Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 49(2); 2015 > Article
-
Original Article
Accuracy of Core Needle Biopsy Versus Fine Needle Aspiration Cytology for Diagnosing Salivary Gland Tumors - In Hye Song, Joon Seon Song, Chang Ohk Sung, Jong-Lyel Roh1, Seung-Ho Choi1, Soon Yuhl Nam1, Sang Yoon Kim1, Jeong Hyun Lee2, Jung Hwan Baek2, Kyung-Ja Cho,
-
Journal of Pathology and Translational Medicine 2015;49(2):136-143.
DOI: https://doi.org/10.4132/jptm.2015.01.03
Published online: March 12, 2015
Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
1Department of Otorhinolaryngology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
2Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- Corresponding Author: Kyung-Ja Cho, M.D. Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 138-736, Korea Tel: +82-2-3010-4545 Fax: +82-2-472-7898 E-mail: kjc@amc.seoul.kr
© 2015 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Frozen Section Analysis in Submandibular Gland Tumors: Optimizing Intraoperative Decision-Making
Amir Bolooki, Felix Johnson, Anna Stenzl, Zhaojun Zhu, Benedikt Gabriel Hofauer
Cancers.2025; 17(5): 895. CrossRef - The Myriad Spectrum of Salivary Gland Lesions: Cytohistological Correlation on Fine Needle Aspiration Cytology, Core Needle Biopsy, and Resections in a 5‐Year Single Institutional Experience of North India
Zachariah Chowdhury, Pallavi Majumdar, Sumeet Narain, Komal Lamba
Diagnostic Cytopathology.2025; 53(8): 391. CrossRef - Salivary Duct Carcinoma: A 12-Year Single Center Experience
Hyowon Ahn, Dongbin Ahn, Ji Hye Kwak
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2025; 68(6): 232. CrossRef - Cytological Spectrum of Salivary Gland Lesions Using the Milan System and their Histopathological Correlation: Retrospective Study in a Peripheral Medical College of Eastern India
Ujjwal Bandyopadhyay, Sarmila Guha Banerjee, Sirshak Dutta
Indian Journal of Otolaryngology and Head & Neck Surgery.2025;[Epub] CrossRef - Giant Pleomorphic Adenoma of Submandibular Gland
Harendra Kumar, Qazi Saquib Rizwan, Mayank Gupta, Tarun Kumar
Indian Journal of Otolaryngology and Head & Neck Surgery.2024; 76(1): 1361. CrossRef - CT-guided core needle biopsies of head and neck tumors: a comprehensive monocenter analysis of safety and outcomes
Thomas Joseph Vogl, Heinrich Johannes Ketelsen, Scherwin Mahmoudi, Jan-Erik Scholtz, Vitali Koch, Leon David Grünewald, Peter Wild, Timo Stoever, Simon Bernatz
European Radiology.2024; 34(8): 5370. CrossRef - Indications for Submandibulectomy Within a 20-Year Period
Amir Bolooki, Anna Stenzl, Christopher Weusthof, Benedikt Hofauer
Ear, Nose & Throat Journal.2024;[Epub] CrossRef - Treatment of Warthin’s Tumors of the Parotid Gland With Radiofrequency Ablation: A Systematic Review of the Current Literature
Kenny Do, Eric Kawana, Sisi Tian, Jo-Lawrence Bigcas
Ear, Nose & Throat Journal.2024;[Epub] CrossRef - Evaluation of the anterior processes of the parotid gland: an ultrasonographic study
Tarık Ali Uğur, Hümeyra Tercanlı
Surgical and Radiologic Anatomy.2024; 46(6): 915. CrossRef - Salivary Gland Fine-Needle Aspiration
Federica Policardo, Antonino Mule’, Esther Diana Rossi
Surgical Pathology Clinics.2024; 17(3): 347. CrossRef - Implementation of the Milan System for Reporting Salivary Gland Cytology: A Two-Year Outcome Cytopathology Data of a Tertiary Care Center
Soudamini Mahapatra, Chinmaya Sundar Ray, Aparajita Mishra, Dileswari Pradhan
Cureus.2024;[Epub] CrossRef - Preoperative cytopathological investigatory aids in the diagnosis of salivary gland lesions
S Vidyalakshmi, K Shanmugasamy
Journal of Oral and Maxillofacial Pathology.2024; 28(2): 172. CrossRef - Machine Learning on Ultrasound Texture Analysis Data for Characterizing of Salivary Glandular Tumors: A Feasibility Study
Li-Jen Liao, Ping-Chia Cheng, Feng-Tsan Chan
Diagnostics.2024; 14(16): 1761. CrossRef - Association of Membranous Basal Cell Adenoma and Basal Cell Adenocarcinoma With Brooke-Spiegler Syndrome
Lexi Goehring, Debby Rampisela, Jordan L Pleitz
Cureus.2024;[Epub] CrossRef - A Retrospective 8‐Year Single Institutional Study in Germany Regarding Diagnosis, Treatment, and Outcome of Malignant Parotid Tumors
S. Andrianopoulou, L. S. Fiedler, B. M. Lippert, O. C. Bulut, Mohamed Rahouma
International Journal of Surgical Oncology.2024;[Epub] CrossRef - High Field MRI in Parotid Gland Tumors: A Diagnostic Algorithm
