of Epstein-Barr Virus Negative Inflammatory Pseudotumor-like Follicular Dendritic Cell Sarcoma Presenting as a Solitary Colonic Mass in a 53-Year-Old Woman; Case Report and Review of Literature
Rossana Kazemimood, Farid Saei Hamedani, Asma Sharif, Sujata Gaitonde, Elizabeth Wiley, Pier Cristoforo Giulianotti, John Vincent Groth
Applied Immunohistochemistry & Molecular Morphology.2017; 25(5): e30. CrossRef - A Case of Inflammatory Pseudotumor-like Follicular Dendritic Cell Sarcoma of the Lymph Node in the Small Bowel Mesentery Accompanied by Myasthenia Gravis
Daichi KITAGUCHI, Katsuji HISAKURA, Taiki SATO, Masanao KURATA, Tatsuya ODA, Nobuhiro OHKOHCHI
Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association).2017; 78(3): 527. CrossRef - Clinicopathological features of inflammatory pseudotumour‐like follicular dendritic cell tumour of the abdomen
Yanyang Chen, Huijuan Shi, Hui Li, Tiantian Zhen, Anjia Han
Histopathology.2016; 68(6): 858. CrossRef - A Rare Case of Follicular Dendritic Cell Sarcoma with Pseudochylous Effusion and Review of Literature From India
Kamal Kant Sahu, Gaurav Prakash, Sandeep Rao, Amanjit Bal, Pankaj Malhotra, Jasmina Ahluwalia, Rakesh K. Vashistha
Indian Journal of Hematology and Blood Transfusion.2015; 31(2): 307. CrossRef - Epstein-Barr virus–associated inflammatory pseudotumor presenting as a colonic mass
Shunyou Gong, Iwona Auer, Rajan Duggal, Stefania Pittaluga, Mark Raffeld, Elaine S. Jaffe
Human Pathology.2015; 46(12): 1956. CrossRef - Response of follicular dendritic cell sarcoma to gemcitabine and docetaxel: report of two cases and literature review
Robert M Conry
Clinical Sarcoma Research.2014;[Epub] CrossRef
- The fifth edition of the WHO classification of mature T cell, NK cell and stroma-derived neoplasms
- Primary Sigmoid Adenocarcinoma Metastasis to the Breast in a 28-Year-Old Female: A Case Study and a Review of Literature
- Amna Ahmad, Kweku Baiden-Amissah, Adegoke Oyegade, Mohammed Absar, Kate Swainson, Sami Titi
- Korean J Pathol. 2014;48(1):58-61. Published online February 25, 2014
- DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.1.58
- 9,338 View
- 52 Download
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Metastasis to the breast from colorectal carcinoma is rare, only a few cases have been reported in the literature, and no cases have been reported in a young, 28-year-old patient. This report confirms the occurrence of the disease in a younger age group. The patient was referred to the Breast Clinic with a history of a gradually increasing lump in her right breast for two weeks' duration. On clinical examination, a 2-cm firm lump was noted in the upper inner quadrant of the right breast, which was clinically benign; however, histological examination of the breast core biopsy together with immunohistochemistry confirmed metastatic colorectal adenocarcinoma. The primary colorectal carcinoma was later confirmed to be a stage pT4N2M1 tumor, and the Duke stage was C1. Histology with immunohistochemistry is very important in the diagnosis of cases of this nature, but the clinical correlation should be taken into consideration at multidisciplinary team meetings to decide the final management of the patient.
-
Citations
Citations to this article as recorded by- Breast mass as the first sign of metastasis from rectal carcinoma: a case report and review of the literature
Jiawei Xu, Chao Liu, Chengdong Yu, Tenghua Yu, Fan Fan, Xiaofang Zhang, Chuansheng Huang, Wen Chen, Zhengkui Sun, Meng Zhou
Frontiers in Oncology.2023;[Epub] CrossRef - A case report of multiple bilateral breast metastases after colorectal cancer
Khaled Arnaout, Nouran Hawa, Sarab Agha, Lama Kadoura, Marwa Aloulou, Kusay Ayoub
International Journal of Surgery Case Reports.2021; 81: 105759. CrossRef - Metastatic Breast Signet-Ring Cell Carcinoma from a Colonic Primary: Review of a Rare Case Report
Ghazaleh Shaker, Hayedeh Haeri, Behnaz Jahanbin
International Journal of Cancer Management.2021;[Epub] CrossRef - Breast metastasis from rectal cancer with BRAF V600E mutation: a case report with a review of the literature
Hiroko Hasegawa, Yoko Nagata, Yuko Sakakibara, Masakazu Miyake, Kiyoshi Mori, Norikazu Masuda, Masayuki Mano, Shoichi Nakazuru, Hisashi Ishida, Eiji Mita
Clinical Journal of Gastroenterology.2020; 13(2): 153. CrossRef - Colonic mucinous adenocarcinoma with secondary in the breast: A case report and literature review
Ameera Balhareth, Abdullah A. AlQatari, Fozan Aldulaijan, Amani Joudeh
International Journal of Surgery Case Reports.2020; 76: 364. CrossRef - Breast metastasis from colorectal cancer treated by multimodal therapy
Tien-Chan Hsieh, Chao-Wen Hsu
Medicine.2019; 98(51): e18016. CrossRef - A case series of metastases to the breast from extramammary malignancies
Tanvi Vaidya, Subhash Ramani, Ashita Rastogi
Indian Journal of Radiology and Imaging.2018; 28(04): 470. CrossRef - Metástasis mamaria de un adenocarcinoma mucinoso de colon: descripción de un caso y revisión de la literatura
Marta Seoane Vigo, María Berdeal Díaz, Lourdes Galán Raposo, Fabio Ares Farpón, Alejandro García Varona, Luis González Crespo
Revista de Senología y Patología Mamaria.2017; 30(1): 28. CrossRef - Metastases of transverse colon cancer to bilateral ovaries (Krukenberg tumor) and the left breast: A case report
Xin-Yu Luo, Jue Wang, Jia Zhao, Rui Chen, Xiao-Ming Zha
Oncology Letters.2017; 14(1): 31. CrossRef - Metastasis of rectal signet ring adenocarcinoma to the breast in a young woman after 10 years, a rare case report and review of the literature
Bita Geramizadeh, Ali Mohammad Bananzadeh, Mohammad Reza Sasani, Asieh Khorshidi, Mahsa Marzban
Cancer Treatment Communications.2016; 7: 58. CrossRef - Metastatic Colonic Adenocarcinoma in Breast: Report of Two Cases and Review of the Literature
Jiten P. Kothadia, Rezina Arju, Monica Kaminski, Arvind Ankireddypalli, Sushil Duddempudi, Jonathan Chow, Shah Giashuddin
Case Reports in Oncological Medicine.2015; 2015: 1. CrossRef
- Breast mass as the first sign of metastasis from rectal carcinoma: a case report and review of the literature
- A Giant Peritoneal Loose Body
- Hyun-Soo Kim, Ji-Youn Sung, Won Seo Park, Youn Wha Kim
- Korean J Pathol. 2013;47(4):378-382. Published online August 26, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.4.378
- 9,791 View
- 65 Download
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF Peritoneal loose bodies (PLBs) are usually discovered incidentally during laparotomy or autopsy. A few cases of giant PLBs presenting with various symptoms have been reported in the literature. Here, we describe a case of a giant PLB incidentally found in the pelvic cavity of a 50-year-old man. Computed tomography revealed a free ovoid mass in the pelvic cavity that consisted of central dense, heterogeneous calcifications and peripheral soft tissue. The mass was an egg-shaped, hard, glistening concretion measuring 7.5×7.0×6.8 cm and weighing 160 g. This concretion consisted of central necrotic fatty tissue surrounded by concentrically laminated, acellular, fibrous material. Small PLBs usually do not require any specific treatment. However, if PLBs cause alimentary or urinary symptoms due to their large size, surgical removal may be recommended. It is essential for clinicians to be aware of this entity and its characteristic features to establish the correct diagnosis.
-
Citations
Citations to this article as recorded by- A giant peritoneal loose body: A case report and updated literature review and data synthesis
Guo-Le Nie, Shicheng Chu, Song Geng, Hong Jiang, Hao Zhan
Medicine.2025; 104(50): e45956. CrossRef - Unveiling the rarity: A case report of giant peritoneal loose body
Abdudin Heru Mehammed, Natnael Alemu Bezabih, Muluken Yifru Gebresilassie, Yohanna Aregawi Hailu, Mengistu Yismie Semahegn, Misganaw Yigletie Damtie
Radiology Case Reports.2024; 19(11): 5492. CrossRef - A Case of a Fixed Giant Peritoneal Loose Body outside the Peritoneum and near the Rectovesical Excavation
Kotaro Nanno, Seiichi Shinji, Takeshi Yamada, Akihisa Matsuda, Ryo Ohta, Hiromichi Sonoda, Takuma Iwai, Kohki Takeda, Kazuhide Yonaga, Koji Ueda, Sho Kuriyama, Toshimitsu Miyasaka, Hiromasa Komori, Yoshinobu Shioda, Hiroshi Yoshida
Journal of Nippon Medical School.2023; 90(3): 276. CrossRef - A Large Intraperitoneal Free Body in a 69-Year-Old Indian Man: a Case Study
Siddhartha Sankar Bhattacharjee, Promit Chakraborty
Indian Journal of Surgery.2022; 84(1): 206. CrossRef - Peritoneal loose body presenting as a hepatic mass: A case report and review of the literature
Yang Wen, Min-jie Shang, Yan-qing Ma, Song-hua Fang, Yuan Chen
Open Medicine.2021; 16(1): 1356. CrossRef - Peritoneal Loose Body in a Patient With Ampullary Adenocarcinoma
A.V. Pradeep, Abdul Razik, Ankur Goyal, Atin Kumar, Virinder Kumar Bansal, Asuri Krishna
ACG Case Reports Journal.2021; 8(11): e00680. CrossRef - Exploratory laparoscopy as first choice procedure for the diagnosis of giant peritoneal loose body: a case report
RuiBin Li, ZhiHeng Wan, HaoTian Li
Journal of International Medical Research.2020;[Epub] CrossRef - A rare peritoneal egg: Case report with literature review
Nilu Malpani Dhoot, Shivaraj Afzalpurkar, Usha Goenka, Vinay Mahendra, Enam Murshed Khan, Arpita Sutradhar, Mahesh Goenka
Radiology Case Reports.2020; 15(10): 1895. CrossRef - A giant peritoneal loose body impacted in the pelvic cavity, a rare and interesting finding during laparotomy
Ayad A. Mohammed
International Journal of Surgery: Global Health.2020; 3(6): e24. CrossRef - Giant Mobile Intraperitoneal Loose Body
Mohd Ilyas, Mohd Yaqoob Wani, Musaib Ahmad Dar, Feroze A. Shaheen
ACG Case Reports Journal.2019; 6(1): e00006. CrossRef - Giant peritoneal loose body in a patient with end-stage renal disease
Nadejda Cojocari, Leonard David
SAGE Open Medical Case Reports.2018;[Epub] CrossRef - Two giant peritoneal loose bodies were simultaneously found in one patient: A case report and review of the literature
Qingxing Huang, Aihong Cao, Jun Ma, Zhenhua Wang, Jianhong Dong
International Journal of Surgery Case Reports.2017; 36: 74. CrossRef - Laparoscopic extraction of a giant peritoneal loose body: Case report and review of literature
Keiso Matsubara, Yuji Takakura, Takashi Urushihara, Takashi Nishisaka, Toshiyuki Itamoto
International Journal of Surgery Case Reports.2017; 39: 188. CrossRef - Symptomatic giant peritoneal loose body in the pelvic cavity: A case report
Andreas Elsner, Mikolaj Walensi, Maya Fuenfschilling, Robert Rosenberg, Robert Mechera
International Journal of Surgery Case Reports.2016; 21: 32. CrossRef - A Case of a Peritoneal Loose Body with the Maximum Diameter of 50 mm
Yoshihiro MOCHIZUKI, Hiroshi IINO, Michio HARA, Syugo SHIBA, Makoto SUDO, Naoki OISHI, Tetsuo KONDO
Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association).2016; 77(10): 2552. CrossRef - Giant peritoneal loose body in the pelvic cavity confirmed by laparoscopic exploration: a case report and review of the literature
Hong Zhang, Yun-zhi Ling, Ming-ming Cui, Zhi-xiu Xia, Yong Feng, Chun-sheng Chen
World Journal of Surgical Oncology.2015;[Epub] CrossRef
- A giant peritoneal loose body: A case report and updated literature review and data synthesis
- Rhabdoid Colorectal Carcinomas: Reports of Two Cases
- Sang Hwa Lee, Hyesil Seol, Wook Youn Kim, So Dug Lim, Wan Seop Kim, Tae Sook Hwang, Hye Seung Han
- Korean J Pathol. 2013;47(4):372-377. Published online August 26, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.4.372
- 9,359 View
- 54 Download
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF Rhabdoid colorectal carcinomas are very rare and only 10 cases have been previously reported. We report two cases of rhabdoid colorectal carcinoma, one arising in the sigmoid colon of a 62-year-old man and another in the rectum of an 83-year-old woman. In both cases, the patients had advanced tumors with lymph node metastases. The tumors mostly showed a diffuse arrangement with rhabdoid features and small glandular regions were combined. Transitional areas from the adenocarcinomas to the rhabdoid tumors were also noted. Adenocarcinoma cells were positive for mixed cytokeratin (CK), CK20 and epithelial membranous antigen (EMA), but focal positive for vimentin. The rhabdoid tumor cells were positive for mixed CK, but focal positive or negative for CK20 and EMA. In addition, they were diffusely positive for vimentin, but negative for desmin. The histological and immunohistologial findings of these two cases suggest that the rhabodid tumor cells originated from dedifferentiated adenocarcinomas.
