Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 49(4); 2015 > Article
-
Original Article
Analysis of Histologic Features Suspecting Anaplastic Lymphoma Kinase (ALK)-Expressing Pulmonary Adenocarcinoma - In Ho Choi, Dong Won Kim, Sang Yun Ha1, Yoon-La Choi1, Hee Jeong Lee2, Joungho Han1
-
Journal of Pathology and Translational Medicine 2015;49(4):310-317.
DOI: https://doi.org/10.4132/jptm.2015.05.13
Published online: June 22, 2015
Department of Pathology, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea
1Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
2Department of Pathology, Gwangmyeong Sungae Hospital, Gwangmyeong, Korea
- Corresponding Author Joungho Han, MD, PhD Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea Tel: +82-2-3410-2800 Fax: +82-2-3410-0025 E-mail: hanjho@skku.edu
© 2015 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background:
- Since 2007 when anaplastic lymphoma kinase (ALK) rearrangements were discovered in non-small cell lung cancer, the ALK gene has received attention due to ALK-targeted therapy, and a notable treatment advantage has been observed in patients harboring the EML4/ALK translocation. However, using ALK-fluorescence in situ hybridization (FISH) as the standard method has demerits such as high cost, a time-consuming process, dependency on interpretation skill, and tissue preparation. We analyzed the histologic findings which could complement the limitation of ALK-FISH test for pulmonary adenocarcinoma.
-
Methods:
- Two hundred five cases of ALK-positive and 101 of ALK-negative pulmonary adenocarcinoma from January 2007 to May 2013 were enrolled in this study. The histologic findings and ALK immunohistochemistry results were reviewed and compared with the results of ALK-FISH and EGFR/KRAS mutation status.
-
Results:
- Acinar, cribriform, and solid growth patterns, extracellular and intracellular mucin production, and presence of signet-ring-cell element, and psammoma body were significantly more often present in ALK-positive cancer. In addition, the presence of goblet cell-like cells and presence of nuclear inclusion and groove resembling papillary thyroid carcinoma were common in the ALK-positive group.
-
Conclusions:
- The above histologic parameters can be helpful in predicting ALK rearranged pulmonary adenocarcinoma, leading to rapid FISH analysis and timely treatment.
- Case selection
- Two hundred five ALK-positive cases identified using IHC or FISH on biopsied and resected specimens from Samsung Medical Center were enrolled from January 2007 to May 2013. The basic characteristics of these cases (age, sex, and tumor stage), method of sampling (biopsy or operation), and results of IHC and FISH were investigated.
- As a control group for comparing the histologic findings, all consecutive cases of pulmonary resection performed during 2012 were collected. The ALK-IHC had been performed in all cases of the control group, regardless of EGFR and KRAS analyses or microscopic findings.
- The Institutional Review Board of Samsung Medical Center approved this study (IRB No. 2014-01-146).
- Review of the histological findings
- All resected tumors were classified according to histologic subtype based on the new International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification; the predominant pattern was determined as lepidic, acinar, papillary, solid, and invasive mucinous. All other components (>5% of tumors) were also noted, including the cribriform growth pattern. Presence of extracellular and intracellular mucin, presence of signetring-cells, abrupt presence of goblet cell-like cells that contain amphophilic mucin like those in the intestinal mucosa, and presence of psammoma body, nuclear inclusion and groove, bizarre nuclei and multilobated nuclei were evaluated. As one of the other cytomorphological parameters, presence of prominent large eosinophilic nucleoli was investigated using a cut-off value of >30% for tumor cells. In the biopsied specimens, we did not determine the predominant subtype, but we recorded all identifiable growth patterns, nuclear features and presence of psammoma body, bizarre nuclei, and nuclear inclusion and groove. We applied the parameter of ‘nuclear inclusion and groove’ to cells showing cytomorphologic features of thyroid papillary carcinoma. All cytomorphological features in all presented growth components were evaluated in three fields of each growth pattern using high magnification (×400).
- IHC and scoring for ALK
- IHC for ALK fusion was performed using formalin-fixed paraffin-embedded (FFPE) tumor tissue (4-μm thickness) and an antibody to NCL-ALK (1:30, clone 5A4, Novocastra, Newcastle upon Tyne, UK). The interpretation of IHC results was based on the a four-tier scoring system: 0 (none), 1+ (faint cytoplasmic staining, ≥10% of tumor cells), 2+ (moderate, smooth cytoplasmic staining), and 3+ (intense, granular cytoplasmic staining). IHC scores of 2+ or 3+ were regarded as ALK-positive results [5].
