Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 50(5); 2016 > Article
-
Original Article
Differential Immunohistochemical Profiles for Distinguishing Prostate Carcinoma and Urothelial Carcinoma - Woo Jin Oh*,, Arthur Minwoo Chung*,, Jee Soon Kim, Ji Heun Han, Sung Hoo Hong1, Ji Yeol Lee1, Yeong Jin Choi
-
Journal of Pathology and Translational Medicine 2016;50(5):345-354.
DOI: https://doi.org/10.4132/jptm.2016.06.14
Published online: August 7, 2016
Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
1Department of Urology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- Corresponding Author Yeong Jin Choi, MD, PhD Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 222 Banpo-daero, Seocho-gu, Seoul 06591, Korea Tel: +82-2-2258-1616 Fax: +82-2-2258-1627 E-mail: mdyjchoi@catholic.ac.kr
- *Woo Jin Oh and Arthur Minwoo Chung contributed equally to this work.
© 2016 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Comparative histologic survey and transcriptomic investigation into canine prostate carcinoma
Nathan K. Hoggard, Said M. Elshafae, Nigel A. Daniels, Jonathan A. Young, Chris Premanandan, John B. Echols, Darshan S. Chandrashekar, Blake E. Hildreth, Michael C. Haffner, Thomas J. Rosol
Research in Veterinary Science.2026; 198: 105981. CrossRef - Impact of hormone sensitivity status on aberrant expression of CK7, CK20, CDX2, GATA3 and TTF1 in prostate cancer
Qing Yin Wang, Nazim Benzerdjeb, Samuel Jaquet, Andreea Stepanov, Mame-Kany Diop, Mirela Birlea, Fred Saad, Dominique Trudel