-
Citations
Citations to this article as recorded by- Undifferentiated Rhabdoid Carcinoma of the Gastrointestinal Tract: A Rare and Aggressive Malignancy
Justin M Hsieh, Zara Summers, Shinn Yeung
Cureus.2025;[Epub] CrossRef - SMARCB1/INI1-Deficient Poorly Differentiated Carcinoma of the Colon With Rhabdoid Features—A Rare Tumor With Serrated Phenotype: Case Report and Review of Literature
Shivali Maurya, Sujata Yadav, Subham Bhowmik, Jasmine Dhal, Lalita Mehra, Raju Sharma, Asuri Krishna, Atul Sharma, Adarsh Barwad, Prasenjit Das
International Journal of Surgical Pathology.2024; 32(1): 187. CrossRef - Emerging and under-recognised patterns of colorectal carcinoma morphologies: a comprehensive review
Yuho Ono, Osman Yilmaz
Journal of Clinical Pathology.2024; 77(7): 439. CrossRef - A Case of Ascending Colon Carcinoma with Rhabdoid Features
Masanari YAMADA, Masanori ICHINOSE, Atsushi HIRATA, Yoshihiro KURATA, Kimiaki FUKASAWA, Hisahiro MATSUBARA
Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association).2024; 85(6): 755. CrossRef - A Rare Case of Undifferentiated Rhabdoid Carcinoma of the Colon
Syed Alishan Nasir, Ronak Patel, Lalaine Ruiz, Michael Bush
Cureus.2022;[Epub] CrossRef - INI1-negative colorectal undifferentiated carcinoma with rhabdoid features and postoperative rapidly growing liver metastases: a case report and review of the literature
Masatsugu Kojima, Toru Miyake, Tomoyuki Ueki, Hiroyuki Ohta, Ryoji Kushima, Masanori Shiohara, Hiroo Mizuta, Hiroya Iida, Tsuyoshi Yamaguchi, Sachiko Kaida, Katsushi Takebayashi, Hiromitsu Maehira, Yusuke Nishina, Tomoharu Shimizu, Eiji Mekata, Masaji Tan
Surgical Case Reports.2021;[Epub] CrossRef - Undifferentiated carcinoma of the transverse colon with rhabdoid features that developed during treatment of non-small cell lung carcinoma with pembrolizumab: a case report
Yuya Ashitomi, Mitsuhiro Yano, Michihisa Kono, Takefumi Suzuki, Ichiro Kawamura, Shinji Okazaki, Yukinori Kamio, Osamu Hachiya, Yuka Urano, Fuyuhiko Motoi
Surgical Case Reports.2020;[Epub] CrossRef - BRAF Mutation in Colorectal Rhabdoid and Poorly Differentiated Medullary Carcinomas
Elena Bolzacchini, Nunzio Digiacomo, Cristina Marrazzo, Nora Sahnane, Roberta Maragliano, Anthony Gill, Luca Albarello, Fausto Sessa, Daniela Furlan, Carlo Capella
Cancers.2019; 11(9): 1252. CrossRef - Pathologic complete response to bevacizumab-FOLFIRI in metastatic colonic undifferentiated carcinoma with rhabdoid features
Tien-Chan Hsieh, Hung-Wei Liu, Chao-Wen Hsu
Journal of Cancer Research and Practice.2019; 6(3): 140. CrossRef - Extraordinary disease-free survival in a rare malignant extrarenal rhabdoid tumor: a case report and review of the literature
Francesco D’Amico, Alessandra Bertacco, Maurizio Cesari, Claudia Mescoli, Giorgio Caturegli, Gabriel Gondolesi, Umberto Cillo
Journal of Medical Case Reports.2018;[Epub] CrossRef - Tumor rabdoide extrarrenal maligno de colon: presentación de 3 casos y revisión de la literatura
María José Sánchez-de las Matas Garre, José García Solano, Pablo Conesa Zamora, Fidel Fernández Fernández, Miguel Pérez-Guillermo
Revista Española de Patología.2016; 49(2): 119. CrossRef - Poorly differentiated cecal adenocarcinoma showing prominent rhabdoid feature combined with appendiceal mucinous cystadenoma: A case report and review of the literature
IN-JU CHO, SUNG-SOO KIM, YOUNG-DON MIN, MUN-WHAN NOH, RAN HONG
Oncology Letters.2015; 9(4): 1527. CrossRef - A Rare Case of Undifferentiated Carcinoma of the Colon with Rhabdoid Features: A Case Report and Review of the Literature
E. Moussaly, J. P. Atallah
Case Reports in Oncological Medicine.2015; 2015: 1. CrossRef - Case Report of Rhabdoid Colon Cancer and Review of Literature
Aparna Kalyan, Gurleen Pasricha, Dulabh Monga, Aatur Singhi, Nathan Bahary
Clinical Colorectal Cancer.2015; 14(1): e5. CrossRef - Malignant Rhabdoid Tumor of the Colon: A Case Report
Elena Romera Barba, Ainhoa Sánchez Pérez, Carlos Duque Pérez, José Antonio García Marcilla, José Luis Vázquez Rojas
Cirugía Española (English Edition).2014; 92(9): 638. CrossRef - Tumor rabdoide maligno de colon: a propósito de un caso☆
Elena Romera Barba, Ainhoa Sánchez Pérez, Carlos Duque Pérez, José Antonio García Marcilla, José Luis Vázquez Rojas
Cirugía Española.2014; 92(9): 638. CrossRef
- Undifferentiated Rhabdoid Carcinoma of the Gastrointestinal Tract: A Rare and Aggressive Malignancy
- SIRT1 Expression Is Associated with Good Prognosis in Colorectal Cancer
- Wonkyung Jung, Kwang Dae Hong, Woon Yong Jung, Eunjung Lee, Bong Kyung Shin, Han Kyeom Kim, Aeree Kim, Baek-hui Kim
- Korean J Pathol. 2013;47(4):332-339. Published online August 26, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.4.332
- 12,220 View
- 81 Download
- 46 Crossref
-
 Abstract
Abstract
 PDF
PDF Background Silent mating type information regulation 2 homolog 1 (SIRT1), an NAD+-dependent deacetylase, might act as a tumor promoter by inhibiting p53, but may also as a tumor suppressor by inhibiting several oncogenes such as β-catenin and survivin. Deleted in breast cancer 1 (DBC1) is known as a negative regulator of SIRT1.
Methods Immunohistochemical expressions of SIRT1, DBC1, β-catenin, surviving, and p53 were evaluated using 2 mm tumor cores from 349 colorectal cancer patients for tissue microarray.
Results Overexpression of SIRT1, DBC1, survivin, and p53 was seen in 235 (67%), 183 (52%), 193 (55%), and 190 (54%) patients, respectively. Altered expression of β-catenin was identified in 246 (70%) patients. On univariate analysis, overexpression of SIRT1 (p=0.029) and altered expression of β-catenin (p=0.008) were significantly associated with longer overall survival. Expression of SIRT1 was significantly related to DBC1 (p=0.001), β-catenin (p=0.001), and survivin (p=0.002), but not with p53. On multivariate analysis, age, tumor stage, differentiation, and expression of SIRT1 were independent prognostic factors significantly associated with overall survival.
Conclusions SIRT1 overexpression is a good prognostic factor for colorectal cancer, and SIRT1 may interact with β-catenin and survivin rather than p53.
-
Citations
Citations to this article as recorded by- Ocular surface squamous neoplasia: Update on genetics, epigenetics and opportunities for targeted therapy
Nefeli Eleni Kounatidou, Evangelos Vitkos, Sotiria Palioura
The Ocular Surface.2025; 35: 1. CrossRef - The Prognostic Impact of SIRT1, STAT3, and YAP1 in Colorectal Carcinoma
Shimaa Elkholy, Aya Abdelbary, Dina Elazab, Mohamed Elkablawy, Asmaa G. Abdou
Applied Immunohistochemistry & Molecular Morphology.2025; 33(1): 29. CrossRef - The NR3C2-SIRT1 signaling axis promotes autophagy and inhibits epithelial mesenchymal transition in colorectal cancer
Feng Li, Xing Wan, Zhigui Li, Liming Zhou
Cell Death & Disease.2025;[Epub] CrossRef - Prognostic and clinicopathological value of dbc1 expression in human cancers: a systematic review and meta-analysis
Haojia Wang, Xinhong Cheng, Bruce Xianzhuo Zhang, Yong Wang, Shuo Gao, Fanghui Ding, Xiaojing Song, Dandan Li, Haixu Ni, Yang Luo, Xun Li
Frontiers in Oncology.2025;[Epub] CrossRef - Glutamine signaling specifically activates c-Myc and Mcl-1 to facilitate cancer cell proliferation and survival
Meng Wang, Fu-Shen Guo, Dai-Sen Hou, Hui-Lu Zhang, Xiang-Tian Chen, Yan-Xin Shen, Zi-Fan Guo, Zhi-Fang Zheng, Yu-Peng Hu, Pei-Zhun Du, Chen-Ji Wang, Yan Lin, Yi-Yuan Yuan, Shi-Min Zhao, Wei Xu
Protein & Cell.2025; 16(11): 968. CrossRef - Targeting TGF-β–Smad2/3–JNK1-mediated SIRT1 activity overcomes the chemoresistance of KRAS mutation lung cancer
Dong Hoon Shin, Minyoung Choi, Chungyong Han, Sang Soo Kim
Experimental & Molecular Medicine.2025; 57(9): 2022. CrossRef - The integrated analysis of SIRT family expression, prognostic value, and potential implications in childhood acute lymphoblastic leukemia
Xusan Xu, Zhendong Wang, Xiaoxia Wang, Wensen Zhang, Zhengqiang Luo, Xiaomei Zheng, Ronghua Pan, Ying Fu, Yajun Wang, Guochun Huang, Riling Chen, Guoda Ma
Frontiers in Oncology.2025;[Epub] CrossRef - ZMIZ1 Regulates Proliferation, Autophagy and Apoptosis of Colon Cancer Cells by Mediating Ubiquitin–Proteasome Degradation of SIRT1
Min Huang, Junfeng Wang, Zhengrong Zhang, Xueliang Zuo
Biochemical Genetics.2024; 62(4): 3245. CrossRef - Research Progress of Biological Function and Prognosis of Colorectal Cancer in Sirtuins Family
瑞阳 李
Journal of Clinical Personalized Medicine.2024; 03(04): 1805. CrossRef - Oncogenic KRAS mutation confers chemoresistance by upregulating SIRT1 in non-small cell lung cancer
Dong Hoon Shin, Jeong Yeon Jo, Minyoung Choi, Kyung-Hee Kim, Young-Ki Bae, Sang Soo Kim
Experimental & Molecular Medicine.2023; 55(10): 2220. CrossRef - Association of β-Catenin, APC, SMAD3/4, Tp53, and Cyclin D1 Genes in Colorectal Cancer: A Systematic Review and Meta-Analysis
Hongfeng Yan, Fuquan Jiang, Jianwu Yang, Ying-Kun Xu
Genetics Research.2022; 2022: 1. CrossRef - Resveratrol-related compounds: Potential for cancer and beyond
MONICA SAVIO, VALENTINA MINOIA, PAOLA FULGHIERI, LUCIA ANNA STIVALA, VIRGINIE SOTTILE
BIOCELL.2022; 46(12): 2525. CrossRef - The relationship between β-catenin and patient survival in colorectal cancer systematic review and meta-analysis
Amna Matly, Jean A. Quinn, Donald C. McMillan, James H. Park, Joanne Edwards
Critical Reviews in Oncology/Hematology.2021; 163: 103337. CrossRef - Trending topics of SIRT1 in tumorigenicity
