Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(3); 2017 > Article
-
Original Article
Clinicopathological Study of 18 Cases of Inflammatory Myofibroblastic Tumors with Reference to ALK-1 Expression: 5-Year Experience in a Tertiary Care Center - Ramesh Babu Telugu, Anne Jennifer Prabhu, Nobin Babu Kalappurayil, John Mathai1, Birla Roy Gnanamuthu2, Marie Therese Manipadam
-
Journal of Pathology and Translational Medicine 2017;51(3):255-263.
DOI: https://doi.org/10.4132/jptm.2017.01.12
Published online: April 17, 2017
Department of General Pathology, Christian Medical College and Hospital, Vellore, India
1Department of Paediatric Surgery, Christian Medical College and Hospital, Vellore, India
2Department of Thoracic Surgery, Christian Medical College and Hospital, Vellore, India
- Corresponding Author Ramesh Babu Telugu, MD Department of General Pathology, Christian Medical College and Hospital, Vellore, Tamilnadu 632004, India Tel: +91-9566434081 Fax: +91-416-2232054 E-mail: dr.rameshtelugu@gmail.com
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Inflammatory myofibroblastic tumor in the liver after bone marrow transplantation: case report and literature review
Shuhui Yang, Yongsheng Tang, Zenan Yuan, Jianwen Zhang
Frontiers in Medicine.2025;[Epub] CrossRef - Inflammatory Myofibroblastic Tumor: An Updated Review
Joon Hyuk Choi
Cancers.2025; 17(8): 1327. CrossRef - Inflammatory Myofibroblastic Tumour of the Bladder in a Young Male: A Rare Case Report
Mohammad Hifzi Mohd Hashim, Suhaila Abdullah, Chin Yiun Lee, Syahril Anuar Salauddin, Hamid Hj Ghazali