Human Pathology.2025; 163: 105877. CrossRef - Unusual Perineal Metastasis in a Case of Prostate Cancer on 68Ga-PSMA-11 PET/CT
Ritanshu Solanki, Bhagwant Rai Mittal, Rajender Kumar, Aravindh Sekar, Narender Kumar
Clinical Nuclear Medicine.2024; 49(2): e73. CrossRef - NKX3.1 Expression in Non-Prostatic Tumors and Characterizing its Expression in Esophageal/Gastroesophageal Adenocarcinoma
Ansa Mehreen, Kiran G. Manjee, Divyangi Paralkar, Gladell P. Paner, Thanh Lan
Advances in Anatomic Pathology.2024; 31(3): 202. CrossRef - Clinical Management of Intraductal Carcinoma of the Prostate
Gabriel Wasinger, Olivier Cussenot, Eva Compérat
Cancers.2024; 16(9): 1650. CrossRef - Adenocarcinomas of the Gynecologic Tract Involving the Urinary Bladder: A Series of 16 Cases Potentially Mimicking Urothelial Malignancy
Daniel H. Russell, Jonathan I. Epstein, Oleksandr N. Kryvenko, Matthew Schlumbrecht, Merce Jorda, Andre Pinto
Archives of Pathology & Laboratory Medicine.2024; 148(6): 705. CrossRef - Assessing the diagnostic impact of P63, PSA and BCL-2 proteins in premalignant and malignant prostate tissues
Aderonke C. Ogunlayi, Victor O. Ekundina, Adedapo O. Kehinde, Linus A. Enye, Adegoke O. Aremu
International Journal of Scientific Reports.2024; 10(6): 188. CrossRef - Concurrent occurrence of adenocarcinoma and urothelial carcinoma of the prostate gland: A case report
Jhe Yuan Hsu, Yi Sheng Lin, Li Hua Huang, Tang Yi Tsao, Chao Yu Hsu, Yen Chuan Ou, Min Che Tung
World Journal of Clinical Cases.2024; 12(26): 5952. CrossRef - Metastatic prostate cancer presenting as a posterior mediastinal mass: A rare presentation