Liz M. Garcia-Peterson, Xiaoling Li
Biochimica et Biophysica Acta (BBA) - General Subjects.2021; 1865(9): 129952. CrossRef - Surtuin 1 as a potential prognostic biomarker in very elderly patients with colorectal cancer
Guk Jin Lee, Yun Hwa Jung, Tae-Jung Kim, Yosep Chong, Seo-Won Jeong, In Kyu Lee, In Sook Woo
The Korean Journal of Internal Medicine.2021; 36(Suppl 1): S235. CrossRef - Survival and Clinicopathological Significance of SIRT1 Expression in Cancers: A Meta-Analysis
Min Sun, Mengyu Du, Wenhua Zhang, Sisi Xiong, Xingrui Gong, Peijie Lei, Jin Zha, Hongrui Zhu, Heng Li, Dong Huang, Xinsheng Gu
Frontiers in Endocrinology.2019;[Epub] CrossRef - SIRT1: a potential tumour biomarker and therapeutic target
Bin Zhao, Xin Li, Liangfu Zhou, Ye Wang, Peng Shang
Journal of Drug Targeting.2019; 27(10): 1046. CrossRef - The clinicopathological significance of SIRT1 expression in colon cancer: An immunohistochemical study and meta-analysis
Won Gi Hong, Jung-Soo Pyo
Pathology - Research and Practice.2018; 214(10): 1550. CrossRef - Sirtuin 1 and oral cancer (Review)
Shajedul Islam, Yoshihiro Abiko, Osamu Uehara, Itsuo Chiba
Oncology Letters.2018;[Epub] CrossRef - A novel SIRT1 inhibitor, 4bb induces apoptosis in HCT116 human colon carcinoma cells partially by activating p53
Ananga Ghosh, Amrita Sengupta, Guru Pavan Kumar Seerapu, Ali Nakhi, E.V. Venkat Shivaji Ramarao, Navneet Bung, Gopalakrishnan Bulusu, Manojit Pal, Devyani Haldar
Biochemical and Biophysical Research Communications.2017; 488(3): 562. CrossRef - SIRT1 gene polymorphisms and its protein level in colorectal cancer
Olfat Gamil Shaker, Miriam Safwat Wadie, Reham Maher Mohamed Ali, Ayman Yosry
Gene Reports.2017; 7: 164. CrossRef - Overexpression of SIRT1 is Associated With Poor Outcomes in Patients With Ovarian Carcinoma
David H. Mvunta, Tsutomu Miyamoto, Ryoichi Asaka, Yasushi Yamada, Hirofumi Ando, Shotaro Higuchi, Koichi Ida, Hiroyasu Kashima, Tanri Shiozawa
Applied Immunohistochemistry & Molecular Morphology.2017; 25(6): 415. CrossRef - SIRT1 suppresses colorectal cancer metastasis by transcriptional repression of miR-15b-5p
Li-Na Sun, Zheng Zhi, Liang-Yan Chen, Qun Zhou, Xiu-Ming Li, Wen-Juan Gan, Shu Chen, Meng Yang, Yao Liu, Tong Shen, Yong Xu, Jian-Ming Li
Cancer Letters.2017; 409: 104. CrossRef - TrpC5 regulates differentiation through the Ca2+/Wnt5a signalling pathway in colorectal cancer
Zhen Chen, Chunlei Tang, Yaodan Zhu, Mingxu Xie, Dongxu He, Qiongxi Pan, Peng Zhang, Dong Hua, Teng Wang, Linfang Jin, Xiaowei Qi, Yifei Zhu, Xiaoqiang Yao, Jian Jin, Xin Ma
Clinical Science.2017; 131(3): 227. CrossRef - Meta-analysis of SIRT1 expression as a prognostic marker for overall survival in gastrointestinal cancer
Shuangjie Wu, Jinghui Jiang, Jun Liu, Xinhai Wang, Yu Gan, Yifan Tang
Oncotarget.2017; 8(37): 62589. CrossRef - Prognostic and clinicopathological significance of SIRT1 expression in NSCLC: a meta-analysis
Yifei Chen, Tao Wang, Wei Wang, Jiahao Hu, Ruiting Li, Shaojun He, Jiong Yang
Oncotarget.2017; 8(37): 62537. CrossRef - The prognostic role of Sirt1 expression in solid malignancies: a meta-analysis
Changwen Wang, Wen Yang, Fang Dong, Yawen Guo, Jie Tan, Shengnan Ruan, Tao Huang
Oncotarget.2017; 8(39): 66343. CrossRef - SIRT1 induces tumor invasion by targeting epithelial mesenchymal transition-related pathway and is a prognostic marker in triple negative breast cancer
Min-Sun Jin, Chang Lim Hyun, In Ae Park, Ji Young Kim, Yul Ri Chung, Seock-Ah Im, Kyung-Hun Lee, Hyeong-Gon Moon, Han Suk Ryu
Tumor Biology.2016; 37(4): 4743. CrossRef - Survivin and SIRT1: can be two prognostic factors in chronic myeloid leukemia?
Fatemeh Salari, Javad Mohammdai-asl, Amal Saki Malehi, Ahmad Ahmadzadeh, Mohammad Ali Jalali far, Zari Tahannejad Asadi, Najmaldin Saki
Comparative Clinical Pathology.2016; 25(2): 415. CrossRef - Clinicopathological significance of SIRT1 expression in colorectal cancer: A systematic review and meta analysis
Guo Zu, Anlong Ji, Tingting Zhou, Ningwei Che
International Journal of Surgery.2016; 26: 32. CrossRef - The small molecule survivin inhibitor YM155 may be an effective treatment modality for colon cancer through increasing apoptosis
Wan Lu Li, Mi-Ra Lee, Mee-Yon Cho
Biochemical and Biophysical Research Communications.2016; 471(2): 309. CrossRef - Nuclear expression and/or reduced membranous expression of β-catenin correlate with poor prognosis in colorectal carcinoma
Shizhen Zhang, Zhen Wang, Jinlan Shan, Xiuyan Yu, Ling Li, Rui Lei, Daozhe Lin, Siqi Guan, Xiaochen Wang
Medicine.2016; 95(49): e5546. CrossRef - Association of SIRT1 and HMGA1 expression in non-small cell lung cancer
SHUANG-YAN LIN, FANG PENG
Oncology Letters.2016; 11(1): 782. CrossRef - SIRT1 is a regulator of autophagy: Implications in gastric cancer progression and treatment
Guanglin Qiu, Xuqi Li, Xiangming Che, Chao Wei, Shicai He, Jing Lu, Zongliang Jia, Ke Pang, Lin Fan
FEBS Letters.2015; 589(16): 2034. CrossRef - Stromal expression of miR-21 in T3-4a colorectal cancer is an independent predictor of early tumor relapse
Won Kyung Kang, Jin Kwon Lee, Seong Taek Oh, Sung Hak Lee, Chan Kwon Jung
BMC Gastroenterology.2015;[Epub] CrossRef - Association of SIRT1 and tumor suppressor gene TAp63 expression in head and neck squamous cell carcinoma
Keiji Kikuchi, Akira Noguchi, Rika Kasajima, Yohei Miyagi, Daisuke Hoshino, Naohiko Koshikawa, Akira Kubota, Tomoyuki Yokose, Yasuo Takano
Tumor Biology.2015; 36(10): 7865. CrossRef - Differential expressions of cancer-associated genes and their regulatory miRNAs in colorectal carcinoma
Murat Kara, Onder Yumrutas, Onder Ozcan, Ozgur Ilhan Celik, Esra Bozgeyik, Ibrahim Bozgeyik, Sener Tasdemir
Gene.2015; 567(1): 81. CrossRef - Distinctive role of SIRT1 expression on tumor invasion and metastasis in breast cancer by molecular subtype
Yul Ri Chung, Hyojin Kim, Soo Young Park, In Ae Park, Ja June Jang, Ji-Young Choe, Yoon Yang Jung, Seock-Ah Im, Hyeong-Gon Moon, Kyung-Hun Lee, Koung Jin Suh, Tae-Yong Kim, Dong-Young Noh, Wonshik Han, Han Suk Ryu
Human Pathology.2015; 46(7): 1027. CrossRef - Expression of ROR1, pAkt, and pCREB in gastric adenocarcinoma
Hyeyoon Chang, Woon Yong Jung, Youngran Kang, Hyunjoo Lee, Aeree Kim, Baek-hui Kim
Annals of Diagnostic Pathology.2015; 19(5): 330. CrossRef - miR-34a inhibits cell proliferation in prostate cancer by downregulation of SIRT1 expression
KUN DUAN, YONG-CHAO GE, XUE-PEI ZHANG, SHU-YI WU, JIN-SHUN FENG, SHI-LIN CHEN, LI ZHANG, ZHI-HAO YUAN, CHAO-HONG FU
Oncology Letters.2015; 10(5): 3223. CrossRef - Immunohistochemical Characterization of Large Intestinal Adenocarcinoma in the Rhesus Macaque (Macaca mulatta)
C. E. Harbison, F. Taheri, H. Knight, A. D. Miller
Veterinary Pathology.2015; 52(4): 732. CrossRef - Correlation and prognostic value of SIRT1 and Notch1 signaling in breast cancer
Yu-Wen Cao, Wen-Qin Li, Guo-Xing Wan, Yi-Xiao Li, Xiao-Ming Du, Yu-Cong Li, Feng Li
Journal of Experimental & Clinical Cancer Research.2014;[Epub] CrossRef - Fentanyl Increases Colorectal Carcinoma Cell Apoptosis by Inhibition of NF-κB in a Sirt1-dependent Manner
Xiu-Lai Zhang, Min-Li Chen, Sheng-Li Zhou
Asian Pacific Journal of Cancer Prevention.2014; 15(22): 10015. CrossRef - Elevated HOXB9 expression promotes differentiation and predicts a favourable outcome in colon adenocarcinoma patients
J Zhan, M Niu, P Wang, X Zhu, S Li, J Song, H He, Y Wang, L Xue, W Fang, H Zhang
British Journal of Cancer.2014; 111(5): 883. CrossRef - Prognostic Factors for Metastatic Colorectal Cancer after First-line Chemotherapy with FOLFOX-4 or FOLFIRI Regimen
Jae Hyun Kim, Pyoung Rak Choi, Seun Ja Park, Moo In Park, Won Moon, Sung Eun Kim, Gyu Won Lee
The Korean Journal of Gastroenterology.2014; 63(4): 209. CrossRef - Down-Regulation of mir-221 and mir-222 Restrain Prostate Cancer Cell Proliferation and Migration That Is Partly Mediated by Activation of SIRT1
Xiao Yang, Yingmei Yang, Rong Gan, Lingxu Zhao, Wei Li, Huaibin Zhou, Xiaojuan Wang, Jianxin Lu, Qing H. Meng, George Calin
PLoS ONE.2014; 9(6): e98833. CrossRef
- Ocular surface squamous neoplasia: Update on genetics, epigenetics and opportunities for targeted therapy
- Colonic Adenocarcinoma Arising from Gastric Heterotopia: A Case Study
- Hyoungsuk Ko, Shin Young Park, Eun Jung Cha, Jang Sihn Sohn
- Korean J Pathol. 2013;47(3):289-292. Published online June 25, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.3.289
- 9,908 View
- 53 Download
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF Heterotopic gastric mucosa occurs in all areas of the gastrointestinal tract including the nasopharynx, tongue, esophagus, small intestine, colon, and rectum. Gastric heterotopia of the large bowel is infrequent, and most cases have been reported in the rectum. Review of the literature has revealed only eight cases involving the colon proximal to the rectum. Little is known of the natural history of gastric heterotopias, except that. It usually presents with gastrointestinal bleeding, though other serious complications such as bowel perforation, intussusceptions, and fistula formation, are possible. Further, it is unclear whether heterotopic gastric mucosa progresses to malignancy. Herein, we describe a case of adenocarcinoma of the transverse colon arising from gastric heterotopia. To the best of our knowledge, this is the first report of adenocarcinoma arising from heterotopic gastric mucosa in the colon.