Cureus.2025;[Epub] CrossRef - Rare case of small bowel inflammatory myofibroblastic tumor
Nemanja Trifunović, Nebojša Mitrović, Dejan Stevanović, Damir Jašarović, Jovana Trifunović
Journal of Surgery and Medicine.2025; 9(10): 00. CrossRef - Clinicopathological Features and Immunochemical Staining of Inflammatory Myofibroblastic Tumor: A Retrospective Study of 48 Cases
Qi-An Wang, Ren-Chin Wu, Chao-Wei Lee, Yu-Hsuan Yeh, Chiao-En Wu, Suraiya Saleem
Analytical Cellular Pathology.2025;[Epub] CrossRef - Treatment and outcomes in pediatric inflammatory myofibroblastic tumors – A systematic review of published studies
Arimatias Raitio, Paul D. Losty
European Journal of Surgical Oncology.2024; 50(7): 108388. CrossRef - Case report: Epithelioid inflammatory myofibroblastic sarcoma treated with an ALK TKI ensartinib
Mengmeng Li, Ruyue Xing, Jiuyan Huang, Chao Shi, Chunhua Wei, Huijuan Wang
Frontiers in Oncology.2023;[Epub] CrossRef - Inflammatory Myofibroblastic Tumour of the Sinonasal Tract with Orbital and Intracranial Extensions Simulating a Malignancy: A Case Report and Review of Literature
Gaveshani Mantri, Subhalaxmi Rautray, Rahul Mohanty, Vinushree Karakkandy
Indian Journal of Otolaryngology and Head & Neck Surgery.2022; 74(S2): 1668. CrossRef - Clinical, pathologic, and molecular features of inflammatory myofibroblastic tumors in children and adolescents
Aurore Pire, Daniel Orbach, Louise Galmiche, Dominique Berrebi, Sabine Irtan, Sabah Boudjemaa, Hervé J. Brisse, Laureline Berteloot, Salma Moalla, Charlotte Mussini, Pascale Philippe‐Chomette, Bogdana Tilea, Gaelle Pierron, Florent Guerin, Véronique Minar