Muhammad Haider, Arun Umesh Mahtani, Bachar Botrus, Foma Munoh Kenne, Madiha Fatima Master
Clinical Case Reports.2023;[Epub] CrossRef - Diagnostic and Prognostic Roles of GATA3 Immunohistochemistry in Urothelial Carcinoma
Daeseon Yoo, Kyueng-Whan Min, Jung-Soo Pyo, Nae Yu Kim
Medicina.2023; 59(8): 1452. CrossRef - Primary high-grade urothelial carcinoma of prostate with prostatic hyperplasia: a rare case report and review of the literature
Liang Liu, Fu-zhen Sun, Pan-ying Zhang, Yu Xiao, Xiao Yue, Dong-Ming Wang, Qiang Wang
The Aging Male.2023;[Epub] CrossRef - Expression of Gata Binding Protein 3 as a Prognostic Factor in Urogenital Lesions and Its Association With Morphology
T Govardhan, Debahuti Mohapatra, Sujata Naik, Prateek Das, Pranita Mohanty, Ankita Pal
Cureus.2023;[Epub] CrossRef - Histological and immunohistochemical investigation of canine prostate carcinoma with identification of common intraductal carcinoma component
Simone de Brot, Jennifer Lothion‐Roy, Llorenç Grau‐Roma, Emily White, Franco Guscetti, Mark A. Rubin, Nigel P. Mongan
Veterinary and Comparative Oncology.2022; 20(1): 38. CrossRef - Urothelial Carcinoma and Prostate-specific Membrane Antigen: Cellular, Imaging, and Prognostic Implications
Arsalan Tariq, Amy E. McCart Reed, Andrew Morton, Sima Porten, Ian Vela, Elizabeth D. Williams, John W. Yaxley, Peter C. Black, Matthew J. Roberts
European Urology Focus.2022; 8(5): 1256. CrossRef - Immunohistochemical Reactivity of Prostate-Specific Membrane Antigen in Salivary Gland Tumors
Haruto Nishida, Yoshihiko Kondo, Takahiro Kusaba, Hiroko Kadowaki, Tsutomu Daa