-
Citations
Citations to this article as recorded by- Anorectal gastric heterotopia as a rare cause of constipation: Case report and review of pediatric literature
Kathryn M. Stephenson, Raj P. Kapur, Jeffrey R. Avansino, Lusine Ambartsumyan
JPGN Reports.2025; 6(4): 407. CrossRef - Intussusception of Heterotopic Gastric Mucosa in the Transverse Colon: A Rare Cause of Perforation and Bleeding
Sho Fujiwara, Ryuichi Nishimura, Nozomi Koyamada
Cureus.2024;[Epub] CrossRef - Gastric heterotopia of colon found cancer workup in liver abscess: A case report
Jun Gi Park, Jeong Ill Suh, Yeo Un Kim
World Journal of Clinical Cases.2022; 10(15): 5012. CrossRef - Gastric heterotopia in the ileum mimicking Meckel's diverticulum
Reza Shojaeian, Negar Nekooei, Paria Dehghanian
Journal of Pediatric Surgery Case Reports.2022; 84: 102361. CrossRef - Sometimes Things Are Not Where They Are Supposed to Be: A Case Report of Gastric Heterotopia in the Rectum
Asher Lippe, Scott Lippe
Physician's Journal of Medicine.2022;[Epub] CrossRef - Gastric heterotopia of the rectum
Eduardo Dantas, Diva Yamaguti, Kendi Yamazaki
Gastroenterología y Hepatología.2021; 44(8): 579. CrossRef - Bleeding Gastric Heterotopia of Cecal Diverticulum in an Adolescent: A Case Report
Hyun-Il Seo, Jae-Young Kwak
Advances in Pediatric Surgery.2021; 27(1): 32. CrossRef - Gastric heterotopia of the rectum
Eduardo Dantas, Diva Yamaguti, Kendi Yamazaki
Gastroenterología y Hepatología (English Edition).2021; 44(8): 579. CrossRef - Polypoid Gastric Heterotopia of Colon
Marcela Adriana Duran Alvarez, Carla Noemi Tafur Sanchez
GE - Portuguese Journal of Gastroenterology.2020; 27(1): 65. CrossRef - Heterotopic Respiratory Mucosa in the Rectum: An Unusual Type and Site of Heterotopia in the Gastrointestinal Tract
Caroline Bsirini, Pratyusha Tirumanisetty, Joseph N. Dytoc, Diana Agostini-Vulaj, Christopher Steevens, Asad Ullah, Aaron R. Huber
International Journal of Surgical Pathology.2019; 27(2): 221. CrossRef - Perforation of Heterotopic Gastric Mucosa in ileal duplication in an adult: A case report
Vaanathi Paulvannan, Seshukumar Bylapudi, Mithun Kumar Ramesh Kumar, Mahesh Nachimuthu, Paulvannan Subramanian
Journal of Surgical Case Reports.2019;[Epub] CrossRef - Mixed adenoneuroendocrine carcinoma of the tongue arising within a congenital enteric cyst
Louis J. Ligthelm, Belinda K. Bunn, Erich J. Raubenheimer, Willie F. P. van Heerden
Head & Neck.2018;[Epub] CrossRef - The outlet patch: gastric heterotopia of the colorectum and anus
Abul A S R Mannan, Michael Vieth, Armen Khararjian, Binny Khandakar, Dora Lam‐Himlin, David Heydt, Feriyl Bhaijee, Henry J Venbrux, Kathleen Byrnes, Lysandra Voltaggio, Norman Barker, Songyang Yuan, Elizabeth A Montgomery
Histopathology.2018; 73(2): 220. CrossRef - Large heterotopic gastric mucosa and a concomitant diverticulum in the rectum: Clinical experience and endoscopic management
Wen-Guo Chen, Hua-Tuo Zhu, Ming Yang, Guo-Qiang Xu, Li-Hua Chen, Hong-Tan Chen
World Journal of Gastroenterology.2018; 24(30): 3462. CrossRef - Gastric heterotopia in the rectum. A rare cause of ectopic gastric tissue
George A. Salem, Javid Fazili, Tauseef Ali
Arab Journal of Gastroenterology.2017; 18(1): 42. CrossRef - Gastric heterotopia in rectum: A literature review and its diagnostic pitfall
Peyman Dinarvand, Ashley A. Vareedayah, Nancy J Phillips, Christine Hachem, Jinping Lai
SAGE Open Medical Case Reports.2017;[Epub] CrossRef - Heterotopic gastric mucosa in the anus and rectum: first case report of endoscopic submucosal dissection and systematic review
Federico Iacopini, Takuji Gotoda, Walter Elisei, Patrizia Rigato, Fabrizio Montagnese, Yutaka Saito, Guido Costamagna, Giampaolo Iacopini
Gastroenterology Report.2016; 4(3): 196. CrossRef
- Anorectal gastric heterotopia as a rare cause of constipation: Case report and review of pediatric literature
- Extrapelvic Uterus-like Masses Presenting as Colonic Submucosal Tumor: A Case Study and Review of Literature
- Ki Yong Na, Gou Young Kim, Kyu Yeoun Won, Hyun-Soo Kim, Sang Won Kim, Chi Hoon Lee, Jae Myung Cha
- Korean J Pathol. 2013;47(2):177-181. Published online April 24, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.2.177
- 9,995 View
- 76 Download
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF A uterus-like mass (ULM) is a central cavity lined by endometrial glands and stroma and surrounded by thick-walled smooth muscles. To date, 31 cases of ULM have been reported in the English literature. ULM typically presents as a single mass and is located in the pelvic cavity. We report here a very rare case of multiple extrapelvic ULMs involving the cecum, descending colon, and mesocolon. After extensive literature research, our case appears to be the first case of multiple ULMs found in extrapelvic sites and the first case of ULM in the colon. The present case suggests that ULM should be included in the differential diagnosis of colonic submucosal tumors in female patients with chronic abdominal pain or menstruation-associated symptoms.
-
Citations
Citations to this article as recorded by- Ultrasonographic Imaging Features of Accessory Cavitated Uterine Malformations and Application to Diagnosis
Ruijie Sun, Xinting Liu, Niya Wei, Xiaokun Li, Ying Zou, Yue Wang
Journal of Clinical Ultrasound.2025; 53(8): 1707. CrossRef - Clinical Diagnosis and Treatment Analysis of Accessory Cavity Uterine Malformation in Adolescents (with a Case Report and Literature Review)
义敏 项
Advances in Clinical Medicine.2025; 15(08): 1098. CrossRef - Accessory cavitated uterine malformation in a perimenopausal woman: Case report and literature review
Yuan Zhang, Hanxue Lv, Congjie Lin, Hua Liu
Journal of International Medical Research.2025;[Epub] CrossRef - Extrauterine adenomyoma: A case report and systematic review of the literature
Matteo Giorgi, Luca Labanca, Gabriele Centini, Lucia Lazzeri, Francesco Giuseppe Martire, Ester Sorrentino, Virginia Mancini, Diego Raimondo, Antonio Raffone, Daniele Neola, Anna Chiara Aru, Nassir Habib, Paolo Casadio, Renato Seracchioli, Errico Zupi
International Journal of Gynecology & Obstetrics.2024; 164(3): 869. CrossRef - Extrapelvic “Uterus Like Mass” Following Laparoscopic Morcellation Hysterectomy - a Consequence of Iatrogenic Implantation?
Neha Bakshi, Shashi Dhawan
International Journal of Surgical Pathology.2023; 31(5): 791. CrossRef - Extrauterine adenomyoma of the lesser omentum: A case report and review of the literature
Yanlin Chen, Liangyong Deng, Jingbo Zhao, Tianwen Luo, Zhong Zuo
Medicine.2022; 101(36): e30240. CrossRef - Pelvic Pain and Adnexal Mass: Be Aware of Accessory and Cavitated Uterine Mass
Pooya Iranpour, Sara Haseli, Pedram Keshavarz, Amirreza Dehghanian, Neda Khalili, Michael S. Firstenberg
Case Reports in Medicine.2021; 2021: 1. CrossRef - Endomyometriosis of the Rectum With Disseminated Peritoneal Leiomyomatosis 8 Years After Laparoscopic Myomectomy: A Case Report
Giorgio La Greca, Cristina Colarossi, Paolo Di Mattia, Cecilia Gozzo, Marco De Zuanni, Eliana Piombino, Lorenzo Memeo
Frontiers in Surgery.2021;[Epub] CrossRef - Imaging Manifestations of Accessory Cavitated Uterine Mass—A Rare Mullerian Anomaly
Tharani Putta, Reetu John, Betty Simon, Kirthi Sathyakumar, Anuradha Chandramohan, Anu Eapen
Indian Journal of Radiology and Imaging.2021; 31(03): 545. CrossRef - A rare case of ovarian adenomyoma mimicking primary invasive ovarian cancer with a contralateral serous borderline ovarian tumor: A case report and review of the literature
Viola Liberale, Alessandra Surace, Lorenzo Daniele, Luca Liban Mariani
Heliyon.2020; 6(7): e04406. CrossRef - Extrauterine adenomyoma located in the inguinal region: a case report of a 44-year-old woman
Winesh Ramphal, Chloé M L Peters, Luthy S M Alcalá, Dennis van Hamont, Paul D Gobardhan
Journal of Surgical Case Reports.2020;[Epub] CrossRef - Accessory and Cavitated Uterine Mass: Is It a Müllerian-Duct Anomaly?
Vani Malhotra, Sonia Dahiya, Smiti Nanda, Meenakshi Chauhan, Vandana Bhuria
Journal of Gynecologic Surgery.2020; 36(6): 350. CrossRef - Uterus-like mass in the right broad ligament
Lei Liu, Hui Yang, Shu-Peng Zhang
Medicine.2019; 98(38): e17246. CrossRef - Extrauterine adenomyoma: a review of the literature
P.G. Paul, Gunjan Gulati, Hemant Shintre, Sumina Mannur, George Paul, Santwan Mehta
European Journal of Obstetrics & Gynecology and Reproductive Biology.2018; 228: 130. CrossRef - Uterus-like mass
Jian He, Jie Xu, Hong-Yan Zhou
Medicine.2016; 95(39): e4961. CrossRef - Endometrioid Adenocarcinoma in an Extrauterine Adenomyoma
Michael A. Ulm, David B. Robins, Edwin M. Thorpe, Mark E. Reed
Obstetrics & Gynecology.2014; 124(2): 445. CrossRef - Endomyometriosis (“Uterus - like mass”) in an XY Male
Raul S. González, Cindy L. Vnencak-Jones, Chanjuan Shi, Oluwole Fadare
International Journal of Surgical Pathology.2014; 22(5): 421. CrossRef
- Ultrasonographic Imaging Features of Accessory Cavitated Uterine Malformations and Application to Diagnosis
- Silent Colonic Malakoplakia in a Living-Donor Kidney Transplant Recipient Diagnosed during Annual Medical Examination
- Go Eun Bae, Nara Yoon, Ha Young Park, Sang Yun Ha, Junhun Cho, Yunkyung Lee, Kyoung-Mee Kim, Cheol Keun Park
- Korean J Pathol. 2013;47(2):163-166. Published online April 24, 2013
- DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.2.163
- 8,252 View
- 61 Download
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Malakoplakia is a characteristic inflammatory condition, which is usually seen in the urogenital tract, and less frequently in the gastrointestinal tract. We present a case of colonic malakoplakia in an immunocompromised patient. A 55-year-old female visited the outpatient clinic for routine cancer surveillance. Her past medical history was significant for kidney transplantation 11 years ago, and she had been taking immunosuppressants. A colonoscopy revealed several depressed flat lesions and elevated polyps, which were 0.3 to 0.4 cm in size and accompanied by whitish exudates. A biopsy revealed an infiltration of histiocytes with ample granular eosinophilic cytoplasm, with some lymphocytes and plasma cells. Many histiocytes had the characteristic morphology, described as Michaelis-Gutmann bodies: one or several round basophilic structures of approximately 1 to 10 µm in size with some being laminated, some appearing homogeneous, and others having a dense central core with a targetoid appearance. These Michaelis-Gutmann bodies were positively stained on von Kossa stain, and were diagnostic for malakoplakia.
-
Citations
Citations to this article as recorded by- Malakoplakia in kidney transplant recipients: Three case reports
Prathap Kumar Simhadri, Renish Contractor, Deepak Chandramohan, Matthew McGee, Udit Nangia, Mohammad Atari, Syed Bushra, Sanjana Kapoor, Ramya Krishna Velagapudi, Pradeep K Vaitla
World Journal of Nephrology.2025;[Epub] CrossRef - Caecal malakoplakia: a rare mimic of malignancy
Jeffrey Li Voon Chong, Noor Ali
BMJ Case Reports.2024; 17(1): e257130. CrossRef - A Surgical Challenge Generated by Colonic Malakoplakia in Disguise as a Locally Advanced Colonic Malignancy—A Case Report
Cristina Șerban, Alexandra Toma, Dragoș Cristian Voicu, Constantin Popazu, Dorel Firescu, George Țocu, Raul Mihailov, Laura Rebegea
Medicina.2023; 59(1): 156. CrossRef - Colonic malakoplakia in a cardiac transplant recipient: A case report
Sadiya Shafijan
Indian Journal of Pathology and Microbiology.2020; 63(2): 322. CrossRef - Immunosuppressive drugs and the gastrointestinal tract in renal transplant patients
Merel M. Tielemans, Gerben A.J. van Boekel, Teun van Gelder, Eric T. Tjwa, Luuk B. Hilbrands
Transplantation Reviews.2019; 33(2): 55. CrossRef - Malakoplakia of the colon following renal transplantation in a 73 year old woman: report of a case presenting as intestinal perforation
Andrew Mitchell, Alexandre Dugas
Diagnostic Pathology.2019;[Epub] CrossRef - Colonic malakoplakia in a liver transplant recipient: A case report
Rana Ajabnoor, Mohammad Mawardi, Abdulmonem Almutawa
Human Pathology: Case Reports.2019; 18: 200323. CrossRef - Malakoplakia after kidney transplantation: Case report and literature review
John Fredy Nieto‐Ríos, Isabel Ramírez, Mónica Zuluaga‐Quintero, Lina María Serna‐Higuita, Federico Gaviria‐Gil, Alejandro Velez‐Hoyos
Transplant Infectious Disease.2017;[Epub] CrossRef - Megalocytic Interstitial Nephritis Following Acute Pyelonephritis with Escherichia coli Bacteremia: A Case Report
Hee Jin Kwon, Kwai Han Yoo, In Young Kim, Seulkee Lee, Hye Ryoun Jang, Ghee Young Kwon
Journal of Korean Medical Science.2015; 30(1): 110. CrossRef
- Malakoplakia in kidney transplant recipients: Three case reports
- Strongyloidiasis of Gastric and Colonic Mucosa in a Patient with Monoclonal Gammopathy of Undetermined Significance: A Case Report.
- Jung Uee Lee, Sang Bum Kang, Hae Joung Sul, Jong Ok Kim
- Korean J Pathol. 2011;45:S75-S78.