- FISH of ALK
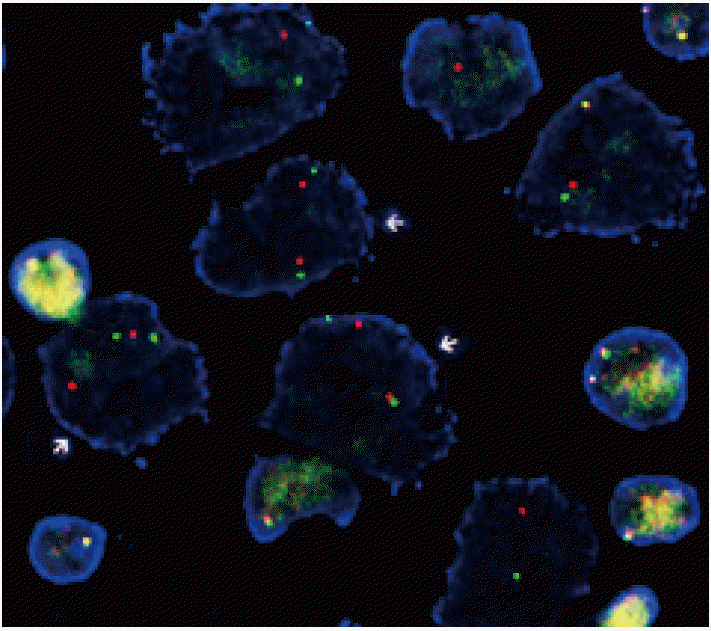
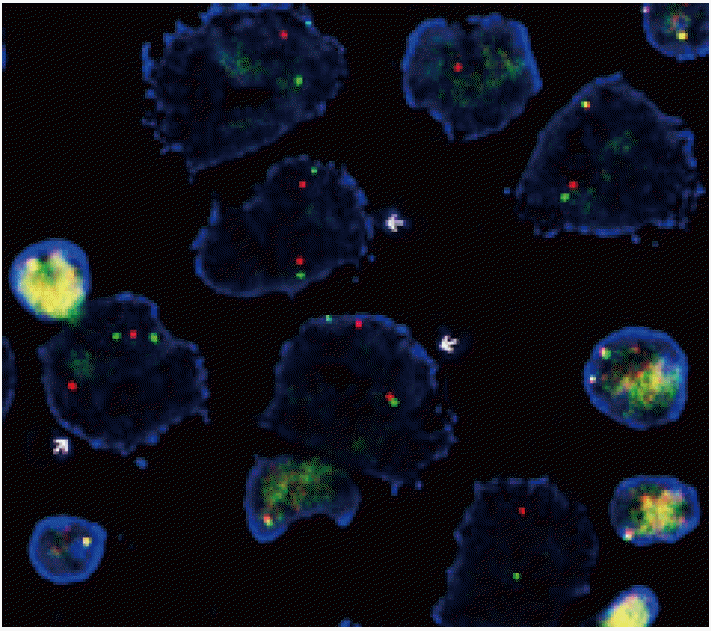
- The FISH analysis for ALK rearrangement on FFPE tissue was examined using a probe specific to the ALK locus (Vysis LSI ALK dual-color, Break-Apart Rearrangement Probe, Abbott Molecular, Abbott Park, IL, USA). At least 50 nonoverlapping tumor cells were examined, and the cut-off value for positive ALK rearrangement was defined as >15% of tumor cells showing split signals or lone 3´ (orange color) signals (Fig. 1).
- EGFR and KRAS mutation status
- EGFR and KRAS mutation data of ALK-negative and ALK-positive groups were investigated. The mutation analyses of EGFR (exon 18, 19, 20, and 21) and KRAS (exons 2 and 3) were examined using direct sequencing-polymerase chain reaction (PCR). Genomic DNA was extracted from the FFPE tissue and purified with a QIAquick PCR purification kit (Qiagen, Hilden, Germany). Bidirectional sequencing was performed using the BigDye Terminator v 1.1 kit (Applied Biosystems, Foster City, CA, USA) on an ABI 3130xl genetic analyzer (Applied Biosystems).
- Statistical analysis
- Fisher exact test was used to compare the features of ALK-positive pulmonary adenocarcinoma according to sex, and linear by linear association was used for age and tumor stage. Normal distribution according to age in each group was tested by Kolmogorove-Smirnove and Shapiro-Wilk test. Chi-square test/Fisher exact test, independent samples t test, and logistic regression analysis were applied where appropriate in order to determine the relationship between morphological features and ALK positivity. A p-value of <.05 was considered to be statistically significant in all statistical tests. All analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
MATERIALS AND METHODS
- Comparison of ALK-IHC and ALK-FISH
- The status of ALK-FISH and ALK-IHC was compared in 78 cases (Table 1). Among 69 ALK-FISH positive cases, 63 cases showed 2+ or 3+ positivity in ALK-IHC staining; therefore, the sensitivity of ALK-IHC (2+ and 3+) was determined to be 91.3% (63/69).
- Characteristics of patients with ALK-positive pulmonary adenocarcinoma
- We settled ALK-positive group (n=205) as ALK-IHC or ALK-FISH positive cases, including 127 cases of ALK-IHC positive (2+ or 3+) without ALK-FISH examination, 63 cases of positive ALK-IHC and ALK-FISH, 9 cases of positive ALK-IHC and not detected or failed ALK-FISH, and 6 cases of negative (0 or 1+) or failed ALK-IHC and positive ALK-FISH.
- The characteristics of patients in the ALK-positive group and control group are presented in Table 2. The ALK-positive group consisted of 129 biopsy cases and 76 resection cases. The sex ratio in the ALK-positive group was male:female=83:122 in contrast to 53:48 in the ALK-negative group. This finding was statistically significant (p=.047) and suggested that ALK-positive pulmonary adenocarcinoma occurs more frequently in women, with odds ratio of 1.623. ALK-positive cancer occurred in patients 7.3 years younger than the ALK-negative group, and this difference was statistically significant (p<.001). The distribution of tumor stage in the ALK-positive biopsy group was one-sided in stage 4 (82.2%). However, a statistically significant difference was not noted between the ALK-positive and -negative resection groups (p=.234).