Head and Neck Pathology.2022; 16(2): 427. CrossRef - Weak NKX3.1 expression in a urothelial carcinoma: A diagnostic pitfall
Maryam Abdo, Robert Hoyt, Ashley Highfill, Daniel Mettman
Human Pathology Reports.2022; 27: 300599. CrossRef - Gene of the month: NKX3.1
Jon Griffin, Yuqing Chen, James W F Catto, Sherif El-Khamisy
Journal of Clinical Pathology.2022; 75(6): 361. CrossRef - Diagnostic Value of GATA3 and Uroplakin 3 in Differentiating Urothelial Carcinoma from Prostatic and Colorectal Carcinoma
Maha Salama, Dina A. Khairy
Open Access Macedonian Journal of Medical Sciences.2022; 10(A): 514. CrossRef - Diagnostic challenges for the distinction of high-grade prostatic adenocarcinoma and high-grade urothelial carcinoma of simultaneous occurrences - A literature review
Shreyas Bhushan Jayade, Manana Jikurashvili
GEORGIAN SCIENTISTS.2022;[Epub] CrossRef - Cytomorphology, immunoprofile, and clinicopathologic correlation of metastatic prostatic carcinoma
Xiaoqi Lin, Qiuying Shi, Ximing J. Yang
Human Pathology.2022; 130: 36. CrossRef - Cutaneous Metastasis of Prostate Adenocarcinoma: A Rare Presentation of a Common Disease
Alexander Dills, Okechukwu Obi, Kevin Bustos, Jesse Jiang, Shweta Gupta
Journal of Investigative Medicine High Impact Case Reports.2021;[Epub] CrossRef - Mining The Cancer Genome Atlas gene expression data for lineage markers in distinguishing bladder urothelial carcinoma and prostate adenocarcinoma
Ewe Seng Ch’ng
Scientific Reports.2021;[Epub] CrossRef - Immunohistochemical analysis of thrombomodulin expression in myocardial tissue from autopsy cases of ischemic heart disease