- DOI: https://doi.org/10.4132/KoreanJPathol.2011.45.S1.S75
- 3,668 View
- 20 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - Here we report a case of Strongyloides stercoralis infection of the gastric and pancolonic mucosa in a 79-year-old female with a monoclonal gammopathy of undetermined significance. Endoscopic biopsies were performed in gastric antrum, cecum, distal ascending colon, and hepatic flexure of the colon. On microscopic examination, there were many adult worms, larvae and eggs in the gastric and colonic mucosa. Worms, larvae, and eggs were located in the crypts and within the lumen of the crypts. The body wall of the adult worm was composed of cuticle and a weak muscle layer. A routine stool examination failed to detect larvae or ova. Based on the histopathologic examination, these parasites were confirmed as S. stercoralis.
-
Citations
Citations to this article as recorded by- Is Gastric Involvement by Strongyloides stercoralis in an Immunocompetent Patient a Common Finding? A Case Report and Review of the Literature
Irene Pecorella, Tom Richard Okello, Gaia Ciardi, David Martin Ogwang
Acta Parasitologica.2022; 67(1): 94. CrossRef
- Is Gastric Involvement by Strongyloides stercoralis in an Immunocompetent Patient a Common Finding? A Case Report and Review of the Literature
- A Metastatic Granulocyte Colony-Stimulating Factor Producing Sarcomatoid Carcinoma of the Lung Causing Jejunal Intussusception: Report of a Case.
- Min Eui Hong, Soon Auck Hong, Gui Young Kwon, Tae Jin Lee, Eon Sub Park, Sung Jae Cha, Jae Hyuk Do, Jae Hyung Yoo
- Korean J Pathol. 2011;45(2):205-208.
- DOI: https://doi.org/10.4132/KoreanJPathol.2011.45.2.205
- 3,550 View
- 26 Download
-
 Abstract
Abstract
 PDF
PDF - A 75-year-old man was referred to our hospital with intestinal obstruction caused by intussusception. Abdominal computed tomography (CT) revealed seven polypoid masses in the small intestine, while chest CT revealed a mass in the right lower lobe. Preoperative laboratory tests showed white blood cell (WBC) and neutrophil differential counts of 63,630/mm3 and 95%, respectively. The serum granulocyte colony-stimulating factor (G-CSF) was 114 pg/mL, which was elevated (normal range, <18.1 pg/mL). After resection of the small bowel, the WBC count decreased to 20,510/mm3. The pathology showed a poorly differentiated carcinoma with sarcomatous components confirmed by positive immunostaining of cytokeratin (AE1/AE3) and vimentin in the small intestine. Furthermore, immunohistochemistry with specific monoclonal antibodies against G-CSF was positive. A lung biopsy revealed the same histological findings as the small intestine lesion. Therefore, the patient was diagnosed as having a G-CSF producing sarcomatoid carcinoma of the lung with metastasis to the small intestine.
- Lethal Giant Larvae2 Expression Is Reduced or Localized at Cytoplasm in Colon Adenomas and Adenocarcinomas.
- Tae Jung Jang
- Korean J Pathol. 2010;44(5):488-492.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.5.488
- 3,347 View
- 24 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The Scribble, Par and Crumbs polarity modules are essential for establishing and maintaining apicobasal cell polarity in epithelial cells. The aim of the present study was to investigate the expression pattern of Lethal giant larvae2 (Lgl2) in normal colonic epithelium and epithelial tumors and to examine the relationship between Lgl2 expression and clinicopathological parameters.
METHODS
We examined Lgl2 expression in 66 primary colon cancers and 20 adenomas by immunohistochemistry.
RESULTS
In normal colonic epithelium, Lgl2 was strongly expressed at the basolateral membrane of cells in the luminal surface but was not expressed at the base of crypts. The expression pattern of E-cadherin in normal epithelium was similar to that of Lgl2. In contrast, tumors did not express Lgl2 or showed diffuse cytoplasmic staining. The Lgl2 positive rate in tumors was significantly lower than in normal epithelium, and its negative rate in tumors was higher in tumors with abnormal E-cadherin expression than in tumors with positive membranous staining. Lgl2 staining intensity was significantly lower in tumor budding sites than in tumor centers. No significant differences were observed between Lgl2 and clinicopathological parameters.
CONCLUSIONS
Lgl2 expression was reduced or localized at the cytoplasm in colon epithelial tumors, suggesting that a perturbation of Lgl2 expression frequently occurs in colon epithelial tumors.
- Mutation and Expression of DNA2 Gene in Gastric and Colorectal Carcinomas.
- Sung Hak Lee, Yoo Ri Kim, Nam Jin Yoo, Sug Hyung Lee
- Korean J Pathol. 2010;44(4):354-359.
- DOI: https://doi.org/10.4132/KoreanJPathol.2010.44.4.354
- 4,805 View
- 35 Download
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Deregulation of DNA repair and replication are involved in cancer development. DNA2 is a nuclease/helicase that plays roles in DNA repair and replication. The aim of this study was to explore DNA2 mutation and DNA2 protein expression in gastric cancers (GCs) and colorectal cancers (CRCs).
METHODS
We analyzed two mononucleotide repeats in DNA2 in 27 GCs with high microsatellite instability (MSI-H), 34 GCs with stable MSI (MSS), 29 CRCs with MSI-H and 35 CRCs with MSS by single-strand conformation polymorphism. We also analyzed DNA2 expression in GCs and CRCs either with MSI-H or MSS.
RESULTS
We found DNA2 mutations in two GCs (7.1%) and two CRCs with MSI-H (6.9%), but not in cancers with MSS. The mutations consisted of three cases of a c.2593delT and one of a c.2592_2593delTT, which would result in premature stopping of amino acid synthesis (p.Ser865Hisfsx6 and p.Ser865Thrfsx20, respectively). DNA2 expression was observed in 16 (80%) of the GCs and 15 (75%) of the CRCs with MSI-H, but all of the cancers with DNA2 frameshift mutations were weak or negative for DNA2.
CONCLUSIONS
Our data indicate that DNA2 mutation and loss of DNA2 expression occur in GCs and CRCs, and suggest that these alterations may contribute to cancer pathogenesis by deregulating DNA repair and replication. -
Citations
Citations to this article as recorded by- Multiple roles of DNA2 nuclease/helicase in DNA metabolism, genome stability and human diseases
Li Zheng, Yuan Meng, Judith L Campbell, Binghui Shen
Nucleic Acids Research.2020; 48(1): 16. CrossRef - Integration of multiple networks and pathways identifies cancer driver genes in pan-cancer analysis
Claudia Cava, Gloria Bertoli, Antonio Colaprico, Catharina Olsen, Gianluca Bontempi, Isabella Castiglioni
BMC Genomics.2018;[Epub] CrossRef - Replication intermediates that escape Dna2 activity are processed by Holliday junction resolvase Yen1
Gizem Ölmezer, Maryna Levikova, Dominique Klein, Benoît Falquet, Gabriele Alessandro Fontana, Petr Cejka, Ulrich Rass
Nature Communications.2016;[Epub] CrossRef
- Multiple roles of DNA2 nuclease/helicase in DNA metabolism, genome stability and human diseases
- Significance of the Expression of Cathepsins B, H, & L in Colonic Epithelial Neoplasms.
- Jae Young Sim, Mi Ja Lee, Keun Hong Kee
- Korean J Pathol. 2009;43(5):408-412.
- DOI: https://doi.org/10.4132/KoreanJPathol.2009.43.5.408
- 3,524 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Cathepsin is associated with tumorigenesis, tumor invasion and metastasis through its ability to induce degradation of extracellular matrix components.
METHODS
To investigate the correlation between cathepsin expression and tumor progression, invasion depth or nodal metastasis, immunohistochemical staining for cathepsins B, H and L were done on 20 hyperplastic polyps, 48 adenomas, and 67 adenocarcinomas of the colon. Evaluation of the expression of cathepsins B, H and L was based on the percentage of neoplastic cells that stained positive for any given cathepsin.
RESULTS
Cathepsin B expression was significantly higher in adenocarcinomas than adenomas (29.33 vs 5.48%), but was not associated with the degree of differentiation, depth of invasion and nodal status of the tumors. Expression of cathepsins H and L was absent or low in both adenomas and adenocarcinomas. CONCLUSIONS: We suggest that cathepsin B is involved in progression of a subset of colonic adenomas, while cathepsins H and L are not.
- Gastrointestinal Stromal Tumor of the Colon Mimicking Inflammatory Fibroid Polyp with a Novel 63 bp c-kit Deletion Mutation: A Case Report.
- In Gu Do, Cheol Keun Park, Sung Hyun Yoon, John Goldblum, Kyoung Mee Kim
- Korean J Pathol. 2009;43(4):374-377.
- DOI: https://doi.org/10.4132/KoreanJPathol.2009.43.4.374
- 3,584 View
- 26 Download
-
 Abstract
Abstract
 PDF
PDF - Colonic gastrointestinal stromal tumors (GISTs) are rare and behave aggressively compared to GISTs in other parts of the gastrointestinal tract. Therefore, accurate diagnosis of GISTs and their distinction from other mesenchymal tumors is important for proper patient management and follow-up. Herein, we present an unusual case of a colonic GIST mimicking an inflammatory fibroid polyp with a novel 63 bp deletion mutation in exon 11 of the c-kit gene, which has not previously been reported. The tumor consisted of loosely arranged spindle cells and many inflammatory cells scattered throughout the tumor. Immunohistochemically, the tumor cells were focally and weakly positive for c-kit and diffusely positive for CD34, but were negative for PKC-theta, SMA, S-100 protein, ALK-1, and desmin. Our case re-emphasizes the broad morphologic spectrum of GISTs.
- Familial Juvenile Polyposis.
- Sun Hee Chang, Shi Nae Lee, Hea Soo Koo, Ok Kyung Kim, Sun Sub Jung, Eung Bum Park
- Korean J Pathol. 1997;31(2):185-188.
- 1,878 View
- 14 Download
-
 Abstract
Abstract
 PDF
PDF - Familial juvenile polyposis is a rare intestinal polyposis characterized by the occurrence of multiple juvenile polyps in the gastrointestinal tract. We report a case of familial juvenile polyposis in a 17-year-old man with a review of the literature. This patient underwent total colectomy due to a 6 years history of rectal bleeding. Grossly, the colon showed 36 variable sized pedunculated polyps, measuring 2.5cm x 2cm from the largest size and 0.2cm x 0.2cm to the smallest size. Histologically, the polyps consisted of cystically dilated glands, lined by normal colonic epithelial cells, scattered in loose, edematous stroma containing inflammatory cell infiltration. There were no areas of tubular adenoma or malignancy in any of the polyp.
- Ectopic Paragonimiasis Presented as Multiple Colonic and Liver Masses.
- Hye Sung Kim, Young Soo Lee, Yun Kyung Kang, Hye Kyung Lee, Jun Hee Kim, Hyuk Sang Lee
- Korean J Pathol. 1997;31(4):357-360.
- 1,959 View
- 15 Download
-
 Abstract
Abstract
 PDF
PDF - Ectopic paragonimiasis has been diagnosed in many organs such as the mesentery, ovary, pleura, central nervous system, subcutis and very rarely in the liver. However, simultaneous involvement of the colon and liver, which mimics colonic cancer with liver metastasis, is quite unusual, and to our knowledge has never been reported. Our case is a 63 year old woman who visited our hospital because of upper abdominal pain. Radiologically, space occupying lesions were detected in the transverse colon, mesocolon and left hepatic lobe. After the radical presection, they were proved to be an ectopic paragonimiasis forming multiple cavitary parasitic granulomas with Charcot-Leyden crystals and degenerating eggs.
- Two Cases of Strongyloidiasis Diagnosed by Colonoscopic Biopsy.
- Sang Chul Nam, Man Hoon Han, Young Su Kim, Yoon Seup Kum, In Soo Suh, Han Ik Bae
- Korean J Pathol. 2007;41(5):343-346.
- 2,305 View
- 23 Download
-
 Abstract
Abstract
 PDF
PDF - Strongyloides stercoralis is an intestinal nematode that is able to infect the host tissue and persist for many years through autoinfection, and it causes life-threatening hyperinfection in immunocompromised hosts. We report here on two cases of strongyloidiasis that were diagnosed by colonoscopic biopsy. One case was a 73-year-old woman who was hospitalized with complaints of melena. She was being treated with corticosteroid due to her asthma and rheumatoid arthritis. The other case was a 63-year-old man who suffered with abdominal discomfort and severe loss of body weight (18 kg) for 2 months. In both cases, colonoscopic examination revealed polyps and petechiae at the entire colon. Microscopically, a small illdefined granuloma with a longitudinally sectioned parasite was seen on the colonoscopic biopsy. Endoscopic examination was done after suspecting parasitic infestation. The gastric and duodenal mucosa showed numerous cross sections of adult worms, eggs and larvae that were developing in crypts. Even if such a patient is in an asymptomatic state, this illness must be treated due to the potential for fatal autoinfection.
- Crohn's Disease Involving Small Intestine and Colon: 2 cases report.