Chiara Gaudino, Andrea Cassoni, Martina Lucia Pisciotti, Resi Pucci, Chiara Veneroso, Cira Rosaria Tiziana Di Gioia, Francesca De Felice, Patrizia Pantano, Valentino Valentini
Cancers.2024; 17(1): 71. CrossRef - Nationwide Incidence Trends of Pediatric Parotid Malignancy in Korea and a Retrospective Analysis of Single-Institution Surgical Experience of Parotidectomy
Hyun Seong Kim, Seo Young Kim, Eun-Jae Chung, Seong Keun Kwon, Soon-Hyun Ahn, Yuh-Seog Jung, Jungirl Seok
Korean Society of Head and Neck Oncology.2024; 40(2): 7. CrossRef - The Usefulness of Ultrasound-Guided Core Needle Biopsy Compared to Fine Needle Aspiration in Pre-Operative Diagnosis of Cystic-Predominant Parotid Tumors
Youn Jin Cho, Young Rok Jo, Hyun Jun Hong, Hye Ran Lee
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2023; 66(8): 532. CrossRef - The Value of Ultrasound-guided Core Needle Biopsy in Differentiating Benign from Malignant Salivary Gland Lesions
Mohammad Ali Kazemi, Farzaneh Amini, Bita Kargar, Maryam Lotfi, Keyvan Aghazadeh, Hashem Sharifian, Behnaz Moradi, Javid Azadbakht
Indian Journal of Otolaryngology and Head & Neck Surgery.2023; 75(2): 266. CrossRef - Schnellschnittdiagnostik bei Tumoren des Trigonum submandibulare
S. Riemann, A. Knopf
HNO.2023; 71(3): 164. CrossRef - Myoepithelial Carcinoma Ex Pleomorphic Adenoma of the Submandibular Gland: A Case Report
Georgia Syrnioti, Antonia Syrnioti, Alharith Abdullah, Xuehui Lui, Ernesto Mendoza
Cureus.2023;[Epub] CrossRef - Intraductal Carcinoma: The Carcinoma In Situ of the Salivary Gland
Rhema Thomas, Tijjani Umar, Farzad Borumandi
Journal of Craniofacial Surgery.2023; 34(5): e432. CrossRef - Fine-Needle Aspiration Cytology for Parotid Tumors
Masataka Taniuchi, Tetsuya Terada, Ryo Kawata
Life.2022; 12(11): 1897. CrossRef - Utility of the Milan System for Reporting Salivary Gland Cytology, with focus on the incidence and histologic correlates of atypia of undetermined significance (AUS) and salivary gland neoplasm of uncertain malignant potential (SUMP): A 3‐year institution
Christopher M. Cormier, Shweta Agarwal
Cancer Cytopathology.2022; 130(4): 303. CrossRef - Percutaneous CT-Guided Core Needle Biopsies of Head and Neck Masses: Review of 184 Cases at a Single Academic Institution, Common and Special Techniques, Diagnostic Yield, and Safety
R.W. Jordan, D.P. Shlapak, J.C. Benson, F.E. Diehn, D.K. Kim, V.T. Lehman, G.B. Liebo, A.A. Madhavan, J.M. Morris, P.P. Morris, J.T. Verdoorn, C.M. Carr
American Journal of Neuroradiology.2022; 43(1): 117. CrossRef - Nodular fasciitis of the submandibular gland
Ting Suen Wong, Richard Wei Chern Gan, Laszlo Karsai, Bun Yin Winson Wong
BMJ Case Reports.2022; 15(4): e245584. CrossRef - Validation of the Milan system for reporting salivary gland cytopathology: a single institution’s 10-year experience
Christopher Felicelli, Joseph Reznicek, Yevgen Chornenkyy, Lucy Jager, Daniel Johnson
Journal of the American Society of Cytopathology.2022; 11(5): 264. CrossRef - Application of the Milan system for reporting salivary gland cytopathology using cell blocks
Grégoire B. Morand, Raihanah Alsayegh, Alex M. Mlynarek, Marianne Plourde, Tiffany Mach, Marco A. Mascarella, Michael P. Hier, Livia Florianova, Marc P. Pusztaszeri
Virchows Archiv.2022; 481(4): 575. CrossRef - Comparisons among the Ultrasonography Prediction Model, Real-Time and Shear Wave Elastography in the Evaluation of Major Salivary Gland Tumors
Ping-Chia Cheng, Wu-Chia Lo, Chih-Ming Chang, Ming-Hsun Wen, Po-Wen Cheng, Li-Jen Liao
Diagnostics.2022; 12(10): 2488. CrossRef - A Novel Sonographic Scoring Model in the Prediction of Major Salivary Gland Tumors
Wu‐Chia Lo, Chih‐Ming Chang, Chi‐Te Wang, Po‐Wen Cheng, Li‐Jen Liao
The Laryngoscope.2021;[Epub] CrossRef - Assessing the diagnostic accuracy for pleomorphic adenoma and Warthin tumor by employing the Milan System for Reporting Salivary Gland Cytopathology: An international, multi‐institutional study
Derek B. Allison, Alexander P. Smith, Daniel An, James Adam Miller, Khurram Shafique, Sharon Song, Kartik Viswanathan, Elizabeth Eykman, Rema A. Rao, Austin Wiles, Güliz A. Barkan, Ritu Nayar, Guido Fadda, Celeste N. Powers, Esther Diana Rossi, Momin T. S
Cancer Cytopathology.2021; 129(1): 43. CrossRef - Magnetic resonance imaging of salivary gland tumours: Key findings for imaging characterisation
Davide Maraghelli, Michele Pietragalla, Cesare Cordopatri, Cosimo Nardi, Anna Julie Peired, Giandomenico Maggiore, Stefano Colagrande