Takeshi Kondo, Motonori Takahashi, Gentaro Yamasaki, Marie Sugimoto, Azumi Kuse, Mai Morichika, Kanako Nakagawa, Makoto Sakurada, Migiwa Asano, Yasuhiro Ueno
Legal Medicine.2021; 51: 101897. CrossRef - Application and Pitfalls of Immunohistochemistry in Diagnosis of Challenging Genitourinary Cases
Jenny Ross, Guangyuan Li, Ximing J. Yang
Archives of Pathology & Laboratory Medicine.2020; 144(3): 290. CrossRef - New Screening Test Improves Detection of Prostate Cancer Using Circulating Tumor Cells and Prostate-Specific Markers
Karin Ried, Tasnuva Tamanna, Sonja Matthews, Peter Eng, Avni Sali
Frontiers in Oncology.2020;[Epub] CrossRef - An Unlikely Culprit: Gastric Metastasis from Primary Prostatic Adenocarcinoma
Eric Omar Then, Spoorthi Nutakki, Andrew Ofosu, Saad Saleem, Vijay Gayam, Tagore Sunkara, Vinaya Gaduputi
Journal of Gastrointestinal Cancer.2020; 51(3): 1081. CrossRef - MRI of prostatic urethral mucinous urothelial carcinoma: Expanding the differential diagnosis for T2 hyperintense prostatic masses
Neel Patel, Bryan R. Foster, Elena K. Korngold, Kyle Jensen, Kevin R. Turner, Fergus V. Coakley
Clinical Imaging.2020; 68: 68. CrossRef - Morphological and Immunohistochemical Biomarkers in Distinguishing Prostate Carcinoma and Urothelial Carcinoma: A Comprehensive Review
Francesca Sanguedolce, Davide Russo, Vito Mancini, Oscar Selvaggio, Beppe Calò, Giuseppe Carrieri, Luigi Cormio