- Shi Nae Lee, Sun Hee Chang, Hee Soo Yoon, Hea Soo Koo, Ok Kyung Kim, Ryung Ah Lee, Eung Beum Park
- Korean J Pathol. 1997;31(4):379-382.
- 2,106 View
- 13 Download
-
 Abstract
Abstract
 PDF
PDF - Crohn's disease was originally described as a small bowel disorder and has been known to involve the large bowel in approximately 40% of all cases with or without concomitant ileal component. We describe two cases of Crohn's diseas of small intestine and colon with a summary of differential diagnosis with ulcerative colitis. Both cases were originally diagnosed and treated as ileal tuberculosis. Grossly, there were skip lesions in both cases with prominent pseudopolyps and ulcerations in colon. Also noted were typical serpentine lesions in ileum as well as in colon. Microscopically, transmural inflammation was confirmed and one case showed scattered noncaseating granulomas in the wall. Submucosal edema and fibrosis with thickening of the wall was not prominent in colon. Polymerase chain reaction performed on paraffin block for the demonstration of Mycobacterium tuberculosis in one case showed negative reaction.
- Expression of bcl-2 Protein in Colorectal Adenoma and Adenocarcinoma and its Relationship with p53 and Apoptosis.
- Ae Ree Kim, Seong Jin Cho, Nam Hee Won, Yang Seok Chae
- Korean J Pathol. 1997;31(5):417-426.
- 1,929 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - Either increased cellular proliferation or decreased death might result in an expansion of their numbers in the oncogenic process. Cellular apoptosis represents an autonomous suicide pathway that helps to restrict the cell number. However bcl-2 and mutant p53 inhibit programmed cell death. To determine whether the bcl-2 gene is activated during colorectal tumorigenesis and whether it has any relationship with p53 and apoptosis, we studied the expression of bcl-2 and p53 in the normal colonic mucosa, in the adenomatous polyps and in the adenocarcinomas using the immunohistochemical method. Also we evaluated the status of apoptosis using the in situ end labeling method. The bcl-2 immunoreactivity was restricted to the basal epithelial cells of all normal colonic mucosa and they were expressed in all adenomas and 86% of adenocarcinomas, especially in the superficial lesion of some tumors. Mutations of p53 were not found in the normal colonic mucosa, but they were present in dysplastic cells of adenomas (52%) and in cancer cells of the adenocarcinomas (47%). Apoptosis was confined to the tips of the normal colonic mucosa. It was more easily detected in the p53-positive adenomas than in the p53-negative adenomas (p=0.010). In the adenocarcinomas, the findings of apoptotic process are not related with p53 mutation (p=0.3) and bcl-2 expression (p=0.187). p53 and bcl-2 are probably one step of several apoptotic processes in the adenocarcinomas.
- Pathologic Characteristics of Colorectal Cancers with DNA Replication Errors.
- Hoguen Kim, Yoon Mi Jeen, Jeong Yeon Shim, Chanil Park
- Korean J Pathol. 1995;29(5):590-595.
- 1,885 View
- 10 Download
-
 Abstract
Abstract
- Unstable microsatellite repeat sequences or DNA replication errors(RER) due to defective mismatch repair genes have been reported in a subset of sporadic colorectal tumors and in most tumors of patients of hereditary nonpolyposis colorectal carcinoma(HNPCC). To elucidate the clinicopathological correlation of these RER-positive cancers, we examined 16 cases of colorectal carcinoma of different histologic subtypes(6 cases of carcinoma with no gland formation, 5 cases of mucinous carcinoma and 5 cases of gland forming carcinoma). We detected RER in five cases. The patients with RER-positive cancers had a marked preponderance of carcinoma with no gland formations out of 6 carcinomas with no gland formation were RER-positive cancers) and of cancers proximal to splenic flexure(all of the RER-positive cases were proximal colon carcinomas). We conclude that RER-positive cancers have wiique pathologic features that may be useful for the screening and counselling of patients with hereditary colon cancers.
- Expression of pS2/TFF1 Protein in Normal Colonic Mucosa, Adenoma and Adenocarcinoma.
- Seoung Wan Chae, Eun Yoon Cho, Eo Jin Kim, Jin Hee Sohn, Young Euy Park
- Korean J Pathol. 2004;38(5):324-329.
- 2,128 View
- 13 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The trefoil factor 1 protein (pS2/TFF1) is a candidate tumor-suppressor protein, and it is a pleiotropic factor involved in the organization and homeostasis of the gastrointestinal tract and various inflammatory or neoplastic diseases. The purpose of this study was to assess the expression of pS2/TFF1 and its clinicopathologic relationship, including the p53 and Ki-67 labeling index, in colorectal carcinogenesis.
METHODS
The expression of pS2/TFF1 protein was evaluated immunohistochemically in 45 samples of normal colonic mucosa, 43 samples of adenoma and 186 samples of colorectal carcinoma.
RESULTS
pS2/TFF1 protein was expressed weakly in 37.8% of normal colonic mucosa samples, and it had a weak to strong expression in 48.8% of adenomas and 28% of colorectal adenocarcinomas. pS2/TFF1 expression in carcinoma was slightly increased in the poorly differentiated group compared with the well to moderately differentiated group (p=0.059). Interestingly, mucinous carcinoma (4/4) and signet ring cell carcinoma (2/3) showed significant increase of pS2/TFF1 expression. pS2/TFF1 expression was inversely correlated with the p53 protein expression and the Ki-67 labelling index (p<0.05). There was no significant correlation with the tumor size, metastasis or pathologic staging.
CONCLUSIONS
Overexpression of pS2/TFF1 expression in colorectal adenocarcinoma was inversely correlated with the Ki-67 labelling index and the p53 expression in cancer. These results suggest that pS2/TFF1 protein may contribute as tumor suppressor factor in colorectal adenocarcinoma.
- Desmoplastic Small Round Cell Tumor of the Sigmoid Colon.
- Kwang Il Kim, Jung Yeol Kim, Insun Kim
- Korean J Pathol. 2001;35(5):451-454.
- 2,063 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - Desmoplastic small round cell tumor (DSRCT) is a distinctive disease with a multidirectional differentiation and an aggressive clinical course. It mostly occurs in mesothelial-lined sites, and tumors originating in the paratesticular region, pleura and central nervous system are rarely reported. We report a case of DSRCT occurring in the sigmoid colon of a 39-year-old man, which was difficult to distinguish from small cell neuroendocrine carcinoma. The tumor was characterized by small round cells with irregular nests, cords, or rosette-like structures in the striking desmoplastic stroma. Some tumor cells had a rhabdoid feature with eosinophilic cytoplasmic globules. The tumor cells showed immunoreactivity for cytokeratin, epithelial membrane antigen, vimentin, desmin, neuron-specific enolase and Leu-7. Electron microscopic finding revealed perinuclear globoid whorls of intermediate filaments pushing the nucleus eccentrically.
- Composite Adenocarcinoma and Choriocarcinoma of the Sigmoid Colon with Hepatic Metastasis of the Choriocarcinomatous Component.
- Young Ha Oh, Won Mee Lee, Kyung Sook Kim, Moon Hyang Park, Jung Dal Lee
- Korean J Pathol. 1997;31(8):788-793.
- 2,091 View
- 28 Download
-
 Abstract
Abstract
 PDF
PDF - A rare case of hepatic metastasis with a choriocarcinomatous component from a composite adenocarcinoma and choriocarcinoma of the sigmoid colon in a 60-year-old man is reported. The hepatic metastasis displayed choriocarcinoma with extensive hemorrhagic necrosis. The tumor cells were poorly differentiated with scattered foci of bizzare syncytiotrophoblastic cells. Retrospective examination of the previous colonic carcinoma proved that the tumor was composed of two distinctive elements. One was a moderately well differentiated adenocarcinoma located in mucosa and submucosa. The other was a deep seated and undifferentiated carcinoma which was made up of hyperchromatic bizzare cells with syncytiotrophoblastic cells. There were transitional foci from adenocarcinoma to undifferentiated carcinoma with trophoblastic cells. Immunohistochemical staining showed beta-hCG expression in the undifferentiated cells of both the primary and the metastatic tumors. Implications for the possible origin and cause of tumor cell heterogeneity are briefly discussed.
- Choriocarcinoma of the Colon.
- Youn Mee Kim, Mee Youn Cho, Soon Won Hong, Soon Hee Jung
- Korean J Pathol. 1997;31(8):794-797.
- 2,202 View
- 40 Download
-
 Abstract
Abstract
 PDF
PDF - Choriocarcinoma of the gastrointestinal tract is rare. Among them, that of the stomach is the most common. Six cases of choriocarcinoma of the colon were found in the review of the literature. All of these previously reported cases had multiple metastatic foci in the liver, lung, lymph nodes and the prognosis seemed to be very poor. Therefore we think that choriocarcinoma of the colon should be distinguished from conventional adenocarcinoma. A 66-year old female patient, described in this case, was operated on under the impression she was suffering from acute appendicitis. The resected ascending colon revealed extensive hemorrhagic necrosis and perforation with fibrous adhesion in the cecum. On the cut section, the mural tumorous thickening was not definite. Histologically, the tumor showed a focus of typical adenocarcinoma arising from glandular epithelial cells, which were transformed into highly anaplastic tumor cells. There were frequent vascular invasions of tumor cells, similar to syncytiotrophoblasts. In the immunohistochemical stains, both glandular and highly anaplastic tumor cells reacted with cytokeratin. The glandular cells were also reactive for carcinoembryonic antigen (CEA) and anaplastic tumor cells for human chorionic gonadotrophin (hCG). This is the first report of choriocarcinoma of the colon in Korea. We describe this case with a review of the literature.
- Mutational Analysis of Proapoptotic bcl-2 Family genes in Colon Carcinomas.
- Young Hwa Soung, Jong Woo Lee, Su Young Kim, Suk Woo Nam, Won Sang Park, Jung Young Lee, Nam Jin Yoo, Sug Hyung Lee
- Korean J Pathol. 2005;39(3):168-171.
- 2,038 View
- 29 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Several lines of evidence have indicated that the deregulation of apoptosis is involved in the mechanisms of cancer development, and somatic mutations of the apoptosisrelated genes have been reported in human cancers. Members of the bcl-2 family proteins regulate the intrinsic apoptosis pathway mainly in the mitochondria. The aim of this study was to explore whether the somatic mutation of the proapoptotic bcl-2 family genes, one of the mechanisms that prolong the survival of cancer cells, occurred in colorectal carcinomas.
METHODS
In the current study, to detect the somatic mutations in the DNA sequences encoding the bcl-2 homology 3 (BH3) domain of the human bak, bid, bik, bim, PUMA, bcl-rambo, bcl-G, and bmf genes in 98 colon adenocarcinomas, we used polymerase chain reaction (PCR), single strand conformation polymorphism (SSCP), and DNA sequencing.
RESULTS
The SSCP analysis detected no evidence of somatic mutations of the genes in the coding regions of the BH3 domain in the cancers.
CONCLUSIONS
The data presented here indicate that the proapoptotic bcl-2 family genes, bak, bid, bik, bim, PUMA, bcl-rambo, bcl-G and bmf may not be somatically mutated in human colorectal carcinomas, and suggest that the colorectal cancers may not utilize mutational events of these proapoptotic bcl-2 family genes in the mechanisms for evading apoptosis.
- Squamous Metaplasia in Tubular Adenoma of Sigmoid Colon: A case report.
- Soo Min Kang, Weon Seo Park, Woo Ho Kim, Yong Il Kim
- Korean J Pathol. 1993;27(6):663-665.
- 4,281 View
- 177 Download
-
 Abstract
Abstract
 PDF
PDF - The occurrence of squamous metaplasia(morule) in colorectal mucosa and adenocarcinoma, althrough rare, has been well documented. In contrast, very little mention has been given to mature squamous cells seen in colorectal polyps or adenomas. A 42-year-old woman presented with a 2-month history of diarrhea and melena. Proctosigmoidoscopy revealed a 4 cm-sized polypoid tumor 20 cm above the anal verge. Colonoscopic biopsy showed tubular adenoma, and a segmental resection of sigmoid colon was done. Microscopically, the tumor was c classical tubular adenoma containing multiple solid nests of squamous cells scattered only in the neoplasm; the squamous nests were generally small, and some showed direct continuity with adenomatous glands. The squamous cells were keratinizing and had regular nuclei with no mitotic activity. The importance of this phenomenon lies in its pathologic recognition, and the findings suggest that awareness of this rare occurrence in colorectal polyps should avert such overdiagnosis, and consequently prevents unnecessary radical surgery.
- Expression of Osteopontin, ZO-1 and E-cadherin in Adenoma and Adenocarcinoma of the Colon.
- Yu Kyung Jeong, Mi Ja Lee, Sung Chul Lim, Keun Hong Kee, Ho Jong Jeon, Chae Hong Suh
- Korean J Pathol. 2005;39(4):242-250.