European Journal of Radiology.2021; 139: 109716. CrossRef - The Milan System, from Its Introduction to Its Current Adoption in the Diagnosis of Salivary Gland Cytology
Esther Diana Rossi
Journal of Molecular Pathology.2021; 2(2): 114. CrossRef - Utility of the Milan system for reporting salivary gland cytopathology during rapid on‐site evaluation (ROSE) of salivary gland aspirates
Aanchal Kakkar, Mukin Kumar, Priyadarsani Subramanian, Arshad Zubair, Rajeev Kumar, Alok Thakar, Deepali Jain, Sandeep R. Mathur, Venkateswaran K. Iyer
Cytopathology.2021; 32(6): 779. CrossRef - Contribution of small tissue biopsy and flow cytometry to preoperative cytological categorization of salivary gland fine needle aspirates according to the Milan System: Single center experience on 287 cases
Tolga Bağlan, Serpil Dizbay Sak, Cevriye Cansız Ersöz, Koray Ceyhan
Diagnostic Cytopathology.2021; 49(4): 509. CrossRef - Is Milan for kids?: The Milan System for Reporting Salivary Gland Cytology in pediatric patients at an academic children's hospital with cytologic‐histologic correlation
Swati P. Satturwar, Maren Y. Fuller, Sara E. Monaco
Cancer Cytopathology.2021; 129(11): 884. CrossRef - Radiographic Interpretation in Oral Medicine and Hospital Dental Practice
Katherine France, Anwar A.A.Y. AlMuzaini, Mel Mupparapu
Dental Clinics of North America.2021; 65(3): 509. CrossRef - Carcinoma ex pleomorphic adenoma of major salivary glands: CT and MR imaging findings
Can Wang, Qiang Yu, Siyi Li, Jingjing Sun, Ling Zhu, Pingzhong Wang
Dentomaxillofacial Radiology.2021; 50(7): 20200485. CrossRef - Salivary gland carcinoma in children and adolescents: The EXPeRT/PARTNER diagnosis and treatment recommendations
Aurore Surun, Dominik T. Schneider, Andrea Ferrari, Teresa Stachowicz‐Stencel, Jelena Rascon, Anna Synakiewicz, Abbas Agaimy, Kata Martinova, Denis Kachanov, Jelena Roganovic, Ewa Bien, Gianni Bisogno, Ines B. Brecht, Frédéric Kolb, Juliette Thariat, Anto
Pediatric Blood & Cancer.2021;[Epub] CrossRef - A Call for Universal Acceptance of the Milan System for Reporting Salivary Gland Cytopathology
Eric Barbarite, Sidharth V. Puram, Adeeb Derakhshan, Esther D. Rossi, William C. Faquin, Mark A. Varvares
The Laryngoscope.2020; 130(1): 80. CrossRef - Preoperative biopsy in parotid malignancies: Variation in use and impact on surgical margins
Liliya Benchetrit, Sina J. Torabi, Elliot Morse, Saral Mehra, Rahmatullah Rahmati, Heather A. Osborn, Benjamin L. Judson
The Laryngoscope.2020; 130(6): 1450. CrossRef - α‐Synuclein Real‐Time Quaking‐Induced Conversion in the Submandibular Glands of Parkinson's Disease Patients
Sireesha Manne, Naveen Kondru, Huajun Jin, Vellareddy Anantharam, Xuemei Huang, Arthi Kanthasamy, Anumantha G. Kanthasamy
Movement Disorders.2020; 35(2): 268. CrossRef - The Accessory Parotid Gland and its Clinical Significance
Mateusz A. Rosa, Dominik P. Łazarz, Jakub R. Pękala, Bendik Skinningsrud, Sigurd S. Lauritzen, Bernard Solewski, Przemysław A. Pękala, Jerzy A. Walocha, Krzysztof A. Tomaszewski
Journal of Craniofacial Surgery.2020; 31(3): 856. CrossRef - Comparison of core needle biopsy and fine‐needle aspiration in diagnosis of ma lignant salivary gland neoplasm: Systematic review and meta‐analysis
Jungheum Cho, Junghoon Kim, Ji Sung Lee, Choong Guen Chee, Youngjune Kim, Sang Il Choi
Head & Neck.2020; 42(10): 3041. CrossRef - The Milan system for reporting salivary gland cytopathology: The clinical impact so far. Considerations from theory to practice
Esther Diana Rossi, William C. Faquin
Cytopathology.2020; 31(3): 181. CrossRef - The role of core needle biopsy in parotid glands lesions with inconclusive fine needle aspiration
Farrokh Heidari, Firouzeh Heidari, Benyamin Rahmaty, Neda Jafari, Kayvan Aghazadeh, Saeed Sohrabpour, Ebrahim Karimi
American Journal of Otolaryngology.2020; 41(6): 102718. CrossRef - Role of Fine Needle Aspiration Cytology in the Diagnosis of Parotid Gland Tumors: Analysis of 193 Cases
Rahim Dhanani, Haissan Iftikhar, Muhammad Sohail Awan, Nida Zahid, Sehrish Nizar Ali Momin
International Archives of Otorhinolaryngology.2020; 24(04): e508. CrossRef - Cytohistological correlation of salivary gland tumours with emphasis on Milan system for reporting: A novel step towards internal quality assurance
Anandraj Vaithy.K, ATM Venkat Raghava, E S Keerthika Sri, K R Umadevi