International Journal of Surgical Pathology.2019; 27(2): 120. CrossRef - A Case of Metastatic Prostate Cancer to the Urethra That Resolved After Androgen Deprivation Therapy
Darren J. Bryk, Kenneth W. Angermeier, Eric A. Klein
Urology.2019; 129: e4. CrossRef - The Homeodomain Transcription Factor NKX3.1 Modulates Bladder Outlet Obstruction Induced Fibrosis in Mice
Mehul S. Patel, Diana K. Bowen, Nicholas M. Tassone, Andrew D. Gould, Kirsten S. Kochan, Paula R. Firmiss, Natalie A. Kukulka, Megan Y. Devine, Belinda Li, Edward M. Gong, Robert W. Dettman
Frontiers in Pediatrics.2019;[Epub] CrossRef - Cancer of unknown primary: Ancillary testing of cytologic and small biopsy specimens in the era of targeted therapy
Morgan L. Cowan, Christopher J. VandenBussche
Cancer Cytopathology.2018; 126(S8): 724. CrossRef - Glandular Tumors of the Urachus and Urinary Bladder: A Practical Overview of a Broad Differential Diagnosis
Alexander S. Taylor, Rohit Mehra, Aaron M. Udager
Archives of Pathology & Laboratory Medicine.2018; 142(10): 1164. CrossRef - S100P as a Marker for Urothelial Histogenesis: A Critical Review and Comparison With Novel and Traditional Urothelial Immunohistochemical Markers
Moushumi Suryavanshi, Julian Sanz-Ortega, Deepika Sirohi, Mukul K. Divatia, Chisato Ohe, Claudia Zampini, Daniel Luthringer, Steven C. Smith, Mahul B. Amin
Advances in Anatomic Pathology.2017; 24(3): 151. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
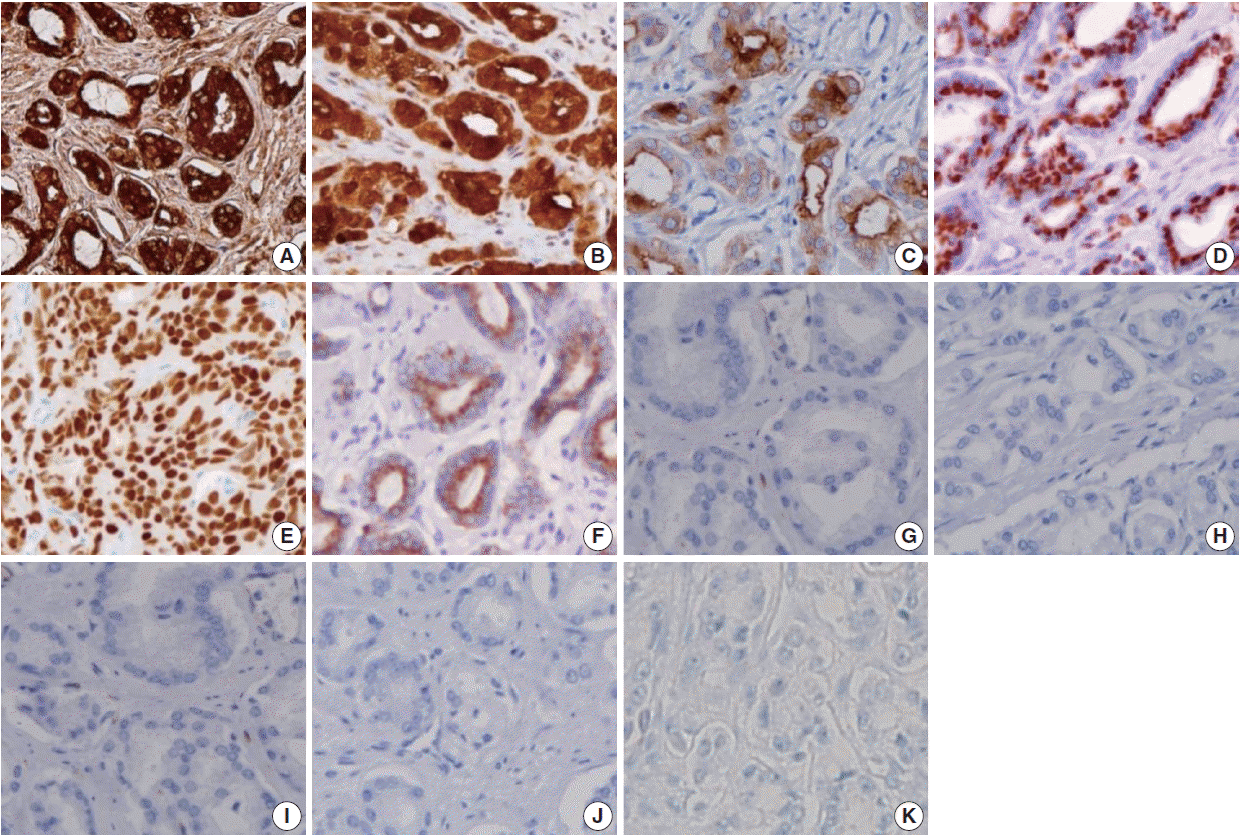
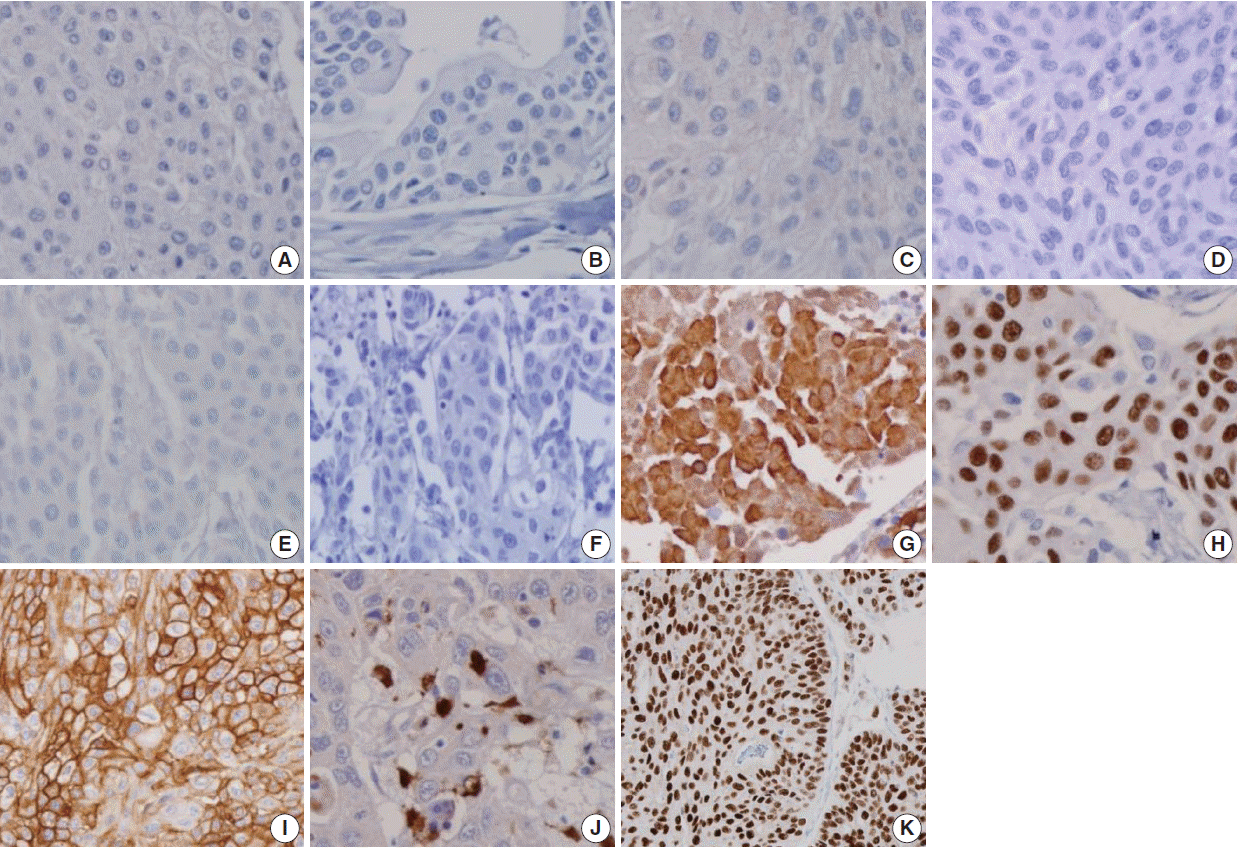
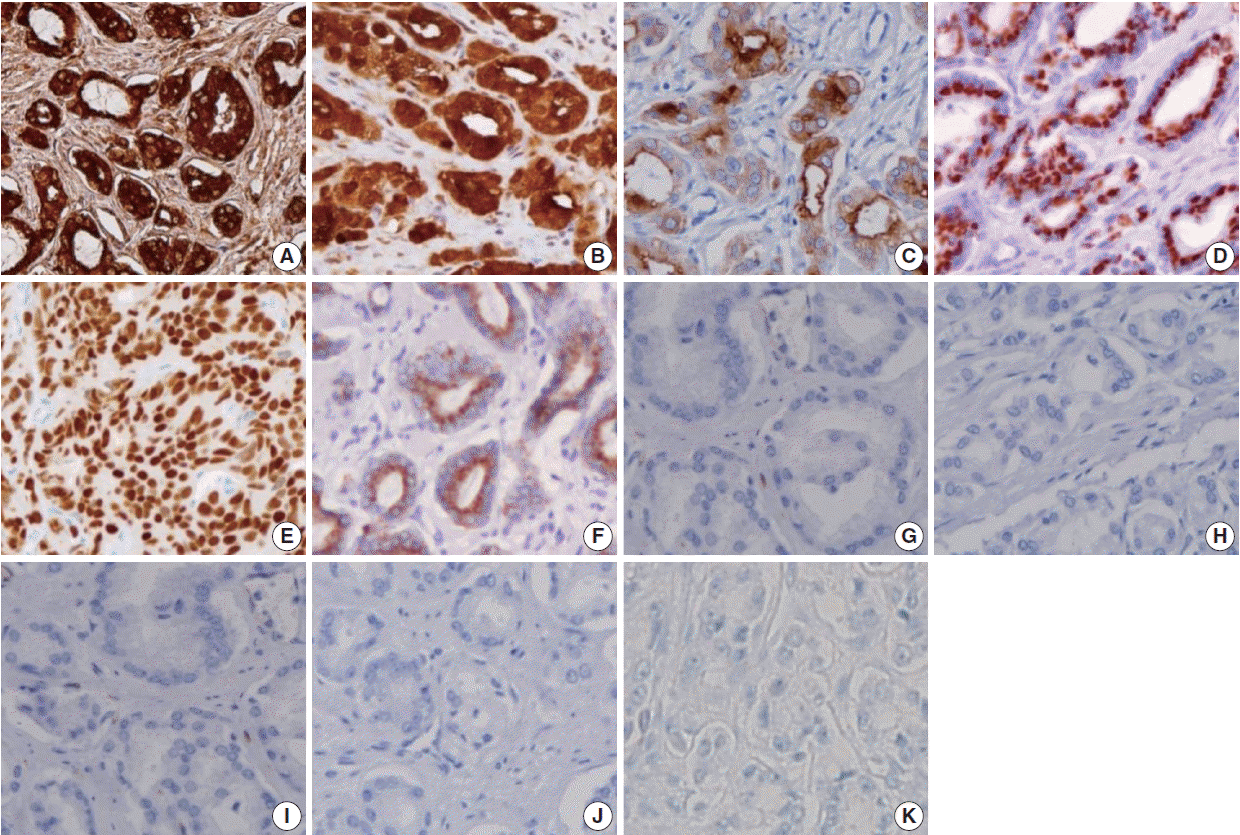
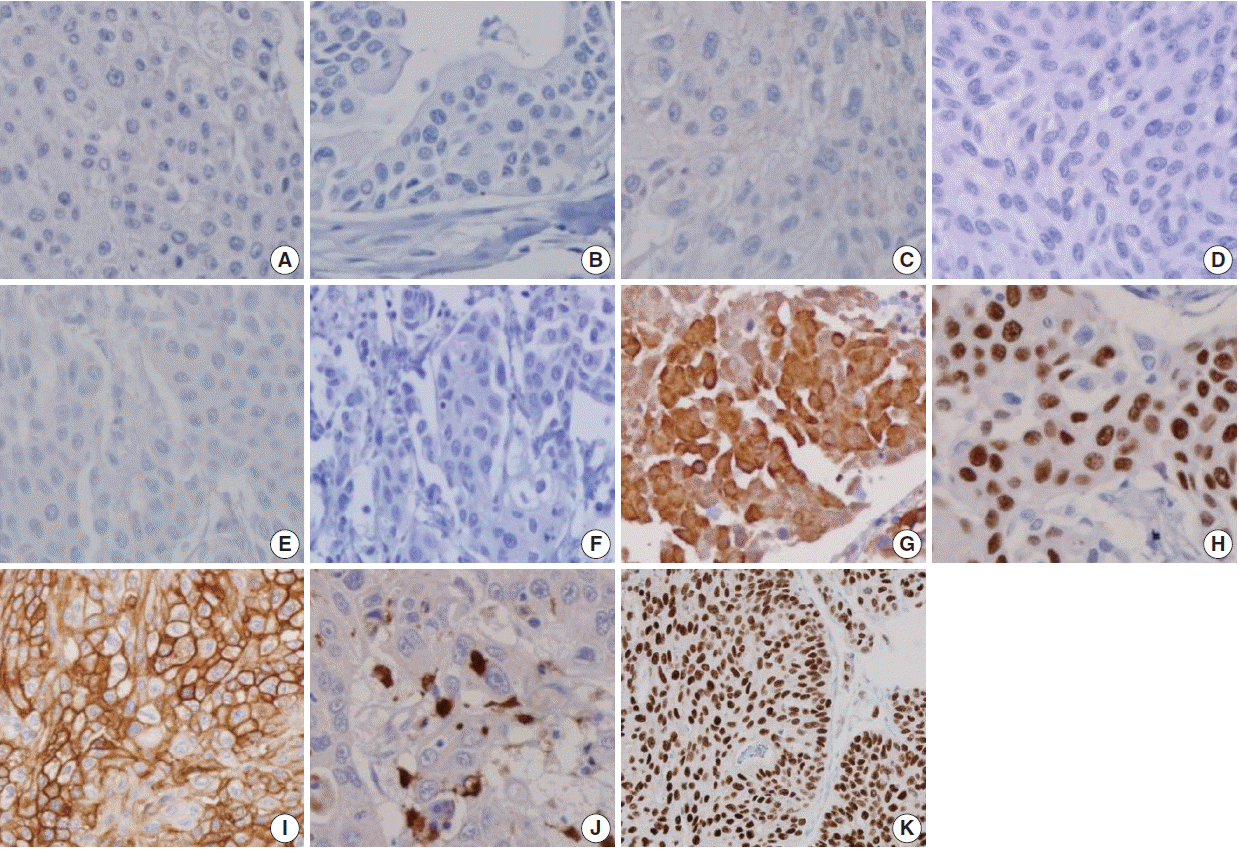
- Figure



Fig. 1.
Fig. 2.
Fig. 3.