- Histological type and characteristic microscopic findings in ALK-positive pulmonary adenocarcinoma
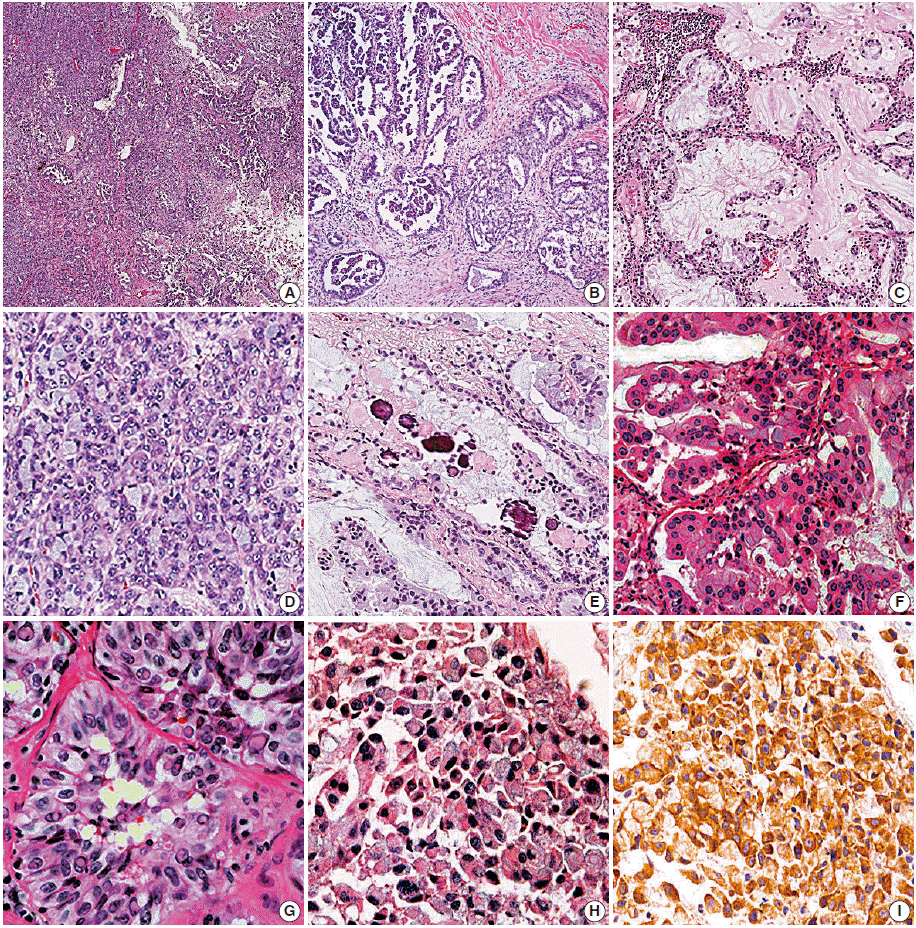
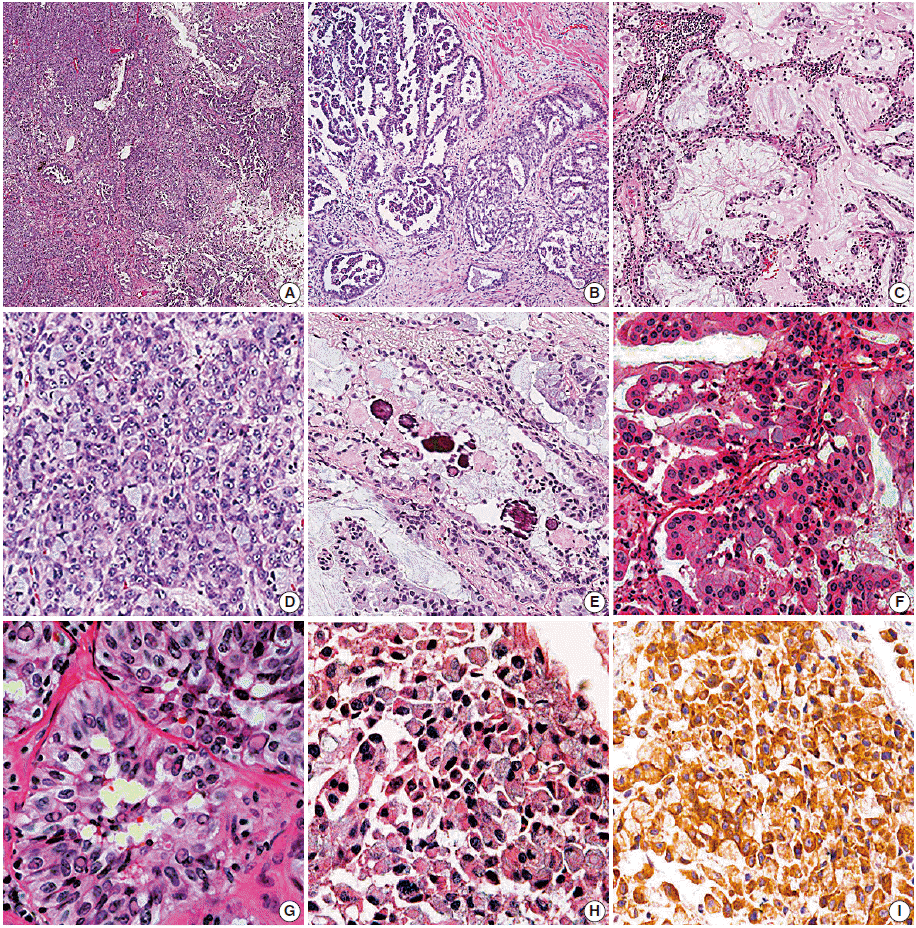
- Histologic subtypes based on predominant growth pattern were investigated in the ALK-positive and -negative resection groups, but there was no statistically significant histologic subtype of ALK-positive pulmonary adenocarcinoma (Table 3). However, acinar, solid, micropapillary, and cribriform growth patterns were more frequent in ALK-positive lung cancer based on the examination of growth patterns (p=.035, p=.002, p=.004, and p<.001 respectively) (Table 3, Fig. 2A, B). The ALK-positive group showed more variable growth patterns at a statistically significant level; 77.7% of cases in the ALK-positive group showed more than 3 types of growth patterns (mean, 3.3). However, cases in the ALK-negative group usually contained 2 or 3 types of growth patterns (75.2%; mean, 2.4) (p<.001) (Table 3, Fig. 2A).
- Among the examined cytomorphologic parameters, extracellular and intracellular mucin production (Fig. 2C), presence of signet-ring-cell element (Fig. 2D), presence of psammoma body (Fig. 2E), abrupt presence of goblet cell-like cells (Fig. 2F), and presence of nuclear inclusion and groove (Fig. 2G) were significantly more frequent in the ALK-positive resection group. Hobnail cells with abundant cytoplasm, bizarre cells, and presence of macronucleoli were more frequently observed in the ALK-negative group.