IP Archives of Cytology and Histopathology Research.2020; 5(4): 283. CrossRef - Diagnosing Recently Defined and Uncommon Salivary Gland Lesions in Limited Cellularity Specimens: Cytomorphology and Ancillary Studies
Esther Diana Rossi, Zubair Baloch, William Faquin, Liron Pantanowitz
AJSP: Reviews and Reports.2020; 25(5): 210. CrossRef - Peripheral T Cell Lymphoma of Parotid Gland: A Diagnostic Challenge
J. G. Aishwarya, Satish Nair, C. N. Patil, Swarna Shivakumar, N. Shrivalli, Ashish Shah
Indian Journal of Otolaryngology and Head & Neck Surgery.2019; 71(S1): 533. CrossRef - Potential utility of core needle biopsy in the diagnosis of IgG4-related dacryoadenitis and sialadenitis
Kenichi Takano, Tsuyoshi Okuni, Keisuke Yamamoto, Ryuta Kamekura, Ryoto Yajima, Motohisa Yamamoto, Hiroki Takahashi, Tetsuo Himi
Modern Rheumatology.2019; 29(2): 393. CrossRef - Retrospective assessment of the effectiveness of the Milan system for reporting salivary gland cytology: A systematic review and meta‐analysis of published literature
Sahar J Farahani, Zubair Baloch
Diagnostic Cytopathology.2019; 47(2): 67. CrossRef - Values of fine‐needle aspiration cytology of parotid gland tumors: A review of 996 cases at a single institution
Manabu Suzuki, Ryo Kawata, Masaaki Higashino, Shuji Nishikawa, Tetsuya Terada, Shin‐Ichi Haginomori, Yoshitaka Kurisu, Yoshinobu Hirose
Head & Neck.2019; 41(2): 358. CrossRef - Positive Surgical Margins in Submandibular Malignancies: Facility and Practice Variation
Liliya Benchetrit, Elliot Morse, Benjamin L. Judson, Saral Mehra
Otolaryngology–Head and Neck Surgery.2019; 161(4): 620. CrossRef - The growth rate and the positive prediction of needle biopsy of clinically diagnosed Warthin’s tumor
Jungirl Seok, Woo-Jin Jeong, Soon-Hyun Ahn, Young Ho Jung
European Archives of Oto-Rhino-Laryngology.2019; 276(7): 2091. CrossRef - The Difference in the Clinical Features Between Carcinoma ex Pleomorphic Adenoma and Pleomorphic Adenoma
Jungirl Seok, Se Jin Hyun, Woo-Jin Jeong, Soon-Hyun Ahn, Hyojin Kim, Young Ho Jung
Ear, Nose & Throat Journal.2019; 98(8): 504. CrossRef - Fine‐needle aspiration cytology and radiological imaging in parotid gland tumours: Our experience in 103 patients
Clare Perkins, Edward Toll, Philip Reece
Clinical Otolaryngology.2019; 44(6): 1124. CrossRef - Application of the Milan system of reporting salivary cytopathology – A retrospective cytohistological correlation study
Ramya Katta, Devi Padmavathi Chaganti
Journal of Dr. NTR University of Health Sciences.2019; 8(1): 11. CrossRef - Ultrasound‐guided core needle biopsy in salivary glands: A meta‐analysis
Hee Joon Kim, Jong Seung Kim
The Laryngoscope.2018; 128(1): 118. CrossRef - Accuracy and effectiveness of ultrasound-guided core-needle biopsy in the diagnosis of focal lesions in the salivary glands
Jose Luis del Cura, Gloria Coronado, Rosa Zabala, Igone Korta, Ignacio López
European Radiology.2018; 28(7): 2934. CrossRef - The Milan System for Reporting Salivary Gland Cytopathology (MSRSGC): an ASC-IAC–sponsored system for reporting salivary gland fine-needle aspiration
Esther Diana Rossi, Zubair Baloch, Marc Pusztaszeri, William C. Faquin
Journal of the American Society of Cytopathology.2018; 7(3): 111. CrossRef - Routine and Advanced Ultrasound of Major Salivary Glands
Kunwar Suryaveer Singh Bhatia, Yuk-Ling Dai
Neuroimaging Clinics of North America.2018; 28(2): 273. CrossRef - The Milan System for Reporting Salivary Gland Cytopathology (MSRSGC): An ASC-IAC-Sponsored System for Reporting Salivary Gland Fine-Needle Aspiration
Esther Diana Rossi, Zubair W. Baloch, Marc Pusztaszeri, William C. Faquin
Acta Cytologica.2018; 62(3): 157. CrossRef - Evaluation and diagnosis of salivary gland neoplasms
Erica Jackson Mayland, Anna M. Pou
Operative Techniques in Otolaryngology-Head and Neck Surgery.2018; 29(3): 129. CrossRef - Feasibility and Safety of Multicenter Tissue and Biofluid Sampling for α-Synuclein in Parkinson’s Disease: The Systemic Synuclein Sampling Study (S4)
Lana M. Chahine, Thomas G. Beach, Nicholas Seedorff, Chelsea Caspell-Garcia, Christopher S. Coffey, Michael Brumm, Charles H. Adler, Geidy E. Serrano, Carly Linder, Sherri Mosovsky, Tatiana Foroud, Holly Riss, Dixie Ecklund, John Seibyl, Danna Jennings, V
Journal of Parkinson’s Disease.2018; 8(4): 517. CrossRef - Preoperative diagnostic of parotid gland neoplasms: fine-needle aspiration cytology or core needle biopsy?