| Antibody | Clone | Dilution | Vendor |
|---|---|---|---|
| PSA | Polyclonal | 1:1,000 | Dako |
| PSMA | Monoclonal | 1:100 | Novocastra |
| PAP | Monoclonal | Prediluted | Dako |
| P501s | Monoclonal | 1:200 | Dako |
| NKX3.1 | Monoclonal | 1:500 | Athena ES |
| AMACR | Monoclonal | 1:200 | Cell Marque |
| CK34βE12 | Monoclonal | 1:50 | Dako |
| p63 | Monoclonal | 1:800 | Lab Vision |
| TM | Monoclonal | 1:1,000 | Dako |
| S100P | Monoclonal | 1:800 | Dako |
| GATA3 | Monoclonal | Prediluted | Cell Marque |
| Variable | PSA | PSMA | PAP | P501s | NKX3.1 | AMACR | CK34βE12 | p63 | TM | S100P | GATA3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PAC | |||||||||||
| Sensitivity | 111/111 (100) | 93/111 (83.8) | 102/111 (91.9) | 104/111 (93.7) | 98/111 (88.3) | 74/111 (66.7) | 2/111 (1.8) | 0/111 (0) | 0/111 (0) | 4/111 (3.6) | 0/111 (0) |
| Specificity | 125/138 (90.6) | 137/138 (99.3) | 112/138 (81.2) | 137/138 (99.3) | 138/138 (100) | 126/138 (91.3) | 34/138 (24.6) | 36/138 (26.1) | 75/138 (54.3) | 107/138 (77.5) | 21/138 (15.2) |
| PPV | 111/124 (89.5) | 93/94 (98.9) | 102/128 (79.7) | 104/105 (99.1) | 98/98 (100) | 74/86 (86.1) | 2/106 (1.9) | 0/102 (0) | 0/63 (0) | 4/35 (11.4) | 0/117 (0) |
| NPV | 125/125 (100) | 137/155 (88.4) | 112/121 (92.6) | 137/144 (95.1) | 138/151 (91.4) | 126/163 (77.3) | 34/143 (23.8) | 36/147 (24.5) | 75/186 (40.3) | 107/214 (50) | 21/132 (15.9) |
| UC | |||||||||||
| Sensitivity | 13/138 (9.4) | 1/138 (0.7) | 26/138 (18.8) | 1/138 (0.7) | 0/138 (0) | 12/138 (8.7) | 104/138 (75.4) | 102/138 (73.9) | 63/138 (45.7) | 31/138 (22.5) | 117/138 (84.8) |
| Specificity | 0/111 (0) | 18/111 (16.2) | 9/111 (8.1) | 7/111 (6.3) | 13/111 (11.7) | 37/111 (33.3) | 109/111 (98.2) | 111/111 (100) | 111/111 (100) | 107/111 (96.4) | 111/111 (100) |
| PPV | 13/124 (10.5) | 1/94 (1.1) | 26/128 (20.3) | 1/105 (0.9) | 0/87 (0) | 12/86 (13.9) | 104/106 (98.1) | 102/102 (100) | 63/63 (100) | 31/35 (88.6) | 117/117 (100) |
| NPV | 0/25 (0) | 18/155 (11.6) | 9/21 (42.9) | 7/144 (4.9) | 13/151 (8.6) | 37/163 (22.7) | 109/143 (76.2) | 111/147 (75.5) | 111/186 (59.7) | 107/214 (50) | 106/127 (84.1) |
| Variable | PSA | PSMA | PAP | P501s | CK34βE12 | p63 | S100P | GATA3 |
|---|---|---|---|---|---|---|---|---|
| PAC | ||||||||
| Sensitivity | 47/47 (100) | 39/47 (83) | 43/47 (91.5) | 44/47 (93.6) | 0/47 (0) | 1/47 (2.1) | 2/47 (4.3) | 0/47 (0) |
| Specificity | 95/110 (86.4) | 109/110 (99.1) | 85/110 (77.3) | 109/110 (99.1) | 23/110 (20.9) | 27/110 (24.6) | 84/110 (76.4) | 17/110 (15.5) |
| PPV | 47/62 (75.8) | 39/40 (97.5) | 43/68 (63.2) | 44/45 (98.8) | 0/87 (0) | 1/84 (1.2) | 2/28 (7.1) | 0/93 (0) |
| NPV | 95/95 (100) | 109/117 (93.2) | 85/90 (95.5) | 109/112 (97.3) | 23/70 (32.9) | 27/73 (37) | 84/129 (65.1) | 17/64 (26.6) |
| UC | ||||||||
| Sensitivity | 15/110 (13.6) | 1/110 (0.9) | 25/110 (22.7) | 1/110 (0.9) | 87/110 (79.1) | 83/110 (75.5) | 26/110 (23.6) | 93/110 (84.5) |
| Specificity | 0/47 (0) | 8/47 (17.0) | 4/47 (8.5) | 3/47 (6.4) | 47/47 (100) | 46/47 (97.9) | 45/47 (95.7) | 47/47 (100) |