Pediatric Blood & Cancer.2022;[Epub] CrossRef - Case Report: Early Distant Metastatic Inflammatory Myofibroblastic Tumor Harboring EML4-ALK Fusion Gene: Study of Two Typical Cases and Review of Literature
Qianqian Han, Xin He, Lijuan Cui, Yan Qiu, Yuli Li, Huijiao Chen, Hongying Zhang
Frontiers in Medicine.2022;[Epub] CrossRef - Inflammatory myofibroblastic tumor: Amulti‐institutionalstudy from the Pediatric Surgical Oncology Research Collaborative
Barrie S. Rich, Joanna Fishbein, Timothy Lautz, Nathan S. Rubalcava, Tanvi Kartal, Erika Newman, Pei En Wok, Rodrigo L. P. Romao, Richard Whitlock, Bindi Naik‐Mathuria, Stephanie F. Polites, Katrine Løfberg, Danny Lascano, Eugene Kim, Jacob Davidson, Andr
International Journal of Cancer.2022; 151(7): 1059. CrossRef - Child inflammatory myofibroblastic tumor of the kidney misdiagnosed as Wilms' tumor: case report
Yu-Feng Bai, Jing-Zhong Liu, Li-Na Yue, Li Chen, Sui-Yi Liu, Rui Liu
Radiology Case Reports.2022; 17(12): 4920. CrossRef - A Common Cell of Origin for Inflammatory Myofibroblastic Tumor and Lung Adenocarcinoma with ALK rearrangement
Vasyl Nesteryuk, Omar Hamdani, Raymond Gong, Nava Almog, Brian M. Alexander, Steffan Soosman, Ken Yoneda, Siraj M. Ali, Alexander D. Borowsky, Jonathan W. Riess
Clinical Lung Cancer.2022; 23(8): e550. CrossRef - An extremely rare case of malignant jejunal mesenteric inflammatory myofibroblastic tumor in a 61-year-old male patient: A case report and literature review