Peter Zbären, Asterios Triantafyllou, Kenneth O. Devaney, Vincent Vander Poorten, Henrik Hellquist, Alessandra Rinaldo, Alfio Ferlito
European Archives of Oto-Rhino-Laryngology.2018; 275(11): 2609. CrossRef - A comparison study of the reporting systems for salivary gland fine needle aspirations: Are they really different?
Diana Montezuma, Sule Canberk, Ozlem Aydın, Mehmet Polat Dermirhas, André F. Vieira, Süha Goksel, Ümit İnce, Fernando Schmitt
Diagnostic Cytopathology.2018; 46(10): 859. CrossRef - Pediatric salivary gland carcinomas: Diagnostic and therapeutic management
Céleste Rebours, Vincent Couloigner, Louise Galmiche, Odile Casiraghi, Cécile Badoual, Sabah Boudjemaa, Anthony Chauvin, Monique Elmaleh, Brice Fresneau, Sylvie Fasola, Erea‐Noël Garabédian, Thierry Van Den Abeele, Daniel Orbach
The Laryngoscope.2017; 127(1): 140. CrossRef - Agreement between rapid on‐site evaluation and the final cytological diagnosis of salivary gland specimens
S. Wangsiricharoen, S. Lekawanvijit, S. Rangdaeng
Cytopathology.2017; 28(4): 321. CrossRef - Mesenchymal neoplasms of the head and neck: a cytopathologic analysis on fine needle aspiration
James Lee, Samia Kazmi, Christopher J. VandenBussche, Syed Z. Ali
Journal of the American Society of Cytopathology.2017; 6(3): 105. CrossRef - Clinical Results of Surgical Treatment in Parotid Tumors
Ahmet Kara
Journal of Otolaryngology-ENT Research.2017;[Epub] CrossRef - Parotid gland metastases of distant primary tumours: A diagnostic challenge
Achim M. Franzen, Thomas Günzel, Anja Lieder
Auris Nasus Larynx.2016; 43(2): 187. CrossRef - Modern Radiology in the Management of Head and Neck Cancer
G.J.C. Burkill, R.M. Evans, V.V. Raman, S.E.J. Connor
Clinical Oncology.2016; 28(7): 440. CrossRef - Fine‐needle aspiration and core needle biopsy: An update on 2 common minimally invasive tissue sampling modalities
Paul A. VanderLaan
Cancer Cytopathology.2016; 124(12): 862. CrossRef - Staging and follow-up of high-grade malignant salivary gland tumours: The role of traditional versus functional imaging approaches – A review
Nicole Freling, Flavio Crippa, Roberto Maroldi
Oral Oncology.2016; 60: 157. CrossRef - Biopsy of parotid masses: Review of current techniques