- 2,174 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - Background
: The expressions of osteopontin (OPN), zonula occludens-1 (ZO-1) and E-cadherin, known as cell adhesion-associated substances, were examined in adenoma and adenocarcinoma of the colon. The relationship of their expressions with clinicopathologic factors was examined to investigate the roles of these proteins in the development, invasion or metas- tasis of colon adenocarcinoma. Methods : The expressions of OPN, ZO-1, and E-cadherin were examined in 54 cases of adenoma and 67 cases of adenocarcinoma of the colon by immunohistochemical staining. Results : The expression of OPN in colon adenocarcinoma correlated with staging (p=0.012) and distant metastasis (p=0.021). The expression of ZO-1 was closely related with tumor cell differentiation (p<0.001), and the reduced expression of E-cadherin was associated with tumor cell differentiation (p=0.05) and lymph node metastasis (p<0.001). Co-expression of ZO-1 and E-cadherin was significantly associated with tumor cell differentiation, and the expressions of ZO-1 and E-cadherin were reduced or lost in all cases (5 cases) of poorly differentiated adenocarcinoma. Conclusions : Our data suggest that OPN is involved in the process of invasion and metastasis of colon adenocarcinoma, and ZO-1- and E-cadherin-mediated cell adhesion may play an important role in the differentiation of colon adenocarcinoma.
- The Differential Expressions of the Epithelial-Mesenchymal Transition Regulator, Slug and the Cell Adhesion Molecule, E-cadherin in Colorectal Adenocarcinoma.
- Ran Hong, Dong Yul Choi, Sung Chul Lim, Chae Hong Suh, Keun Hong Kee, Mi Ja Lee
- Korean J Pathol. 2008;42(6):351-357.
- 2,476 View
- 24 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Slug is a member of the Snail family of transcription factors, and it plays a crucial role in the regulation of the epithelial-mesenchymal transition by suppression of several epithelial proteins and adhesion molecules, including E-cadherin. METHODS: The aim of the present study was to examine the significance between the expression of Slug in colorectal adenocarcinoma (CRA) specimens and the clinicopathological parameters of CRA, as determined by immunohistochemical analysis, and to determine the correlation between the Slug and E-cadherin expressions in non-neoplastic colorectal mucosa (n=45), primary CRA (n= 109) and metastatic CRA (n=17). A semiquantitative scoring system was applied based on the intensity and extent of the positive immunohistochemical staining. RESULTS: The expressions of Slug and E-cadherin were associated with the depth of tumor invasion (pT) (p=0.019, p=0.001, respectively), and these expressions showed a significant inverse correlation (p<0.001) each other. CONCLUSIONS: Our results demonstrated a positive role for Slug in the development of CRA, and Slug is a mediator of tumor invasion in CRA. In addition, an up-regulated Slug expression is significantly correlated with the loss of an E-cadherin expression, which suggests that Slug may play some role in the epithelial-mesenchymal transition (EMT) by down-regulating the E-cadherin expression.
- Angiodysplasia Arising in the Bowels: Two cases report.
- Soo Kee Min, Hee Jeung Cha, Joon Mee Kim, Young Chae Chu
- Korean J Pathol. 1997;31(12):1308-1313.
- 2,087 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - Gastrointestinal angiodysplasia is a distinct disease entity which causes frequent gastrointestinal bleeding. It predominantly arises at the stomach and duodenum in the upper gastrointestinal tract and cecum and ascending colon in the lower gastrointestinal tract. The general histological finding of the angiodysplasia is a submucosal vascular ectasia and tortuosity. We have experienced two cases of the intestinal angiodysplasia. The first case occurred on a jejunum in a 22-year-old woman who had anemia. The second case occurred on a sigmoid colon in a 59-year-old man who had constipation. In addition to the general histologic finding of the angiodysplasia, the microscopic findings of the first case revealed some capillary hemangioma-like areas; and in the second case, there was a marked ischemic change and the thickening of the wall.
- Malignant Peripheral Nerve Sheath Tumor in Descending Colon: A Case Report.
- Young S Park, Sung Jing Lim, Woo Ho Kim, Eui Keun Ham
- Korean J Pathol. 2002;36(3):179-183.
- 2,139 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - We report a unique case of malignant peripheral nerve sheath tumor (MPNST) of colon, not associated with neurofibromatosis or parasite infection. The tumor presented as an encircling mass in descending colon causing obstruction with nuberous metastatic lesions in a 43-year-old man. The tumor was largely composed of spindle cells which showed strong positivity for vimemtin, S-100 protein and Leu-7. The tumor often exhibited epithelioid feature where tumor cells were weakly positive for cytokeratin.
- Study on the Stromal Response of Colon Cancer in Relation with the Stage of the Cancer.
- Dong Soo Suk
- Korean J Pathol. 1987;21(2):82-89.
- 1,890 View
- 12 Download
-
 Abstract
Abstract
 PDF
PDF - Total 74 cases of colon cancer were examined in the Inje Medical College, Pusan Paik-hospital, which were collected from 1979 to the April 1986. The stromal histopathological findings are as followings; 1) The highest frequency of the good reactions of the four parameters of the stromal response (i.e., good general stromal reaction, peripheral tumor disintergration, and each inflammatory cell infiltration and fibrous proliferation alone) was found in Dukes class B1 and the least frequency in Dukes C1. The good general stromal response and the peripheral tumor disintegration are more closely related with the stage than the other two parameters. They are about 60% in class B1, 30% in class B2 and C2, and 20% in class C1. 2) The insistent poorest stromal response of the Dukes C1 in all parameters may be explained from the fact that the cancer cells of this particular stage are very aggressive biologically because the cancer cells are capable to invade the regional lymph nodes before the main tumor can infiltrate all through the layers of the wall. 3) Among the four types of the colon cancer, the better stromal reactions are observed in more than half of the cases of the well and moderately differentiated adenocarcinoma while this trend is completely opposite in the mucinous and poorly differentiated types. 4) The above findings indicate that how closely the stroma influences upon the progress of the colon cancer, or how closely it represents the status of the individual immunological force.
- Metastatic Adenocarcinoma of Colon in Meningioma: A case report.
- Yung Suk Lee, Hyun I Cho, Jong Sang Choi, In Sun Kim
- Korean J Pathol. 1994;28(2):173-178.
- 2,305 View
- 30 Download
-
 Abstract
Abstract
 PDF
PDF - Cases of metastases from extracranial tumor to intracranial tumor are very rare. The world wide review of the literatures until 1992 revealed 44 cases of primary intracranial tumors containing metastatic tumors which are unrelated extracranial primary malignant tumors; the intracranial recipient tumor is a meningioma in 35 cases among them. Carcinomas of the lung and the breast are the most common extracranial donor tumors. Metastases from colon cancer to meningioma are extremely rare. A 74 year-old-female presented with headache for 2 weeks. CT revealed a round mass with high signal intensity, measuring 4 cm in diameter, which is located in the left parietal lobe. The patient had colon cancer 2 years ago and lymphoma I year ago. On operation, the tumor is relatively well delineated and attached to the meninx. Microscopically, the tumor is composed of fascicles of long slender, fibroblast-like spindle cells with indistinct cytoplasmic border, variable amount of collagen deposit and many psammoma bodies. A few scattered glands are present in periphery of the meningioma. The tumor glands are composed of columnar cells with basally located hyperchromatic nuclei and similiar to the glands of the adenocarcinoma of the colon.
- The Effect of Uremic Plasma on the Proliferative Activity of CFU-GM in in-vitro Culture of Mouse Bone Marrow.
- Chang Soo Park, Joo Yong Yoo
- Korean J Pathol. 1987;21(4):215-226.
- 1,790 View
- 12 Download
-
 Abstract
Abstract
 PDF
PDF - The increased susceptibility in patients of chronic renal failure to infection has been reported to be attributed to defects in granulocyte and lymphocyte function and proliferative activity of hematopoietic cells. The definite cause of the frequent infection in uremic patients, however, is still controversial. The effect of uremic plasma on the aspect of the hematopoietic cells has been scarcely been studied. In the present study, mouse bone marrow was cultured with uremic plasma, to evaluate the effect of uremic plasma on the proliferative activity and morphological features of CFU-GM. The results obtained were as follows. 1) The number of colonies in group co-cultured with uremic plasma was more reduced than that of normal plasma group. 2) There was no difference between the group cultured with predialytic uremic plasma and that of postdialytic plasma in number of colonies, macroclusters and microclusters. 3) The forms of colony were granulocytic and monocytic forms at 5 day of culture. Electron microscopically, granulocytes disclosed electron dense azurophilic granules and electrolucent specific granules in the cytoplasm, and monocyte showed numerous vesicles and vacuoles in the cytoplasm which had finger-like projections. 4) The molecular weight of inhibitory factor in the uremic plasma was supposed to be less than 50,000 daltons.
- Aberrant Crypt Foci: Histopathologic Classification and Profiles of Mucin Secretion.
- Aeree Kim, Jong Sang Choi, Won Jun Choi, Hong Young Moon
- Korean J Pathol. 2000;34(1):50-55.
- 2,316 View
- 36 Download
-
 Abstract
Abstract
 PDF
PDF - Aberrant crypt foci (ACF) are grossly unidentifiable lesions of the colon and visible only with low-power microscopic examinations after methylene blue stain. To establish the role of ACF in colorectal carcinogenesis, we evaluated the distribution, frequency, histopathological classification, and patterns of mucin secretion of ACF in the colon. A total of 142 aberrant crypt foci were found in 41 colectomy specimen for adenocarcinoma (36 cases) and benign diseases of colon (5 cases). Ten of 142 ACFs were in the ascending and transverse colon, 39 in the descending and sigmoid colon, and 93 in the rectum. The mean number of ACFs in the rectum (0.13 0.11/cm2) was higher than in the ascending and transverse colons (0.019 0.018/ cm2) and descending and sigmoid colon (0.10 0.14/cm2). ACFs were found only in cancer patients. One hundred and twenty ACFs among 142 ACFs identified by topology, were identified on histological examination. We classified ACFs into simple (48.3%), hyperplastic (42.5%), and dysplastic (9.2%) types. All ACFs were infiltrated by the lymphocytes in the stroma and 18 of these accompanied the lymphoid follicles. ACFs have variable histopathologic features and mucin profiles. Some variants of ACFs are at the early stage of the spectrum between benign and malignant.
- The Usefulness of Cytokeratin 7 and Colon Ovarian Tumor Antigen in the Differential Diagnosis of Primary and Metastatic Ovarian Tumors.
- Eung Seok Lee, Hyun Deuk Cho, In Sun Kim
- Korean J Pathol. 1998;32(3):201-207.
- 2,084 View
- 13 Download
-
 Abstract
Abstract
 PDF
PDF - Cytokeratin 7 has been known to be present in various types of human epithelial cells including the ovarian neoplasms, but not in colon cancers. The antibody to colon ovarian tumor antigen (COTA) has been introduced as a marker of colon and ovarian tumors. The aim of this study was to evaluate the usefulness of cytokeratin 7 and COTA in the differential diagnosis between ovarian primary and metastatic tumors. Nineteen primary ovarian epithelial tumors, seven metastatic carcinomas of the ovary from the stomach, three metastatic carcinomas of the ovary from the colon, one mucinous tumor of the ovary associated with a mucinous tumor of the appendix and pseudomyxoma peritonei, and nineteen colonic and twenty gastric adenocarcinomas were stained with monoclonal antibodies to cytokeratin 7 and COTA. The results are summerized as follows; In the primary ovarian tumors, 94.4% were positive for cytokeratin 7 and 50% were positive for COTA. In the primary colonic adenocarcinomas, 94.7% were negative for cytokeratin 7 and 68% were positive for COTA. In the metastatic ovarian tumor from the colonic adenocarcinomas, 100% were negative for cytokeratin 7 and positive for COTA. In the primary gastric adenocarcinomas, 40% were negative for cytokeratin 7 and 85% were negative for COTA. In the metastatic ovarian tumor from the gastric adenocarcinomas, 43% were negative for cytokeratin 7 and 14% were negative for COTA. From the results of this study, it could be concluded that in the differential diagnosis of primary ovarian tumors from metastatic colonic carcinomas, positive reaction for cytokeratin 7 suggests a primary ovarian tumor but a negative reaction for cytokeratin 7 and positive reaction for COTA suggest metastatic colonic carcinomas. The results of this study also reveal that cytokeratin 7 and COTA are not useful in the differential diagnosis of primary ovarian tumors from metastatic gastric carcinomas.
- roded Polypoid Hyperplasia of the Rectosigmoid Colon: Report of 2 cases with special reference to its relation to mucosal prolapse syndrome.
- Nam Hoon Cho, Hee Jeong Ahn, Chan Il Park
- Korean J Pathol. 1994;28(3):297-301.
- 2,251 View
- 10 Download
-
 Abstract
Abstract
- Polypoid prolapse of mucosal folds can occur at various sites and in various conditions predominantly associated with strain during defecation. There are two well known types of mucosal prolapse syndrome(MPS), the inflammatory cloacogenic polyp(ICP) and the mucosal redundant polyp associated with diverticular disease(N4RPD). ICP is a mucosal prolapse of the anorectal junction and MRPD is a proximal analogue involving the sigmoid colon. We experienced two cases of eroded polypoid hyperplasia(EPH) of the rectosigmoid colon which manifested as a huge gyriform mass simulating the gross features of gastrointestinal lymphomas or other malignant tumors. The EPH consisted of confluent polypoid mucosal folds with rolled-up submucosa to form stalk, The polypoid lesion represented hyperplastic epithelium, erosion of the mucosal surface and congestive vascular ectasia of lamina propria and submucosa. To explain the whole morphologic features, the initial phenomenon should be the mucosal prolapse. Vascular stretching with ischemic erosion of the mucosal surface and compensatory epithelial hyperplasia ensue as the result. The ominous endoscopic and gross features of EPH should be kept in mind to avoid erroneous radical surgery.