Hamdi Al Shenawi, Salamah A. Al-Shaibani, Suhair K. Al Saad, Fedaa Al-Sindi, Khalid Al-Sindi, Noor Al Shenawi, Yahya Naguib, Rami Yaghan
Frontiers in Medicine.2022;[Epub] CrossRef - Primary inflammatory myofibroblastic tumor of stomach—report of a very rare case
Ranendra Hajong, Kewithinwangbo Newme, Donkupar Khongwar
Journal of Family Medicine and Primary Care.2021; 10(1): 552. CrossRef - Complicated course of biliary inflammatory myofibroblastic tumor mimicking hilar cholangiocarcinoma: A case report and literature review
Sandra Strainiene, Kotryna Sedleckaite, Juozas Jarasunas, Ilona Savlan, Juozas Stanaitis, Ieva Stundiene, Tomas Strainys, Valentina Liakina, Jonas Valantinas
World Journal of Clinical Cases.2021; 9(21): 6155. CrossRef - Epithelioid Inflammatory Myofibroblastic Sarcoma Presenting as Gastrointestinal Bleed
Alexandra Giannaki, Dimitrios Doganis, Panagiota Giamarelou, Anastasia Konidari
JPGN Reports.2021;[Epub] CrossRef - Complete response to alectinib following crizotinib in an ALK-positive inflammatory myofibroblastic tumor with CNS involvement
Camila B. Xavier, Felipe S.N.A. Canedo, Fabíola A.S. Lima, Raíssa R. Melo, Luiz Guilherme C.A. Lima, José Flávio G. Marin, Ciro E. Souza, Olavo Feher
Current Problems in Cancer: Case Reports.2021; 4: 100117. CrossRef - Urinary Bladder Inflammatory Myofibroblastic Tumor With Mutated TP53 and PPFIBP1-ALK Gene Fusion
Andreia N. Barbieri, Christopher T. Tallman, Raj Satkunasivam, Joseph Annunziata, Jessica S. Thomas, Randall J. Olsen, Steven S. Shen, Michael J. Thrall, Mary R. Schwartz