Sananda Haldar, Joseph D Sinnott, Kemal M Tekeli, Samuel S Turner, David C Howlett
World Journal of Radiology.2016; 8(5): 501. CrossRef - Review on the applications of ultrasonography in dentomaxillofacial region
Şehrazat Evirgen
World Journal of Radiology.2016; 8(1): 50. CrossRef - Comprehensive Cytomorphologic Analysis of Pulmonary Adenoid Cystic Carcinoma: Comparison to Small Cell Carcinoma and Non-pulmonary Adenoid Cystic Carcinoma
Seokhwi Kim, Jinah Chu, Hojoong Kim, Joungho Han
Journal of Pathology and Translational Medicine.2015; 49(6): 511. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
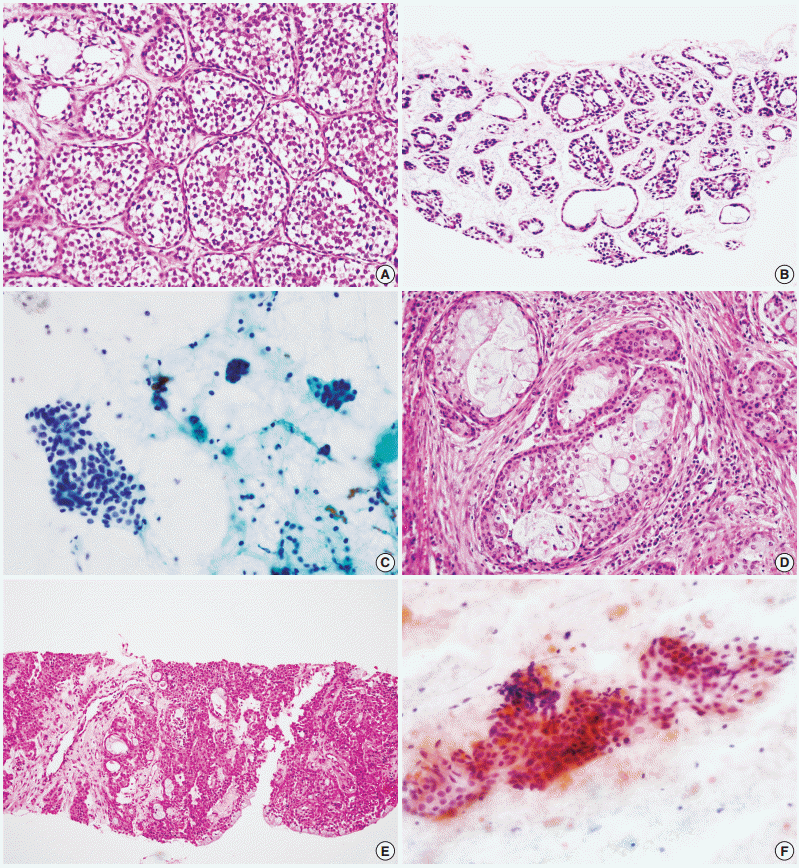
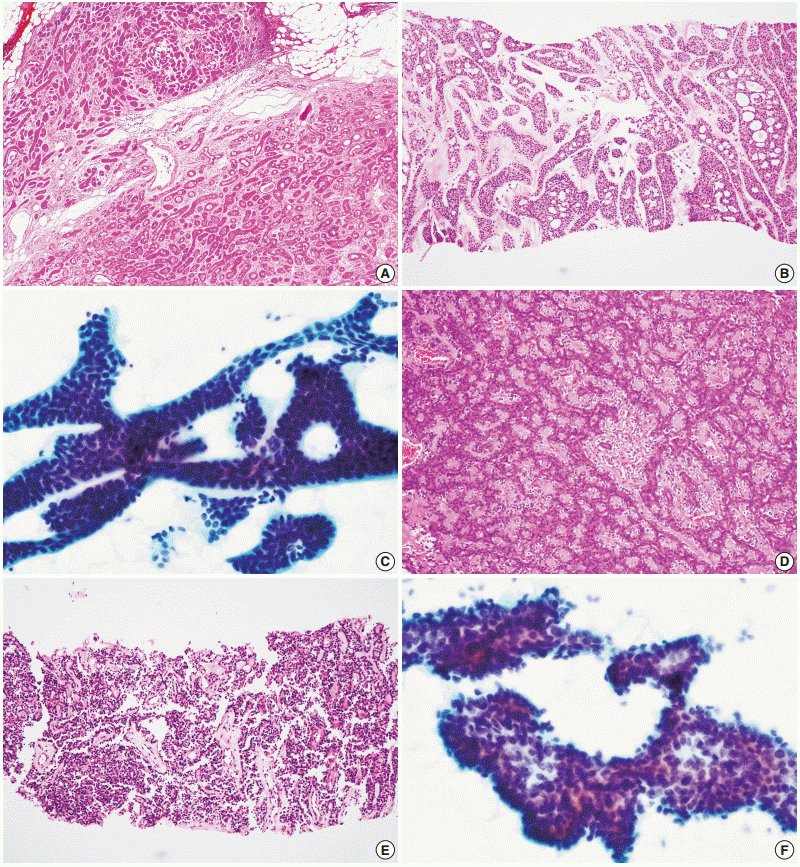
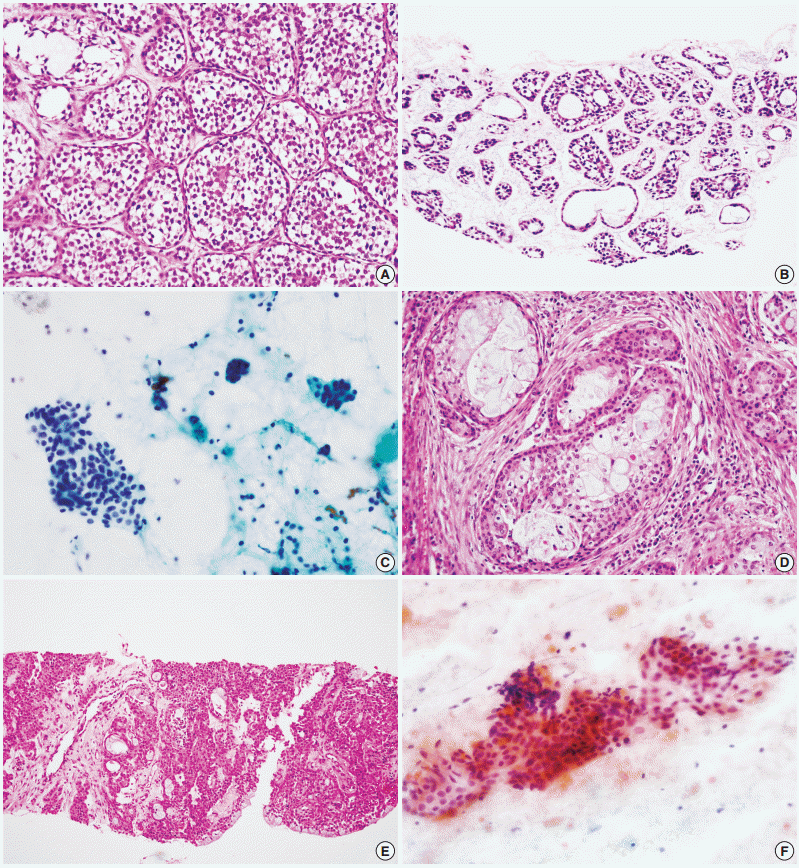
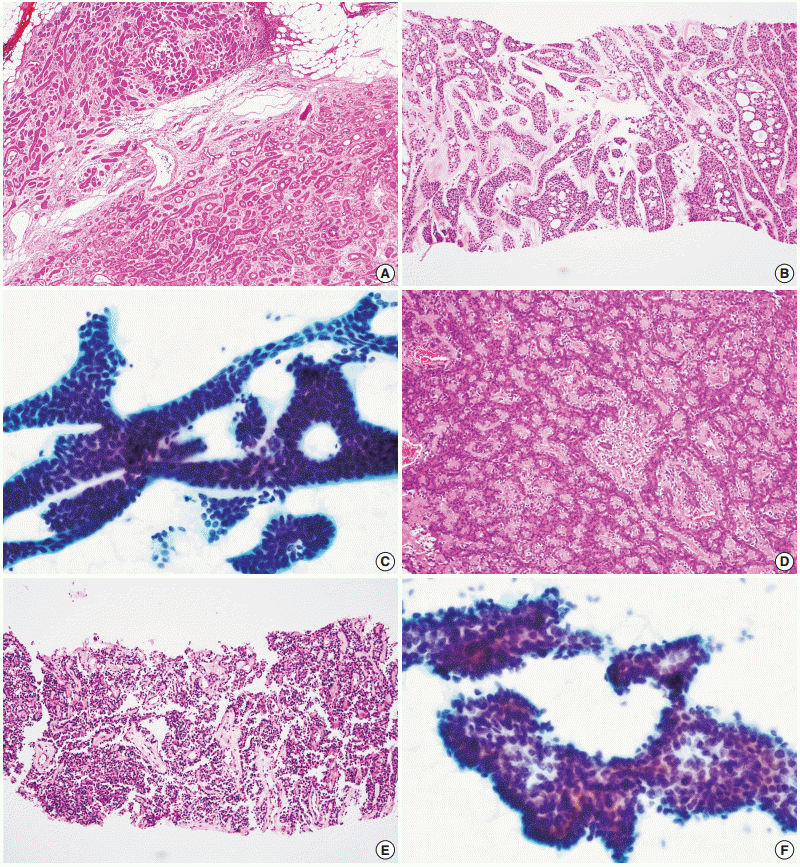
- Figure


Fig. 1.
Fig. 2.
| Characteristic | CNB (n = 228) | FNAC (n = 371) | p-value | |
|---|---|---|---|---|
| Malignant:benign tumor | 54:174 | 62:309 | .479 | |
| Size (mean ± SD, cm) | 2.57 ± 1.22 | 2.85 ± 1.21 | .006 | |
| Site | Parotid | 171 (75.0) | 329 (88.7) | .150 |
| SMG | 56 (24.6) | 40 (10.8) | ||
| SLG | 1 (0.4) | 2 (0.5) | ||
| Laterality | Left | 126 (55.2) | 193 (52.0) | .687 |
| Right | 100 (43.9) | 175 (47.2) | ||
| Bilateral | 2 (0.9) | 3 (0.8) | ||
| Multiplicity | 14 (6.1) | 13 (3.5) | .560 |
| Histologic diagnoses | Unsatisfactory rates |
Rates for multiple procedures | ||
|---|---|---|---|---|
| CNB | FNAC | |||
| Malignancy | ACC | 0/9 | 1/6 | 3/12 |
| AciCC | 1/4 | 0/7 | 1/10 | |
| ANOS | 0/2 | 1/3 | 0/5 | |
| BADC | 0/2 | 1/5 | 0/7 | |
| CPA | 0/5 | 1/13 | 1/17 | |
| CystADC | - | 1/2 | 0/1 | |
| EMC | 0/2 | 0/2 | 1/3 | |
| MEC | 1/15 | 1/7 | 1/21 | |
| OC | - | 0/2 | 0/2 | |
| SCC | 1/3 | 0/1 | 1/3 | |
| SDC | 0/10 | 1/10 | 3/17 | |
| ML | 0/1 | 0/2 | 0/3 | |
| RMS | 0/1 | - | 0/1 | |
| UPS | - | 0/1 | 0/1 | |
| Subtotal | 3/54 (5,6) | 7/62 (11,3) | ||
| Benign | PA | 1/117 | 10/199 | 11/305 |
| WT | 2/33 | 3/70 | 6/96 | |
| BA | 0/16 | 0/24 | 2/37 | |
| LA | 0/1 | - | 0/1 | |
| ME | 0/2 | 0/6 | 1/7 | |
| Oncocytoma | 0/2 | 1/2 | 1/3 | |
| NT | 0/3 | 1/3 | 1/5 | |
| VT | - | 0/3 | 0/3 | |
| Lipoma | - | 1/2 | 0/2 | |