- According to logistic regression analysis (Table 4), acinar, solid, micropapillary, and cribriform growth patterns were more frequent in ALK-positive group, and the significant cytomorphologic features included presence of signet-ring-cell elements, extracellular mucin production, hobnail cells with abundant cytoplasm, and psammoma body.
- Morphological features of small biopsy specimens compared with resection specimens
- To evaluate whether ALK-specific morphologic features in the resection group can be applied to small biopsy specimens, cytomorphologic findings were compared between the ALK-positive biopsy and resection groups (Table 5). The parameters except for extracellular mucin production, psammoma body, and nuclear inclusion and groove showed no differences between the biopsy and resection groups (p>.05).
RESULTS
- Since the presence of ALK rearrangement has become an important predictive biomarker for the use of the ALK-targeted inhibitor ‘crizotinib’ for NSCLC, the detection of ALK rearrangements is also becoming increasingly important. However, ALK-FISH as a standard method for detecting ALK rearrangement is expensive, time-consuming, and influenced by many factors (skill and material). On the other hand, ALK-IHC has been used as a screening method due to its lower specificity compared to ALK-FISH. We evaluated the histologic findings that can be helpful in predicting ALK rearrangement and in saving time until ALK-targeted inhibitor treatment.
- Although there might have been a selection bias due to the excessive number of biopsied cases in stage IV, the ALK-positive group showed more frequent occurrence of lung adenocarcinoma in an advanced tumor stage than did the ALK-negative group (stage IV: 56.1% vs 13.9%). Although the ALK-positive resection group showed no statistically significant difference in the distribution of tumor stage compared with the ALK-negative group (p=.234), ALK-positive lung cancer is suspected to show more aggressive behavior, considering that ALK-positive lung cancer presents in an advanced stage with low resectability, leading to the need for chemotherapy or palliative therapy instead of surgical treatment.
- There have been several studies on the morphologic findings of ALK-rearranged NSCLCs. Some authors [7-9] have suggested that acinar growth pattern and extracellular mucin production are typical of ALK-rearranged pulmonary adenocarcinoma. Another author recommended signet-ring-cell elements associated with solid growth as a feature of ALK-rearranged pulmonary adenocarcinoma [10]. In the present study, histologic subtype based on ‘predominant’ growth pattern was not effective in discriminating between the ALK-positive and -negative groups. However, ALK-positive pulmonary adenocarcinoma showed more variable growth patterns (Fig. 2A) with significantly more frequent presence of acinar, solid, micropapillary, and cribriform growth patterns (Fig. 2A, B, D) compared with non-ALK rearranged adenocarcinoma (Table 3).
- As reported by Jokoji et al. [7] and Inamura et al. [9], who observed that mucin production was one of the important findings of EML4-ALK-positive lung adenocarcinoma, our study also showed that extracellular mucin production was statistically significant in the ALK-positive resection group (p<.001).
- Rodig et al. [10] showed that tumor cells forming a solid or sheetlike pattern were easily distinguishable from other growth patterns in ALK-rearranged adenocarcinoma, and that the majority (56%) of ALK-rearranged tumors showed a solid growth pattern with >10% signet-ring cells. Our study also showed frequent solid growth (45/76 cases, 59.2%) in the ALK-positive resection group, and signet-ring-cell elements were extremely frequent in the ALK-positive resection group (20/76 cases, 26.3% vs 4/101 cases, 4.0%) (Table 3, Fig. 2D). Indeed, the signet-ring-cell element was significantly more frequent in the ALK-positive resection group showing solid growth than in the ALK-negative group (odds ratio, 21.9 vs 1.9).
- A recent study about histomorphologic features of ALK-rearranged lung adenocarcinoma by Kim et al. [11] showed several significant microscopic features, including cribriform formation, presence of extracellular mucin, presence of mucin-containing cells, close relation to adjacent bronchioles, presence of psammoma body, presence of cholesterol cleft, and solid predominant pattern. Their data are similar to those of the present study except for two parameters that we did not evaluate close relation to adjacent bronchioles and presence of cholesterol cleft. However, we are more interested in the abrupt presence of goblet cell-like cells and presence of nuclear inclusion and groove resembling papillary thyroid carcinoma.
- Abrupt presence of goblet cell-like cells (Fig. 2F) was frequent in the ALK-positive resection group (64.5% vs 32.7%, p<.001). We also found that many cases with a signet-ring-cell element in the ALK-positive resection group showed abrupt presence of goblet cell-like cells (16/20 cases, 80.0%). Considering the cytomorphologic similarity between signet-ring cells and goblet cell-like cells, we supposed that presence of goblet cell-like cells is part of a process to form signet-ring-cell and is predictive parameter for ALK-positive pulmonary adenocarcinoma. However, this relation was not statistically significant (p=.091).
- One of the notable findings of our study was that the presence of nuclear inclusion and groove resembling papillary thyroid carcinoma (Fig. 2G) was noted more often observed in the ALK-positive lung cancer cases compared to the negative cases. We hypothesized that this feature is valuable in predicting ALK-positive lung cancer. However, there is no available data on why papillary thyroid carcinoma-like features are more frequent in ALK-positive lung cancer, suggesting additional study cases are needed.
- On the correlation between biopsy and resection cases in ALK-positive tumors (Table 5), we found that some parameters, such as presence of signet-ring-cell elements, intracellular mucin content, and presence of goblet cell-like cells, showed no statistically significant differences between the biopsy and resection groups. However, these features are believed to be helpful for predicting ALK positivity in small biopsy specimens. Furthermore, presence of signet-ring-cell, psammoma body, nuclear inclusion and groove, and goblet cell-like cells are meaningful in small biopsy sections, if present (Fig. 2H).