AJSP: Reviews and Reports.2021; 26(1): 45. CrossRef - Therapeutic options in inoperable ROS1-rearranged inflammatory myofibroblastic tumor of the tongue in a child: a case report and literature review
Malgorzata Styczewska, Agastya Patel, Joanna Jaskulowska, Jan Godzinski, Dominik Swieton, Bartosz Wasag, Juliea Dass, Ewa Bien, Malgorzata A. Krawczyk
Anti-Cancer Drugs.2021; 32(10): 1111. CrossRef - Non-squamous cell carcinoma diseases of the larynx: clinical and imaging findings
Serap Doğan, Alperen Vural, Güven Kahriman, Hakan İmamoğlu, Ümmühan Abdülrezzak, Mustafa Öztürk
Brazilian Journal of Otorhinolaryngology.2020; 86(4): 468. CrossRef - Successful treatment of pulmonary inflammatory myofibroblastic tumor with platinum‐pemetrexed: The first report of two cases
Xiaoyan Si, Hanping Wang, Xiaotong Zhang, Mengzhao Wang, Yan You, Li Zhang
Thoracic Cancer.2020; 11(8): 2339. CrossRef - Rare presentation of inflammatory myofibroblastic tumor in the kidney
Hiba Abduljawad, Ahmet Aslan, Khalifa Aldoseri, Erdem Yilmaz, Wael Ibrahim
Radiology Case Reports.2020; 15(8): 1266. CrossRef - Histopathological landscape of rare oesophageal neoplasms
Gianluca Businello, Carlo Alberto Dal Pozzo, Marta Sbaraglia, Luca Mastracci, Massimo Milione, Luca Saragoni, Federica Grillo, Paola Parente, Andrea Remo, Elena Bellan, Rocco Cappellesso, Gianmaria Pennelli, Mauro Michelotto, Matteo Fassan
World Journal of Gastroenterology.2020; 26(27): 3865. CrossRef - Non‐squamous cell carcinoma diseases of the larynx: clinical and imaging findings