| Subtotal | 3/174 (1,7) | 16/309 (5,2) | ||
| Total | 6/228 (2,6) | 23/371 (6,2) | ||
| Characteristic | CNB | FNAC | p-value |
|---|---|---|---|
| Total No. of cases | 228 | 371 | - |
| No. of adequate specimens, n (%) | 222 (97.4) | 348 (93.8) | - |
| No. of unsatisfactory specimens, n (%) | 6 (2.6) | 23 (6.2) | .078 |
| No. of adequate malignant cases | 51 | 55 | - |
| No. of preop. Dx as malignancy | 45 | 32 | - |
| No. of adequate benign cases | 171 | 293 | - |
| No. of preop. Dx as benign | 170 | 289 | - |
| Sensitivity (%) | 88.2 | 58.2 | .006 |
| Specificity (%) | 99.4 | 98.6 | .742 |
| Positive predictive value (%) | 97.8 | 88.9 | .253 |
| Negative predictive value (%) | 96.6 | 92.6 | .121 |
| Histologic diagnoses | CNB | FNAC |
|---|---|---|
| Malignancy (false-negative results) | ||
| ACC | - | PA (n = 1), benign cyst (n = 1), mucocele (n = 1) |
| AciCC | - | Oncocytoma (n = 1) |
| ANOS | - | WT (n = 1) |
| BADC | BA (n = 2) | BA (n = 2), benign cyst (n = 1) |
| CPA | PA (n = 2) | PA (n = 7) |
| EMC | BA (n = 1), PA (n = 1) | PA (n = 1), benign lesion (n = 1) |
| MEC | - | PA (n = 2), benign cyst (n = 1), mucocele (n = 1) |
| OC | - | Oncocytoma vs WT (n = 1) |
| ML | - | Benign lymphoid lesion (n = 1) |
| Benign (false-positive results) | ||
| PA | MEC (n = 1) | CPA (n = 1), LG malignancy (n = 1) |
| ME | - | ACC (n = 2) |
| Histologic diagnoses | CNB | FNAC | p-value | |
|---|---|---|---|---|
| Malignancy | ACC | 9/9 | 2/5 | |
| AciCC | 3/3 | 4/7 | ||
| ANOS | 2/2 | 0/2 | ||
| BADC | 0/2 | 0/4 | ||
| CPA | 2/5 | 0/12 | ||
| CystADC | - | 0/1 | ||
| EMC | 0/2 | 0/2 | ||
| MEC | 12/14 | 2/6 | ||
| OC | - | 0/2 | ||
| SCC | 2/2 | 0/1 | ||
| SDC | 7/10 | 1/9 | ||
| ML | 1/1 | 1/2 | ||
| RMS | 1/1 | - | ||
| UPS | - | 0/2 | ||
| Subtotal | 39/51 (76.5) | 10/55 (18.2) | .002 | |
| Benign | PA | 111/116 | 170/189 | |
| WT | 31/31 | 53/66 | ||
| BA | 12/16 | 10/24 | ||
| LA | 0/1 | - | ||
| ME | 0/2 | 2/6 | ||
| Oncocytoma | 1/2 | 0/2 | ||
| NT | 2/3 | 1/2 | ||
| VT | - | 0/3 | ||
| Lipoma | - | 0/1 | ||
| Subtotal | 157/171 (91.8) | 236/293 (80.5) | .003 | |
| Total | 196/222 (88.3) | 246/348 (70.7) | <.001 |