- Paik et al. [4] reported that the sensitivity and specificity of ALK-IHC was 100% and 95.8%, respectively, with a well-organized interpretation flow and concluded that ALK-IHC might be useful as a screening method to identify ALK rearrangements; a 91.3% sensitivity of ALK-IHC was noted in our study. However, ALK-IHC alone is not yet adequate to replace ALK-FISH and is applicable only as a screening method. For example, we excluded 4 cases showing discordance between the result of ALK and that of EGFR and KRAS before the present study. These cases included three EGFR-mutated cases (two ALK-IHC- and EGFR-positive cases with no examination by ALK-FISH and one concurrent EGFR-mutated and ALK-FISH-positive case) and one concurrent ALK-IHC- and KRAS-positive case without examination by ALK-FISH; KRAS missense mutation in the 12th codon : c.34G>T (p.G12C). Upon reviewing a case showing EGFR missense mutation in exon 18 (G719X), ALK-IHC-positivity (2+), and ALK-FISH-positivity, there was a suspicion of over-interpretation of ALK-FISH analysis; about 20% of tumor cells showed only ‘lone 3´ (orange color) signals’ without ‘split signals.’ The reasons for the other three false positive cases by ALK-IHC could not be determined, although they showed definite 2+ and 3+ ALK-IHC scoring.
- However, ALK-FISH has some demerits, such as high cost, time-consuming process, dependency on many conditions, including skill of interpretation, and preparation of material such as quantity and quality of tumor cells. In our study, 9 cases showed no detection or failed results on ALK-FISH, and they were all negative for EGFR and KRAS, despite having 2+ or 3+ ALK-IHC scores (Table 1). After review of ALK-IHC and ALK-FISH results, the 6 cases with no detection were also confirmed as failures due to interpretation difficulty of ALK-FISH caused by excessive mucin content and low cellularity. In the practice, ALK-FISH is usually the second choice after analyses of both major mutations (EGFR and KRAS), and it leads to scarcity of tissue availability for testing from small biopsied specimens. Therefore, we believe that ALK-FISH could be complemented for rapid treatment, and the microscopic findings might be predictors of ALK rearrangement. If the morphologic findings are suggestive of ALK-rearranged pulmonary adenocarcinoma and are supported by a positive result in ALK-IHC, the patients can benefit from timely treatment. For example, we encountered a patient who showed dramatic response to ‘crizotinib’ before confirmation by ALK-FISH using only positive ALK-IHC and suspicious histological findings via biopsy (Fig. 2H, I).
- Conclusively, we found that acinar, micropapillary, cribriform, and solid growth patterns with intracellular mucin content, signet-ring-cell element, and psammoma body were useful in predicting ALK rearrangement. Indeed, the notable findings of our study were that presence of goblet cell-like cells or nuclear inclusion or groove resembling papillary thyroid carcinoma was more common in ALK-positive adenocarcinoma of the lung, although these findings should be evaluated further. In the biopsied specimens, presence of signet-ring-cell elements, goblet cell-like cells, intracellular mucin content, and psammoma body can be helpful in predicting ALK rearrangement. Additionally, there is a limitation of our study as the histologic comparison between FISH-positive cases and IHC-positive cases could not be made due to weighted biopsied specimens.
- We hope to clarify the histologic findings of ALK rearrangement in a prospective cohort study and to evaluate the histologic findings with newly appearing genes in ALK rearrangement.
DISCUSSION
| Total cases (n=306) |
ALK-positive |
ALK-negative |
p-value | |||
|---|---|---|---|---|---|---|
| Biopsy (n = 129) | Resection (n = 76) | Total (n = 205) | Resection (n = 101) | |||
| Sex (M:F) | 47:82 | 36:40 | 83:122 | 53:48 | .047a | |
| .501b | ||||||
| Age (yr) | 53.9 ± 11.5 | 55.1 ± 11.7 | 54.4 ± 11.5 | 61.7 ± 8.8 | < .001a | |
| < .001b,c | ||||||
| TNM stage, n (%) | a | 1 (0.8) | 29 (38.2) | 30 (14.6) | 20 (19.8) | .234b,d |
| 1b | 1 (0.8) | 4 (5.3) | 5 (2.4) | 14 (13.9) | ||
| 2a | 4 (3.1) | 8 (10.5) | 12 (5.9) | 12 (11.9) | ||
| 2b | 1 (0.8) | 0 | 1 (0.5) | 8 (7.9) | ||
| 3a | 7 (5.4) | 23 (30.3) | 30 (14.6) | 32 (31.7) | ||
| 3b | 9 (7.0) | 3 (3.9) | 12 (5.9) | 1 (1.0) | ||
| 4 | 106 (82.2) | 9 (11.8) | 115 (56.1) | 14 (13.9) | ||
| ALK-positive resection (n = 76) | ALK-negative resection (n = 101) | p-value | |
|---|---|---|---|