| PPV | 15/62 (24.2) | 1/40 (2.5) | 25/68 (36.8) | 1/45 (2.2) | 87/87 (100) | 83/84 (98.8) | 26/28 (92.9) | 93/93 (100) |
| NPV | 0/95 (0) | 8/117 (6.8) | 4/89 (4.5) | 3/112 (2.7) | 47/70 (67.1) | 46/73 (63) | 45/129 (34.9) | 46/63 (74.6) |
| Immunohistochemical marker | Current study (2016) |
Chuang et al. (2007) [3] |
Kunju et al. (2006) [6] |
Mhawech et al. (2002) [5] |
Genega et al. (2000) [10] |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| PAC | UC | PAC | UC | PAC | UC | PAC | UC | PAC | UC | |
| PSA | 111/111 (100) | 16/138 (11.6) | 37/38 (97.4) | 0/35 (0) | 40/42 (95.2) | 0/36 (0) | 34/40 (85.0) | 0/45 (0) | 32/34 (94.1) | 0/46 (0) |
| PSMA | 93/111 (83.8) | 1/138 (0.7) | 35/38 (92.1) | 0/35 (0) | - | - | - | - | - | - |
| PAP | 102/111 (91.9) | 26/138 (18.8) | - | - | 40/42 (95.2) | 4/36 (11.1) | 38/40 (95.0) | 0/45 (0) | 32/34 (94.1) | 0/46 (0) |
| P501s | 104/111 (93.7) | 1/138 (0.7) | 38/38 (100) | 2/35 (5.7) | - | - | - | - | - | - |
| NKX3.1 | 98/111 (88.3) | 2/138 (1.4) | 36/38 (94.7) | 0/35 (0) | - | - | - | - | - | |
| AMACR | 74/111 (66.7) | 12/138 (8.7) | - | - | 37/42 (88.1) | 13/36 (36.1) | - | - | - | - |
| CK34βE12 | 2/111 (1.8) | 104/138 (75.4) | 3/38 (7.9) | 32/35 (91.4) | 1/42 (2.4) | 35/36 (97.2) | - | - | 2/34 (5.9) | 30/46 (65.2) |
| p63 | 0/111 (0) | 102/138 (73.9) | 0/38 (0) | 29/35 (82.9) | 0/42 (0) | 33/36 (91.7) | - | - | - | - |
| TM | 0/111 (0) | 63/138 (45.7) | 2/38 (5.3) | 24/35 (68.6) | - | - | 0/40 (0) | 22/45 (48.8) | - | - |
| S100P | 4/111 (3.6) | 31/138 (22.5) | 3/38 (7.9) | 25/35 (71.4) | - | - | - | - | - | - |
| GATA3 | 0/111 (0) | 117/138 (95.9) | - | - | - | - | - | - | - | - |
| CK7 | - | - | - | - | 4/42 (9.5) | 34/36 (94.4) | 11/40 (27.5) | 39/45 (86.6) | 4/34 (11.8) | 38/46 (82.6) |
| CK20 | - | - | - | - | 2/42 (4.8) | 19/36 (52.8) | 4/40 (10.0) | 30/45 (66.6) | 8/34 (23.5) | 10/46 (21.7) |
| Uroplakin III | - | - | - | - | - | - | 0/40 (0) | 27/45 (60) | - | - |
PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; PAP, prostate acid phosphatase; AMACR, α-methylacyl coenzyme A racemase; TM, thrombomodulin; GATA3, GATA binding protein 3.
Values are presented as number (%). PAC, prostatic adenocarcinoma; UC, urothelial carcinoma; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; PAP, prostate acid phosphatase; AMACR, α-methylacyl coenzyme A racemase; TM, thrombomodulin; GATA3, GATA binding protein 3; PPV, positive predictive value; NPV, negative predictive value.
Values are presented as number (%). PAC, prostatic adenocarcinoma; UC, urothelial carcinoma; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; PAP, prostate acid phosphatase; GATA3, GATA binding protein 3; PPV, positive predictive value; NPV, negative predictive value.
Values are presented as number (%). PAC, prostatic adenocarcinoma; UC, urothelial carcinoma; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; PAP, prostate acid phosphatase; AMACR, α-methylacyl coenzyme A racemase; TM, thrombomodulin; GATA3, GATA binding protein 3; CK, cytokeratin.

 E-submission
E-submission