Serap Doğan, Alperen Vural, Güven Kahriman, Hakan İmamoğlu, Ümmühan Abdülrezzak, Mustafa Öztürk
Brazilian Journal of Otorhinolaryngology (Versão em Português).2020; 86(4): 468. CrossRef - Laryngeal Inflammatory Myofibroblastic Tumor : Case Series and Literature Review
Ki-Ik Park, Sung-hoon Kim, In-Suh Park, Ji Won Kim
Journal of The Korean Society of Laryngology, Phoniatrics and Logopedics.2019; 30(1): 57. CrossRef - Anaplastic lymphoma kinase-negative pulmonary inflammatory myofibroblastic tumor with multiple metastases and its treatment by Apatinib
Qiuxia Liu, Jianguo Wei, Xizhong Liu, Jianfang Wang
Medicine.2019; 98(52): e18414. CrossRef - Inflammatory myofibroblastic tumor
Veronika Marcináková, Hana Dittrichová, Karel Franěk, Pavel Hanek
Urologie pro praxi.2019; 20(1): 40. CrossRef - Ureteral inflammatory myofibroblastic tumor
Faping Li, Hui Guo, Heping Qiu, Yuchuan Hou
Medicine.2018; 97(46): e13177. CrossRef - Pulmonary inflammatory myofibroblastic tumour misdiagnosed as a round pneumonia
Samira Naime, Anjum Bandarkar, Gustavo Nino, Geovanny Perez
BMJ Case Reports.2018; 2018: bcr-2017-224091. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
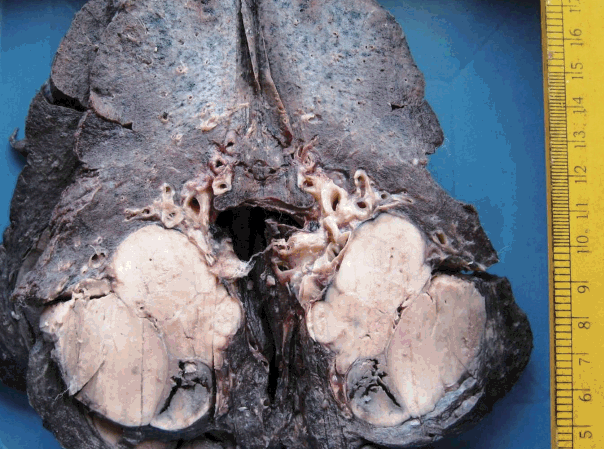
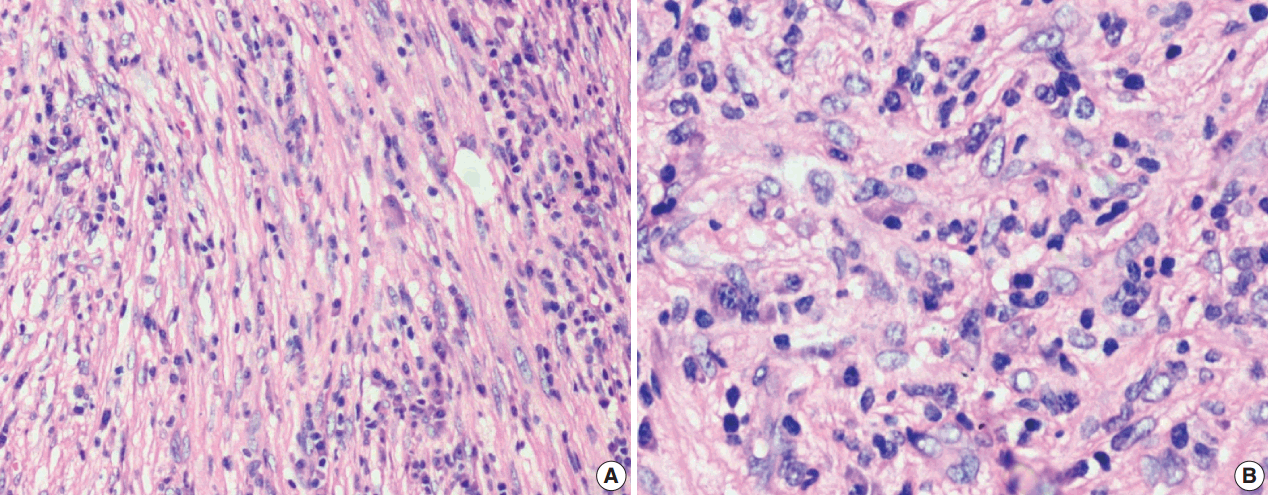
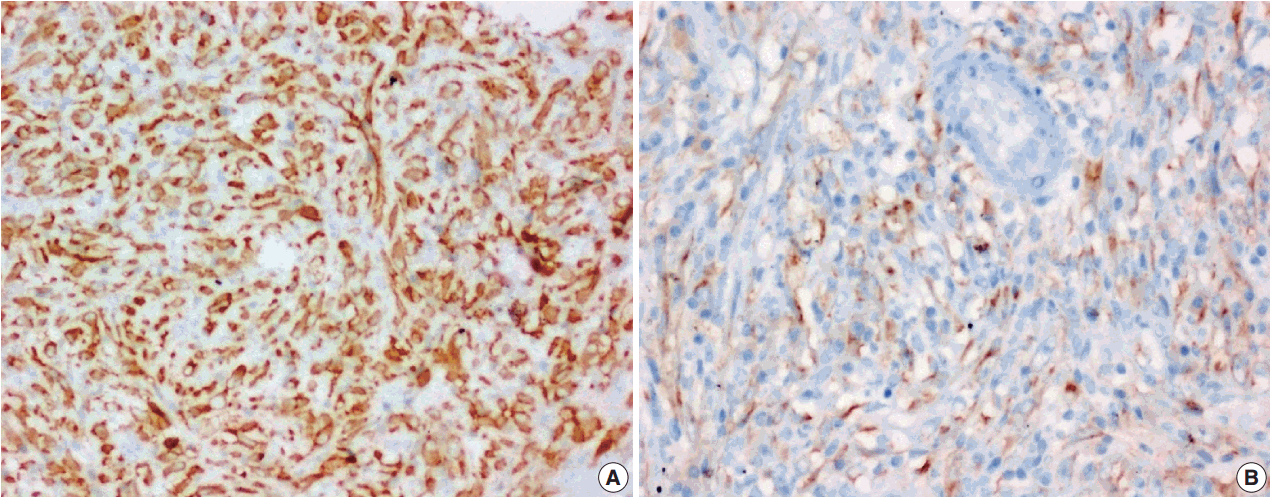
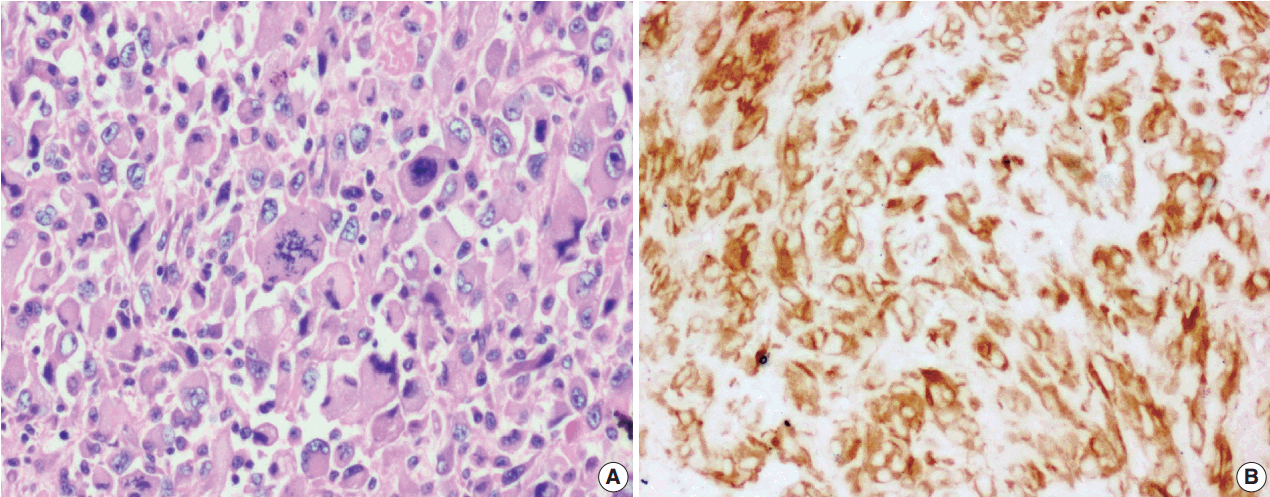
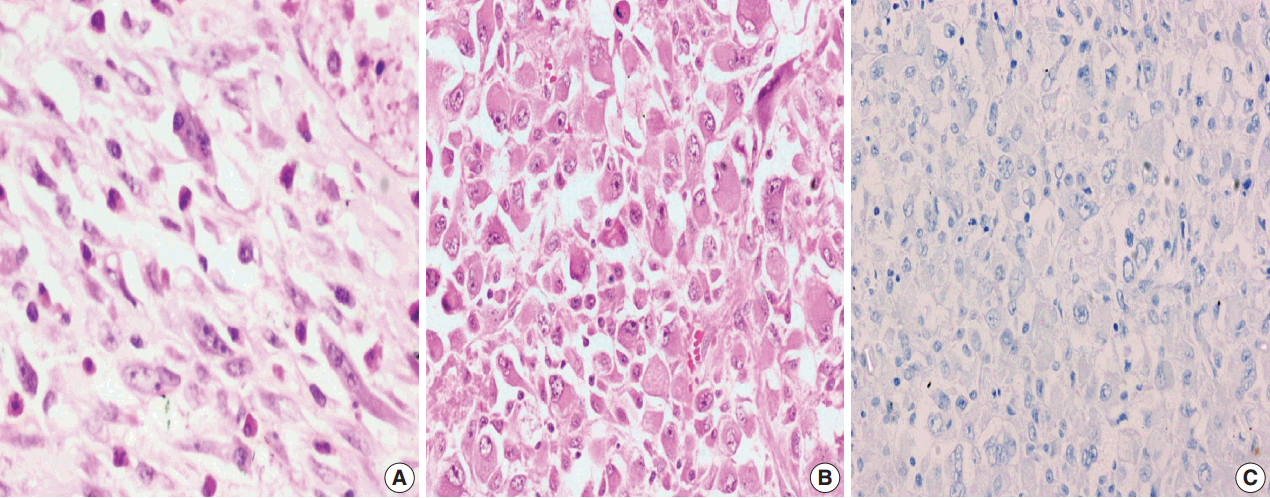
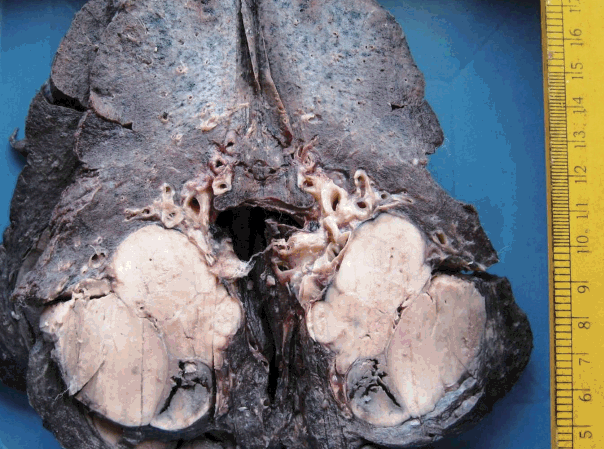
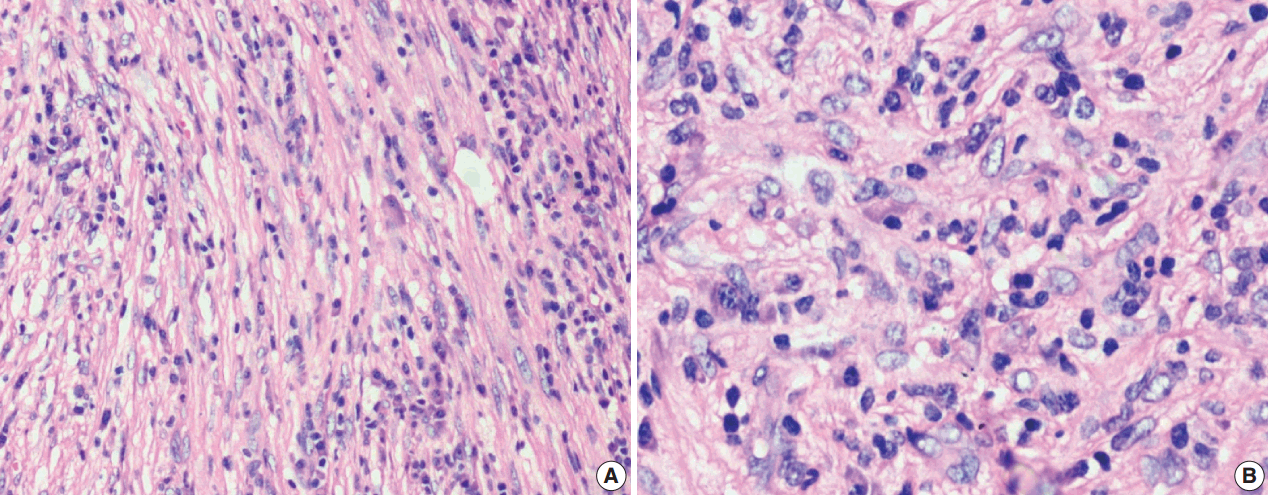
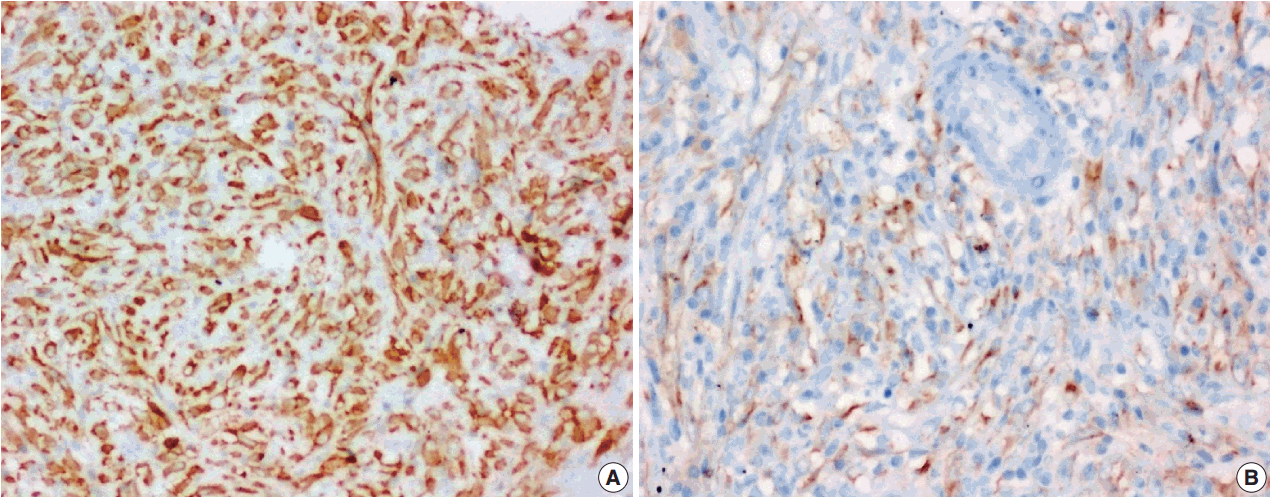
- Figure






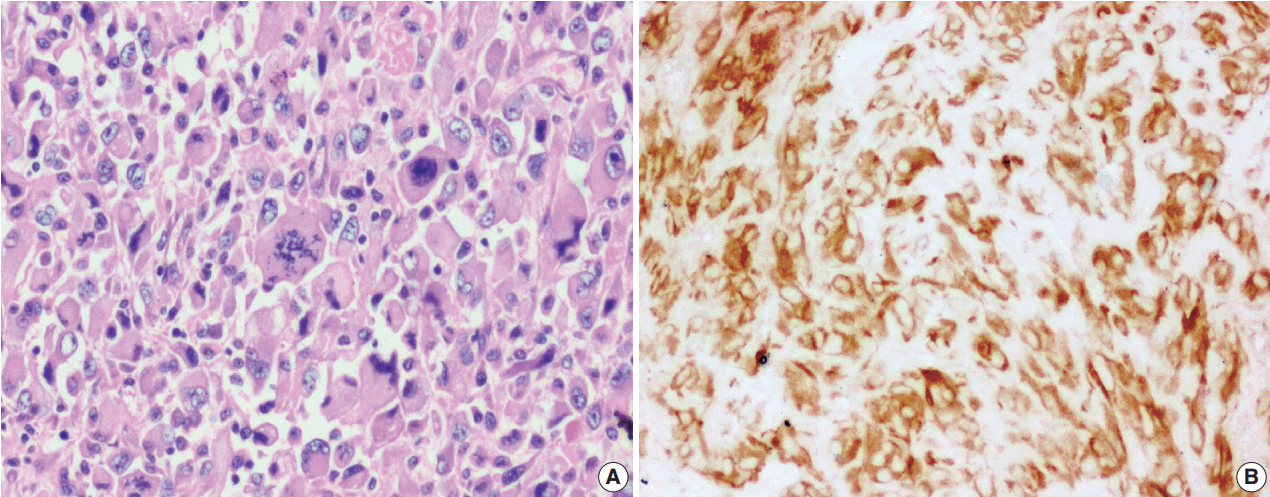
Fig. 1.
Fig. 2.