| Histolologic subtype (predominant) | |||
| Acinar | 31 (40.8) | 35 (34.7) | .403 |
| Papillary | 24 (31.6) | 36 (35.6) | .572 |
| Solid | 19 (25.0) | 23 (22.8) | .730 |
| Micropapillary | 0 | 3 (3.0) | .130 |
| Lepidic | 0 | 3 (3.0) | .130 |
| Invasive mucinous | 2 (2.3) | 1 (1.0) | .402 |
| Growth pattern (> 5% of entire tumor) | |||
| Acinar | 65 (85.5) | 73 (72.3) | .035 |
| Papillary | 52 (68.4) | 66 (65.3) | .668 |
| Solid | 45 (59.2) | 36 (35.6) | .002 |
| Micropapillary | 31 (40.8) | 21 (20.8) | .004 |
| Lepidic | 7 (9.2) | 20 (19.8) | .052 |
| Cribriform | 51 (67.1) | 29 (28.7) | < .001 |
| No. of growth patternsa | |||
| 1 | 3 (3.9) | 14 (13.9) | < .001b |
| 2 | 14 (18.4) | 44 (43.6) | |
| 3 | 26 (34.2) | 32 (31.7) | |
| 4 | 23 (30.3) | 9 (8.9) | |
| 5 | 10 (13.2) | 1 (1.0) | |
| 6 | 0 | 1 (1.0) | |
| Microscopic findings | |||
| Presence of signet-ring-cell element | 20 (26.3) | 4 (4.0) | < .001 |
| Extracellular mucin production | 34 (44.7) | 18 (17.8) | < .001 |
| Intracellular mucin content | 58 (76.3) | 57 (56.4) | .006 |
| Hobnail cells with abundant cytoplasm | 4 (5.3) | 35 (34.7) | < .001 |
| Abrupt presence of goblet cell-like cells | 49 (64.5) | 33 (32.7) | < .001 |
| Psammoma body | 33 (43.4) | 5 (5.0) | < .001 |
| Presence of bizarre cells | 19 (25.0) | 43 (42.6) | .015 |
| Presence of multilobated cells | 5 (6.6) | 6 (5.9) | .862 |
| Nuclear inclusion and groove | 16 (21.1) | 10 (9.9) | .038 |
| Prominent macronucleoli | 58 (46.6) | 78 (53.4) | .034 |
- 1. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007; 448: 561-6. ArticlePubMedPDF
- 2. Sun JM, Lira M, Pandya K, et al. Clinical characteristics associated with ALK rearrangements in never-smokers with pulmonary adenocarcinoma. Lung Cancer 2014; 83: 259-64. ArticlePubMed
- 3. O’Bryant CL, Wenger SD, Kim M, Thompson LA. Crizotinib: a new treatment option for ALK-positive non-small cell lung cancer. Ann Pharmacother 2013; 47: 189-97. ArticlePubMedPDF
- 4. Paik JH, Choe G, Kim H, et al. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: correlation with fluorescence in situ hybridization. J Thorac Oncol 2011; 6: 466-72. ArticlePubMed
- 5. Yi ES, Boland JM, Maleszewski JJ, et al. Correlation of IHC and FISH for ALK gene rearrangement in non-small cell lung carcinoma: IHC score algorithm for FISH. J Thorac Oncol 2011; 6: 459-65. ArticlePubMed
- 6. Yoshida A, Tsuta K, Nakamura H, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol 2011; 35: 1226-34. ArticlePubMed
- 7. Jokoji R, Yamasaki T, Minami S, et al. Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4-ALK-positive lung adenocarcinoma. J Clin Pathol 2010; 63: 1066-70. ArticlePubMed
- 8. Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol 2008; 3: 13-7. ArticlePubMed
- 9. Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 2009; 22: 508-15. ArticlePubMedPDF
- 10. Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 2009; 15: 5216-23. ArticlePubMedPMCPDF
- 11. Kim H, Jang SJ, Chung DH, et al. A comprehensive comparative analysis of the histomorphological features of ALK-rearranged lung adenocarcinoma based on driver oncogene mutations: frequent expression of epithelial-mesenchymal transition markers than other genotype. PLoS One 2013; 8: e76999. Article
REFERENCES
Figure & Data
References
Citations

- Evidential deep learning-based ALK-expression screening using H&E-stained histopathological images
Sai Chandra Kosaraju, Sai Phani Parsa, Dae Hyun Song, Hyo Jung An, Yoon-La Choi, Joungho Han, Jung Wook Yang, Mingon Kang
npj Digital Medicine.2025;[Epub] CrossRef - Clinicopathological significances of cribriform pattern in lung adenocarcinoma
Jung-Soo Pyo, Byoung-Hoon Lee, Kyueng-Whan Min, Nae Yu Kim
Pathology - Research and Practice.2024; 253: 155035. CrossRef - Clinicopathological features and prognostic significance of pulmonary adenocarcinoma with signet ring cell components: meta-analysis and SEER analysis
Yang Tan, Ying-he Huang, Jia-wen Xue, Rui Zhang, Run Liu, Yan Wang, Zhen-Bo Feng
Clinical and Experimental Medicine.2023; 23(8): 4341. CrossRef - Lung-Cancer Risk in Mice after Exposure to Gamma Rays, Carbon Ions or Neutrons: Egfr Pathway Activation and Frequent Nuclear Abnormality
Kenshi Suzuki, Shunsuke Yamazaki, Ken-ichi Iwata, Yutaka Yamada, Takamitsu Morioka, Kazuhiro Daino, Mutsumi Kaminishi, Mari Ogawa, Yoshiya Shimada, Shizuko Kakinuma
Radiation Research.2022;[Epub] CrossRef - Pathological cytomorphologic features and the percentage of ALK FISH-positive cells predict pulmonary adenocarcinoma prognosis: a prospective cohort study