- Effects of Genistein and Daidzein on the Growth of Human Colon Cancer HCT-116 Cells.
- Jong Heon Shin, Ku Seong Kang, Joung Ok Kim, Ghil Suk Yoon, Tae Gyun Kwon, Jung Wan Kim, Yoon Kyung Sohn
- Korean J Pathol. 2006;40(1):46-51.
- 2,241 View
- 92 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Genistein and daidzein are two major soybean isoflavones. They have received increasing attention because of their possible roles for cancer prevention. However, their mechanisms of action and molecular targets on the human colon cancer cells are not fully understood.
METHODS
Human colon cancer HCT-116 cells were treated with genistein and daidzein to investigate their effects on the cell growth and this was analyzed with MTT assay. TUNEL assay and Hoechst33342 stain were carried out to identify apotosis.
RESULTS
Daidzein was able to inhibit cell proliferation and induce apoptosis of the HCT-116 cells, but genistein didn't affect the cell growth. The ER antagonist ICI182780 didn't attenuate the antiproliferative and proapoptotic effects of daidzein: this means the effect of daidzein on the HCT-116 cells may not be dependent on the ER pathway. The other soybean isoflavone, genistein, attenuated the effects of daidzein on the HCT-116 cells and its mechanism should be elucidated.
CONCLUSIONS
These data suggest that daidzein may act as a preventive agent on human colon cancer, and its mechanism of action doesn't involve the ER-dependent pathway.
- Interpretation of DNA Histogram in Flow Cytometry: A Comparative Study of DNA Ploidy in Fresh and Paraffin-embedded Tissues of Colorectal Adenocarcinomas.
- Eun Sook Nam, Soon Hee Jung, Yeon Lim Suh, Woo Hee Jung, Keung Min Kim, In Sun Kim
- Korean J Pathol. 1994;28(4):341-349.
- 2,105 View
- 31 Download
-
 Abstract
Abstract
 PDF
PDF - As flow cytometric analysis using paraffin-embedded tissue was developed by Hedley et al in 1983, retrospective study with large amount of archival material was possible. Many literatures reported that the result of paraffin embedded tissue was compatible with that of fresh tissue. We compared the DNA histograms of 26 cases of colorectal adenocarcinoma in which the analysis was done in both fresh and paraffin-embedded tissues. Aneuploidy in fresh and paraffin-embed-ded tissues was 73.0% and 50.0%, respectively. The concordance rate of fresh and paraffin-em-bedded tissues was 76.8% and six interpreters were agreed in 73.0% of the cases. Because flow cytometric DNA analysis using fresh tissues can detect more aneuploid population than in paraffin-embedded tissue, the former is strongly recommeded in DNA ploidy study. Also careful observation using standard criteria may improve the interpretation of DNA histogram.
- Cytologic Findings of Colon Lavage Fluid in Colon Cancer.
- Hye Kyung Lee, Myung Jin Joo, Kwang Min Lee, Dong Kyu Chung, Yong Woo Choi
- J Pathol Transl Med. 1996;7(1):103-106.
- 1,980 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - Although exfoliative colonic cytology for the diagnosis of colorectal cancer has been largely abandoned due to the widespread use of colonoscopy, some authors still insiston the usefulness of colon lavage fluid. We tried evaluating the diagnostic feasibility of colon lavage fluid cytology using an orally administered balanced electrolyte solution. We collected colon lavage fluids in 106 patients prior to colonoscopy and reviewed the slides. Cytologic examination revealed neoplastic cells in 7 of 16(44%) cases of endoscopically proven adenocarcinoma patients. Therefore, we think cytologic study of colon lavage fluid may be considered as one of the noninvasive diagnostic tools in colorectal cancer.
- Pseudolipomatosis of the Gastrointestinal Mucosa: Report of 6 cases with analysis of possible factors involved during endoscopic procedure.
- Ghee Young Choe, Yong Il Kim, Kyoo Wan Choi, Kee Suk Hong
- Korean J Pathol. 1992;26(1):10-16.
- 2,626 View
- 23 Download
-
 Abstract
Abstract
 PDF
PDF - Pseudolipomatosis of the colonic mucosa has been recognized as a lesion featured with aggregations of gaseous spaces in the lamina propria, but its pathogenesis remains still unclear. This paper describes 6 cases of pseudolipomatosis occurring in the mucosa of stomach and large intestine, and the possible factors involved in gastrointestinal endoscopic procedure to produce gaseous entrapment in the lamina propria were analysed. All cases received either gastroscopy or colonoscpy before endoscopic biopsy. Mucosal tissues from both stomach and recto-sigmoid colon revealed multiple aggregations of small air-spaces resembling fatty infiltration in the lamina propria. Rarely were similar infiltrations within the muscularis mucosae and adjacent lymphoid follicles. Evidence for pneumatosis cystoides intestinalis or ulcerative colonic lesion was not associated, although one showed a small gastric ulcer nearby. Repeated inflations and deflations of the stomach or colon during the endoscopic procedure with miner mechanical trauma by instrument to the mucosa seem to contribute to its pathogenesis.
- Gastrointestinal Polyposis in Koreans: A Nationwide Survey of Clinicopathologic Analysis of 112 Surgically Resected Cases.
- Mee Soo Chang, Hoguen Kim, Woo Ho Kim, Chan Il Park, Eun Kyung Hong, Han Kyeom Kim, In Soo Suh, Byung Kee Kim, Ja June Jang, Woon Sub Han, Hyung Sik Shin, So Young Jin, Dae Young Kang, Yong Il Kim
- Korean J Pathol. 1998;32(6):404-412.
- 2,354 View
- 11 Download
-
 Abstract
Abstract
- Gastrointestinal polyposis (GIP) is a rare disease characterized by formation of the numerous polyps in the gastrointestinal tract and presenting several extraintestinal manifestations. Most of the diseases are transmitted in an autosomal dominant pattern. In Korea, the epidemiological study as well as the pathological analysis of the GIP is not well established. We therefore analysed 38 items of GIP using surgically resected specimens. The materials in this study were collected from the 12 institutions and case reports in Korean literature between 1980 and 1991. The clinicopathologic findings were reevaluated by several members of the study group for gastrointestinal pathology. The results are as follows: (1) A total of 112 cases were included in this study: 83 cases were collected from 12 institutions and 29 cases were collected from Korean literature. The cases were classified as familial adenomatous polyposis (FAP), 59 cases; Gardner's syndrome, 3 cases; juvenile polyposis, 12 cases; Peutz-Jeghers syndrome, 35 cases; multiple colonic adenomas, 3 cases. (2) Among 59 cases of FAP, the range of age at operation was 14 to 61 years, and a family history was positive in 25 cases. The number of polyps in colorectum was 100~8,000. Of the 37 cases in which the examination of polyp density was available, 16 cases (43%) showed the highest density in the rectum and the sigmoid colon. The carcinomatous change within polyp(s) was present in 18 cases (31%), and associated advanced single or multiple colonic carcinomas existed in 37 cases (63%). Twenty-six (45%) tumors out of total 58 carcinomas were in the rectum. Twenty-five patients were evaluated for the upper gastrointestinal lesions, and 11 patients (44%) had pathologic lesions; multiple fundic gland polyps in 3 cases (12%), gastric and duodenal adenomas in 2 cases (8%), gastric adenomas in 2 cases (8%), duodenal adenomas in 2 cases (8%), gastric carcinoma and adenoma in 1 case (4%), gastric carcinoma in 1 case (4%). (3) Among 3 cases of Gardner's syndrome, the range of age at operation was 25 to 31 years, a family history was identified in 2 cases. One case was associated with an advanced colonic carcinoma and carcinomatous change within polyp. Extra gastrointestinal lesions were sebaceous cyst, epidermal cyst, osteoma and desmoid tumor. (4) Among 12 juvenile polyposis, the range of age at operation was 8 to 51 years and 5 patients had a family history. The carcinomatous change within polyp was found in 2 cases (17%) and associated advanced colonic carcinoma was in 4 cases (33%). The associated different type of polyps was tubular adenomas in 9 cases (75%), hyperplastic polyps in 4 cases (33%) and villous adenomas in 2 cases (17%). (5) Among 35 Peutz-Jeghers syndrome, the range of age at first operation was 6 to 42 years, family history was positive in 11 cases. The carcinomatous change within polyp was found in 1 case (3%), and associated advanced colonic carcinoma in 1 case (3%). The epithelial misplacement was observed in 4 cases (11%), and tubular or villous adenomatous feature in 4 cases (11%). In summary, the most frequent GIP for the surgical resection in Korea is FAP and the FAP is associated with high incidence of coexisting advanced and intramucosal carcinomas. Hamartomatous polyposis syndromes, such as juvenile polyposis and Peutz-Jeghers syndrome are another frequent disease for the surgical resection and are also associated with an increased risk of cancer.
- Heterotopic Enchondral Ossification in Metastatic Colonic Adenocarcinoma: A case report .
- So Yeon Park, Yong Il Kim, Woo Ho Kim
- Korean J Pathol. 2000;34(7):531-533.
- 1,994 View
- 12 Download
-
 Abstract
Abstract
 PDF
PDF - Calcification and ossification of colon cancer is frequently encountered, especially in the mucinous carcinoma. However, cartilage formation or enchondral ossification has rarely been described in human colon cancer. This report describes a case of a 59-year-old man with retroperitoneal metastasis of mucinous adenocarcinoma of colon, which showed a widespread heterotopic ossification through membranous or enchondral ossification. The ossification appeared in apposition to tumor cell nests and in the organized mucin pool. In our knowledge, this is the first case showing enchondral ossification in gastrointestinal carcinoma in Korea.
- Primary Appendiceal Papillary Adenocarcinoma of Colonic Type: Report of a case.
- Yun Kyung Kang, Ghee Young Choe, Yong Il Kim, Kuk Jin Choe
- Korean J Pathol. 1992;26(3):306-309.
- 2,125 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - We report a case of colonic type-papillary adenocarcinoma of appendiceal origin in a 73-year-old male patient. The patient presented with right lower quadrant mass and was operated for a preoperative diagnosis of inflammatory small bowel mass. The mid one-third of the appendix showed a 3.5x3.3 cm sized, broad-based, intraluminal papillary mass. Microscopically, it was a well differentiated papillary adenocarcinoma and revealed a strong immunoreactivity to carcinoembryonic antigen. Tumor desmoplasia and acute inflammatory change were prominent.
- Adenocarcinoma of Urinary Bladder: 2 cases report.
- Ki Kwon Kim, Eunk Sook Chang, Chai Hong Chung
- Korean J Pathol. 1988;22(4):456-461.
- 2,041 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - Two cases of adenocarcinoma of the urinary bladder with clinical and pathological features, and brief review of the literatureare presented. Case 1: The patient, a 52 year-old man, was admitted to this hospital because of intermittent painless total gross hematuria for 15 years. Cystoscopy was done, and showing a cauliflower mass with broad based diffuse infiltrating lesion at the right anten or portion of bladder. TUR-B was performed. Microscopically, the lesion consisted of colonic metaplastic epithelium with atypical glands and cystic dilatation and adenocarcinoma. Case 2: The patient, a 52-year-old woman, was admitted to this hospital because of total painless gorss hematuria for 1 year. Cystoscopy was done showing a sessile diffuse mass with ulceration on the dome area. Total cystectomy was performed. Grossly, the tumor showed an ulcerative tumor mass with elevated nodular margin at the dome of the bladder. Microscopically, the lesion consisted of anaplastic glands with back to back arrangement and branching glands through the entire thickness of the bladder wall.

 E-submission
E-submission

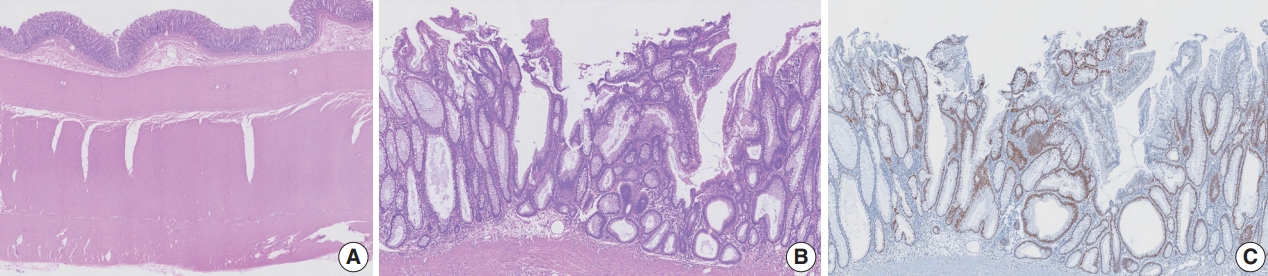


 First
First Prev
Prev