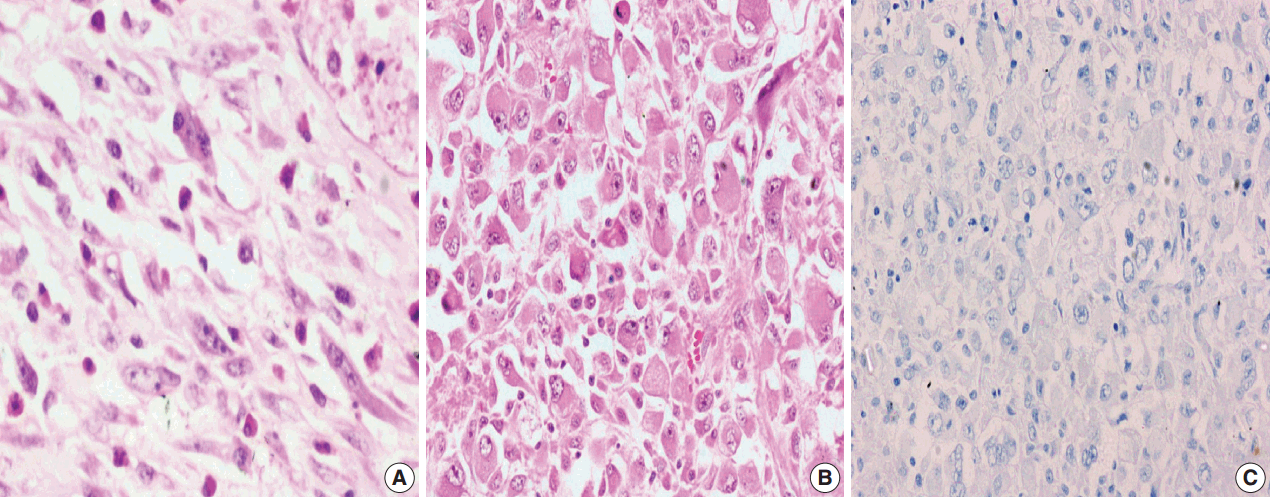
Fig. 3.
Fig. 4.
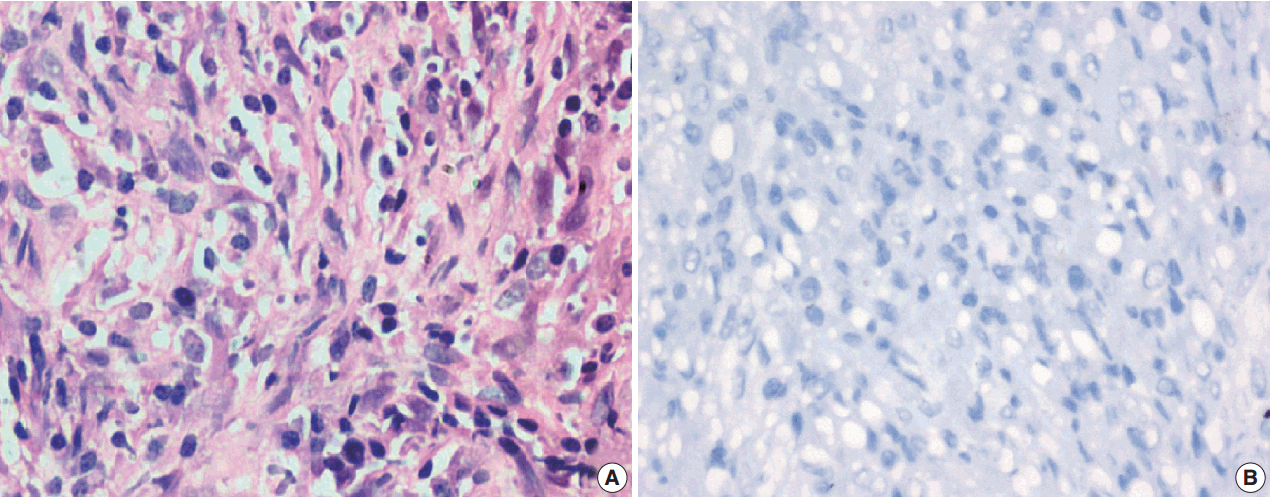
Fig. 5.
