Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(2); 2017 > Article
-
Original Article
Higher Expression of Toll-like Receptors 3, 7, 8, and 9 in Pityriasis Rosea - Mostafa Abou El-Ela, Mohamed El-Komy, Rania Abdel Hay, Rehab Hegazy, Amin Sharobim1, Laila Rashed2, Khalda Amr3
-
Journal of Pathology and Translational Medicine 2017;51(2):148-151.
DOI: https://doi.org/10.4132/jptm.2016.09.09
Published online: February 13, 2017
Department of Dermatology, Faculty of Medicine, Cairo University, Cairo, Egypt
1Department of Dermatology, National Research Center (NRC), Cairo University, Cairo, Egypt
2Department of Clinical Biochemistry, Faculty of Medicine, Cairo University, Cairo, Egypt
3Department of Molecular Genetics, National Research Center (NRC), Cairo University, Cairo, Egypt
- Corresponding Author Rania Abdel Hay, MD Department of Dermatology, Faculty of Medicine, Cairo University, 13th Abrag Othman, Kournish el Maadi, Cairo 11431, Egypt Tel: +20-1006084132 Fax: +20-23596724 E-mail: raniamounir@kasralainy.edu.eg
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Toll-like Receptor-Mediated Immunomodulation of Th1-Type Response Stimulated by Recombinant Antigen of Type 2 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV-2)
Rika Wahyuningtyas, Mei-Li Wu, Wen-Bin Chung, Hso-Chi Chaung, Ko-Tung Chang
Viruses.2023; 15(3): 775. CrossRef - Pityriasis Rosea: An Updated Review
Alexander K.C. Leung, Joseph M. Lam, Kin Fon Leong, Kam Lun Hon
Current Pediatric Reviews.2021; 17(3): 201. CrossRef - Double-blind randomized placebo-controlled trial to evaluate the efficacy and safety of short-course low-dose oral prednisolone in pityriasis rosea
Sidharth Sonthalia, Akshy Kumar, Vijay Zawar, Adity Priya, Pravesh Yadav, Sakshi Srivastava, Atula Gupta
Journal of Dermatological Treatment.2018; 29(6): 617. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

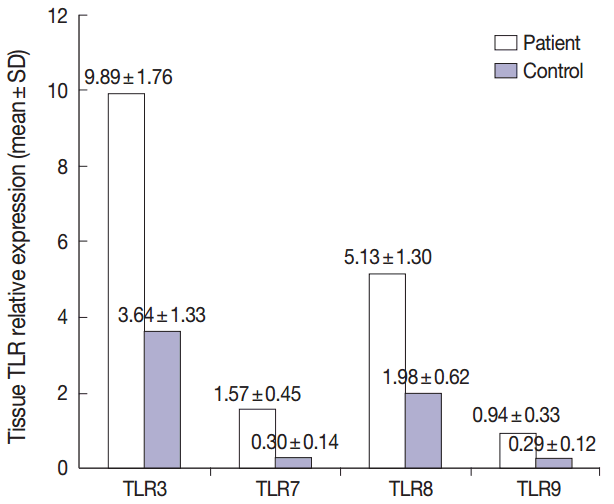
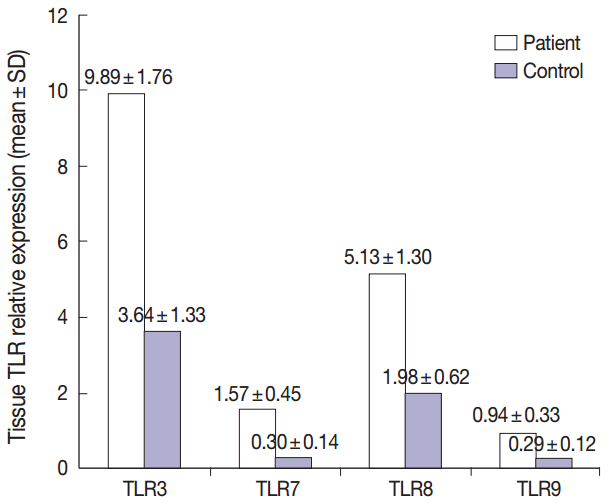
Fig. 1.
| Primer sequence | ||
|---|---|---|
| TLR3 | Forward | 5'- GCATTTGTTTTCTCACTCTTT-3' |
| Reverse | 5'-TTAGCCACTGAAAAGAAAAAT-3' | |
| TLR7 | Forward | 5'-AAACTCCTTGGGGCTAGATG-3' |
| Reverse | 5'-AGGGTGAGGTTCGTGGTGTT-3' | |
| TLR8 | Forward | 5'-CTGTGAGTTATGCGCCGAAGA-3' |
| Reverse | 5'-TGGTGCTGTACATTGGGGTTG-3' | |
| TLR9 | Forward | 5'-CGCCCTGCACCCGCTGTCTCT-3' |
| Reverse | 5'-CGGGGTGCTGCCATGGAGAAG-3' |
| Variable | Patient (n = 24) | Control (n = 24) | p-value |
|---|---|---|---|
| Hb (mg) | 12.69 ± 1.27 (10.8–14.9) | 12.97 ± 1.31 (10.8–14.9) | .431 |
| WBC (mm3) | 6.77 ± 1.63 (4.8–9.8) | 6.46 ± 1.60 (3.9–9.8) | .512 |
| ESR1 (mm/hr) | 14.01 ± 6.27 (6–27) | 12.04 ± 5.36 (6–25) | .251 |
| ESR2 (mm/hr) | 26.25 ± 7.62 (15–40) | 23.37 ± 7.60 (15–40) | .198 |
| ALT (U/L) | 19.41 ± 10.40 (10–47) | 21.91 ± 12.12 (10–47) | .081 |
| AST (U/L) | 20.41 ± 8.37 (10–40) | 21.87 ± 7.90 (12–40) | .126 |
| Urea (mg/dL) | 28.29 ± 9.89 (15–48) | 24.70 ± 8.62 (15–50) | .092 |
| Creatinine (mg/dL) | 0.88 ± 0.23 (0.5–1.3) | 0.89 ± 0.28 (0.5–1.5) | .436 |
PCR, polymerase chain reaction; TLR, Toll-like receptor.
Values are presented as mean ± standard deviation (range). Normal routine laboratory reference values: hemoglobin (Hb; male: 13–18 mg/female: 12–17 mg), white blood cells (WBCs; 4,000–12,000 mm3), erythrocyte sedimentation rate first hour (ESR1; up to 8 mm/hr), erythrocyte sedimentation rate second hour (ESR2; up to 18 mm/hr), alanine aminotransferase (ALT; 5–40 U/L), aspartate aminotransferase (AST; 7–56 U/L), urea (20–50 mg/dL), creatinine (0.5–1.5 mg/dL).

 E-submission
E-submission