Fenge Jiang, Congcong Wang, Ping Yang, Ping Sun, Jiannan Liu
World Journal of Surgical Oncology.2021;[Epub] CrossRef - Cribriform pattern in lung invasive adenocarcinoma correlates with poor prognosis in a Chinese cohort
Yang Qu, Haifeng Lin, Chen Zhang, Kun Li, Haiqing Zhang
Pathology - Research and Practice.2019; 215(2): 347. CrossRef - Incidence of brain metastasis in lung adenocarcinoma at initial diagnosis on the basis of stage and genetic alterations
Bumhee Yang, Hyun Lee, Sang-Won Um, Kyunga Kim, Jae Il Zo, Young Mog Shim, O Jung Kwon, Kyung Soo Lee, Myung-Ju Ahn, Hojoong Kim
Lung Cancer.2019; 129: 28. CrossRef - Qualitative and quantitative cytomorphological features of primary anaplastic lymphoma kinase‐positive lung cancer
Ryuko Tsukamoto, Hiroyuki Ohsaki, Sho Hosokawa, Yasunori Tokuhara, Shingo Kamoshida, Toshiko Sakuma, Tomoo Itoh, Chiho Ohbayashi
Cytopathology.2019; 30(3): 295. CrossRef - Double Trouble: A Case Series on Concomitant Genetic Aberrations in NSCLC
Nele Van Der Steen, Yves Mentens, Marc Ramael, Leticia G. Leon, Paul Germonpré, Jose Ferri, David R. Gandara, Elisa Giovannetti, Godefridus J. Peters, Patrick Pauwels, Christian Rolfo
Clinical Lung Cancer.2018; 19(1): 35. CrossRef - Update on the potential significance of psammoma bodies in lung adenocarcinoma from a modern perspective
Akio Miyake, Koji Okudela, Mai Matsumura, Mitsui Hideaki, Hiromasa Arai, Shigeaki Umeda, Shoji Yamanaka, Yoshihiro Ishikawa, Michihiko Tajiri, Kenichi Ohashi
Histopathology.2018; 72(4): 609. CrossRef - Integrin β3 Inhibition Enhances the Antitumor Activity of ALK Inhibitor in ALK-Rearranged NSCLC
Ka-Won Noh, Insuk Sohn, Ji-Young Song, Hyun-Tae Shin, Yu-Jin Kim, Kyungsoo Jung, Minjung Sung, Mingi Kim, Sungbin An, Joungho Han, Se-Hoon Lee, Mi-Sook Lee, Yoon-La Choi
Clinical Cancer Research.2018; 24(17): 4162. CrossRef - An anaplastic lymphoma kinase-positive lung cancer microlesion: A case report
Tetsuo Kon, Youichiro Baba, Ichiro Fukai, Gen Watanabe, Tomoko Uchiyama, Tetsuya Murata
Human Pathology: Case Reports.2017; 7: 11. CrossRef - The prevalence of ALK rearrangement in pulmonary adenocarcinomas in an unselected Caucasian population from a defined catchment area: impact of smoking
Birgit G Skov, Paul Clementsen, Klaus R Larsen, Jens B Sørensen, Anders Mellemgaard
Histopathology.2017; 70(6): 889. CrossRef - Ciliated muconodular papillary tumor of the lung harboring ALK gene rearrangement: Case report and review of the literature
Yan Jin, Xuxia Shen, Lei Shen, Yihua Sun, Haiquan Chen, Yuan Li
Pathology International.2017; 67(3): 171. CrossRef - Molecular breakdown: a comprehensive view of anaplastic lymphoma kinase (ALK)‐rearranged non‐small cell lung cancer
Ka‐Won Noh, Mi‐Sook Lee, Seung Eun Lee, Ji‐Young Song, Hyun‐Tae Shin, Yu Jin Kim, Doo Yi Oh, Kyungsoo Jung, Minjung Sung, Mingi Kim, Sungbin An, Joungho Han, Young Mog Shim, Jae Ill Zo, Jhingook Kim, Woong‐Yang Park, Se‐Hoon Lee, Yoon‐La Choi
The Journal of Pathology.2017; 243(3): 307. CrossRef - Anaplastic lymphoma kinase immunohistochemistry in lung adenocarcinomas: Evaluation of performance of standard manual method using D5F3 antibody
D Jain, K Jangra, PS Malik, S Arulselvi, K Madan, S Mathur, MC Sharma
Indian Journal of Cancer.2017; 54(1): 209. CrossRef - Clinicopathological Features and Therapeutic Responses of Chinese Patients with Advanced Lung Adenocarcinoma Harboring an Anaplastic Lymphoma Kinase Rearrangement
Danxia Lin, De Zeng, Chen Chen, Xiao Wu, Miaojun Wang, Jiongyu Chen, Hui Lin, Xihui Qiu
Oncology Research and Treatment.2017; 40(1-2): 27. CrossRef - A Validation Study for the Use of ROS1 Immunohistochemical Staining in Screening for ROS1 Translocations in Lung Cancer
Patrizia Viola, Manisha Maurya, James Croud, Jana Gazdova, Nadia Suleman, Eric Lim, Tom Newsom-Davis, Nick Plowman, Alexandra Rice, M. Angeles Montero, David Gonzalez de Castro, Sanjay Popat, Andrew G. Nicholson
Journal of Thoracic Oncology.2016; 11(7): 1029. CrossRef - Non-small Cell Lung Cancer with Concomitant EGFR, KRAS, and ALK Mutation: Clinicopathologic Features of 12 Cases
Taebum Lee, Boram Lee, Yoon-La Choi, Joungho Han, Myung-Ju Ahn, Sang-Won Um
Journal of Pathology and Translational Medicine.2016; 50(3): 197. CrossRef - ALK gene rearranged lung adenocarcinomas: molecular genetics and morphology in cohort of patients from North India
Amanjit Bal, Navneet Singh, Parimal Agarwal, Ashim Das, Digambar Behera
APMIS.2016; 124(10): 832. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


Fig. 1.
Fig. 2.