Values are presented as number (%) unless otherwise indicated. CNB, core needle biopsy; FNAC, fine needle aspiration cytology; SD, standard deviation; SMG, submandibular gland; SLG, sublingual gland.
Values in parentheses are presented as percentage. CNB, core needle biopsy; FNAC, fine needle aspiration cytology; ACC, adenoid cystic carcinoma; AciCC, acinic cell carcinoma; ANOS, adenocarcinoma, not otherwise specified; BADC, basal cell adenocarcinoma; CPA, carcinoma ex pleomorphic adenoma; CystADC, cystadenocarcinoma; EMC, epithelial-myoepithelial carcinoma; MEC, mucoepidermoid carcinoma; OC, oncocytic carcinoma; SCC, squamous cell carcinoma; SDC, salivary duct carcinoma; ML, malignant lymphoma; RMS, rhabdomyosarcoma; UPS, undifferentiated pleomorphic sarcoma; PA, pleomorphic adenoma; WT, Warthin tumor; BA, basal cell adenoma; LA, lymphadenoma; ME, myoepithelioma; NT, neurogenic tumor; VT, vascular tumor.
CNB, core needle biopsy; FNAC, fine needle aspiration cytology; preop., preoperative; Dx, diagnosis.
CNB, core needle biopsy; FNAC, fine needle aspiration cytology; ACC, adenoid cystic carcinoma; PA, pleomorphic adenoma; AciCC, acinic cell carcinoma; ANOS, adenocarcinoma, not otherwise specified; WT, Warthin tumor; BADC, basal cell adenocarcinoma; BA, basal cell adenoma; CPA, carcinoma ex pleomorphic adenoma; EMC, epithelial-myoepithelial carcinoma; MEC, mucoepidermoid carcinoma; OC, oncocytic carcinoma; ML, malignant lymphoma; ME, myoepithelioma; LG, low grade.
Values in parentheses are presented as percentage. CNB, core needle biopsy; FNAC, fine needle aspiration cytology; ACC, adenoid cystic carcinoma; AciCC, acinic cell carcinoma; ANOS, adenocarcinoma, not otherwise specified; BADC, basal cell adenocarcinoma; CPA, carcinoma ex pleomorphic adenoma; CystADC, cystadenocarcinoma; EMC, epithelial-myoepithelial carcinoma; MEC, mucoepidermoid carcinoma; OC, oncocytic carcinoma; SCC, squamous cell carcinoma; SDC, salivary duct carcinoma; ML, malignant lymphoma; RMS, rhabdomyosarcoma; UPS, undifferentiated pleomorphic sarcoma; PA, pleomorphic adenoma; WT, Warthin tumor; BA, basal cell adenoma; LA, lymphadenoma; ME, myoepithelioma; NT, neurogenic tumor; VT, vascular tumor.

 E-submission
E-submission