| ALK-FISH |
No. of cases (n = 78) | |||
|---|---|---|---|---|
| Rearranged (n = 69) | Not detected (n = 6) | Failed (n = 3) | ||
| ALK-IHC | ||||
| Negative | 1 (1.4) | 0 | 0 | 1 |
| 1+ | 3 (4.3) | 0 | 0 | 3 |
| 2+ | 42 (60.9) | 3 (50.0) | 3 (100) | 48 |
| 3+ | 21 (30.4) | 3 (50.0) | 0 | 24 |
| Failed | 2 (2.9) | 0 | 0 | 2 |
| Sampling method | ||||
| Biopsy | 56 (81.2) | 2 (33.3) | 0 | 58 |
| Resection | 13 (18.8) | 4 (66.7) | 3 (100) | 20 |
| Total cases (n=306) | ALK-positive |
ALK-negative |
p-value | |||
|---|---|---|---|---|---|---|
| Biopsy (n = 129) | Resection (n = 76) | Total (n = 205) | Resection (n = 101) | |||
| Sex (M:F) | 47:82 | 36:40 | 83:122 | 53:48 | .047 |
|
| .501 |
||||||
| Age (yr) | 53.9 ± 11.5 | 55.1 ± 11.7 | 54.4 ± 11.5 | 61.7 ± 8.8 | < .001 |
|
| < .001 |
||||||
| TNM stage, n (%) | a | 1 (0.8) | 29 (38.2) | 30 (14.6) | 20 (19.8) | .234 |
| 1b | 1 (0.8) | 4 (5.3) | 5 (2.4) | 14 (13.9) | ||
| 2a | 4 (3.1) | 8 (10.5) | 12 (5.9) | 12 (11.9) | ||
| 2b | 1 (0.8) | 0 | 1 (0.5) | 8 (7.9) | ||
| 3a | 7 (5.4) | 23 (30.3) | 30 (14.6) | 32 (31.7) | ||
| 3b | 9 (7.0) | 3 (3.9) | 12 (5.9) | 1 (1.0) | ||
| 4 | 106 (82.2) | 9 (11.8) | 115 (56.1) | 14 (13.9) | ||
| ALK-positive resection (n = 76) | ALK-negative resection (n = 101) | p-value | |
|---|---|---|---|
| Histolologic subtype (predominant) | |||
| Acinar | 31 (40.8) | 35 (34.7) | .403 |
| Papillary | 24 (31.6) | 36 (35.6) | .572 |
| Solid | 19 (25.0) | 23 (22.8) | .730 |
| Micropapillary | 0 | 3 (3.0) | .130 |
| Lepidic | 0 | 3 (3.0) | .130 |
| Invasive mucinous | 2 (2.3) | 1 (1.0) | .402 |
| Growth pattern (> 5% of entire tumor) | |||
| Acinar | 65 (85.5) | 73 (72.3) | .035 |
| Papillary | 52 (68.4) | 66 (65.3) | .668 |
| Solid | 45 (59.2) | 36 (35.6) | .002 |
| Micropapillary | 31 (40.8) | 21 (20.8) | .004 |
| Lepidic | 7 (9.2) | 20 (19.8) | .052 |
| Cribriform | 51 (67.1) | 29 (28.7) | < .001 |
| No. of growth patterns |
|||
| 1 | 3 (3.9) | 14 (13.9) | < .001 |
| 2 | 14 (18.4) | 44 (43.6) | |
| 3 | 26 (34.2) | 32 (31.7) | |
| 4 | 23 (30.3) | 9 (8.9) | |
| 5 | 10 (13.2) | 1 (1.0) | |
| 6 | 0 | 1 (1.0) | |
| Microscopic findings | |||
| Presence of signet-ring-cell element | 20 (26.3) | 4 (4.0) | < .001 |
| Extracellular mucin production | 34 (44.7) | 18 (17.8) | < .001 |
| Intracellular mucin content | 58 (76.3) | 57 (56.4) | .006 |
| Hobnail cells with abundant cytoplasm | 4 (5.3) | 35 (34.7) | < .001 |
| Abrupt presence of goblet cell-like cells | 49 (64.5) | 33 (32.7) | < .001 |
| Psammoma body | 33 (43.4) | 5 (5.0) | < .001 |
| Presence of bizarre cells | 19 (25.0) | 43 (42.6) | .015 |
| Presence of multilobated cells | 5 (6.6) | 6 (5.9) | .862 |
| Nuclear inclusion and groove | 16 (21.1) | 10 (9.9) | .038 |
| Prominent macronucleoli | 58 (46.6) | 78 (53.4) | .034 |
| ALK-positive resection (n = 76) | ALK-negative resection (n = 101) | Odds ratio | 95% CI | |
|---|---|---|---|---|
| Growth pattern (> 5% of entire tumor) | ||||
| Acinar | 65 (85.5) | 73 (72.3) | 3.178 | 1.298–7.779 |
| Solid | 45 (59.2) | 36 (35.6) | 2.694 | 1.301–5.577 |
| Micropapillary | 31 (40.8) | 21 (20.8) | 3.414 | 1.581–7.372 |
| Cribriform | 51 (67.1) | 29 (28.7) | 3.771 | 1.877–7.575 |
| Microscopic findings | ||||
| Presence of signet-ring-cell element | 20 (26.3) | 4 (4.0) | 5.466 | 1.488–20.080 |
| Extracellular mucin production | 34 (44.7) | 18 (17.8) | 3.372 | 1.411–8.057 |
| Hobnail cells with abundant cytoplasm | 4 (5.3) | 35 (34.7) | 0.096 | 0.026–0.356 |
| Psammoma body | 33 (43.4) | 5 (5.0) | 16.548 | 5.303–51.641 |
| ALK-positive biopsy (n = 129) | ALK-positive resection (n = 76) | p-value | |
|---|---|---|---|
| Presence of signet-ring-cell element | 40 (31.0) | 20 (26.3) | .476 |
| Extracellular mucin production | 15 (11.6) | 34 (44.7) | < .001 |
| Intracellular mucin content | 101 (78.3) | 58 (76.3) | .743 |
| Hobnail cells with abundant cytoplasm | 5 (3.9) | 4 (5.3) | .640 |
| Abrupt presence of goblet cell-like cells | 69 (53.5) | 49 (64.5) | .124 |
| Psammoma body | 20 (15.5) | 33 (43.4) | < .001 |
| Nuclear inclusion and groove | 7 (5.4) | 16 (21.1) | .001 |
Values are presented as number (%). ALK, anaplastic lymphoma kinase; FISH, fluorescence
ALK, anaplastic lymphoma kinase; M, male; F, female. p-value between the ALK-positive and -negative group; p-value between the ALK-positive and -negative resected group; p-value by Student’s t test; Linear by linear association by chi-square test.
Values are presented as number (%). ALK, anaplastic lymphoma kinase. Number of all presented growth patterns presented in ‘Growth pattern (> 5% of entire tumor)’; p-value by Student’s t-test.
Values are presented as number (%). ALK, anaplastic lymphoma kinase; CI, confidence interval.
Values are presented as number (%). ALK, anaplastic lymphoma kinase.

 E-submission
E-submission