Fig. 6.
| Antibody | Poly/Monoclonal | Source | Dilution |
|---|---|---|---|
| ALK-1 | Monoclonal | Leica | Prediluted |
| SMA | 1A4 | Dako | Concentrated |
| MSA | HHF35 | Dako | Concentrated |
| Vimentin | V9 | Dako | Concentrated |
| Desmin | D33 | Dako | Concentrated |
| EMA | E29 | Dako | Concentrated |
| CK | AE1/AE3 | Dako | Concentrated |
| Myogenin | F5D | Biosb | Concentrated |
| CD34 | Q-bend 10 | Dako | Concentrated |
| CD21 | 1F8 | Dako | Concentrated |
| CD117 | C-kit | Dako | Concentrated |
| H-Caldesmon | H/CD | Dako | Concentrated |
| TLE-1 | M-101 | Sigma | Concentrated |
| CD30 | Ber H2 | Dako | Concentrated |
| CD15 | Carb-3 | Dako | Concentrated |
| Case No. | Age (yr) | Sex | Anatomic site | Size (cm) | Treatment | Recurrence (mo) | Metastases | Follow-up status (mo) |
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | F | Suprarenal | 15 | SE + CT | 1 | Liver and Lung | 8 (DOD) |
| 2 | 27 | M | Colon | 13 | SE | NA | NA | NA |
| 3 | 37 | M | Lung | 3.2 | SE | No | No | 4 (ANED) |
| 4 | 26 | F | Mastoid antrum | 1 | SE | No | No | 38 (ANED) |
| 5 | 29 | F | Lung | 4.2 | SE + CT | No | No | 11 (ANED) |
| 6 | 25 | F | Breast | 6.5 | SE | 1 | No | 6 (ANED) |
| 7 | 27 | F | Colon | 4.5 | SE | No | No | 3 (ANED) |
| 8 | 25 | M | Sphenoid wing | 2 | SE + CT + RT | 24 | No | 51 (ANED) |
| 9 | 18 | M | Bronchus | 5 | SE | 10 | No | 65 (ANED) |
| 10 | 26 | F | Lung | 1 | SE | 6 | No | 16 (ANED) |
| 11 | 10 | M | Omentum | 7 | SE | No | No | 14 (ANED) |
| 12 | 37 | F | Lung | 6 | SE | No | No | 8 (ANED) |
| 13 | 41 | M | Jejunum, ileocaeccum, mesentry, and omentum | 8.5 | SE + RT | 6 | No | 9 (ANED) |
| 14 | 24 | M | Dorsum of nose | 2 | SE | No | No | 8 (ANED) |
| 15 | 13 | M | Pelvic mass, omentum, and bladder | 11 | SE + CT | No | Pelvic bone | 7 (ANED) |
| 16 | 4 | M | Mediastinum | 2.5 | SE | No | No | NA |
| 17 | 44 | F | Lung | 7 | SE | No | No | 7 (ANED) |
| 18 | 12 | F | Distal CBD, cystic duct, and periportal lymph nodes | 1 | SE | No | No | 3 (ANED) |
| Marker (No. of tests) | Positive | Negative |
|---|---|---|
| ALK-1 (n = 18) | 10 (55.6) | 8 (44.4) |
| SMA (n = 16) | 12 (75.0) | 4 (25.0) |
| MSA (n = 6) | 3 (50.0) | 3 (50.0) |
| Vimentin (n = 8) | 8 (100) | 0 |
| Desmin (n = 11) | 5 (45.5) | 6 (54.5) |
| EMA (n = 4) | 2 (50.0) | 2 (50.0) |
| CK (n = 6) | 0 | 6 (100) |
| Myogenin (n = 3) | 0 | 3 (100) |
| CD34 (n = 7) | 0 | 7 (100) |
| CD21 (n = 3) | 0 | 3 (100) |
| CD117 (n = 2) | 0 | 2 (100) |
| H Caldesmon (n = 4) | 0 | 4 (100) |
| TLE-1 (n = 4) | 0 | 4 (100) |
| CD30 (n = 3) | 0 | 3 (100) |
| CD15 (n = 2) | 0 | 2 (100) |
| Case No. | Sex | Histological pattern | Atypia | ALK-1 expression | Recurrence (mo) | Metastases |
|---|---|---|---|---|---|---|
| 1 | F | C | Yes | 3+ | 1 | Liver and lung |
| 2 | M | C | Yes | 0 | NA | NA |
| 3 | F | C | No | 2+ | No | No |
| 4 | F | Fi | No | 0 | No | No |
| 5 | F | My | No | 0 | No | No |
| 6 | F | C | Yes | 0 | 1 | No |
| 7 | F | C | No | 2+ | No | No |
| 8 | M | C | No | 3+ | 24 | No |
| 9 | M | My | No | 0 | 10 | No |
| 10 | F | C | No | 2+ | 6 | No |
| 11 | M | C | No | 3+ | No | No |
| 12 | F | C | No | 0 | No | No |
| 13 | M | C | Yes | 0 | 6 | No |
| 14 | M | C | No | 0 | No | No |
| 15 | M | Fi | No | 3+ | No | Pelvic bone |
| 16 | M | C | No | 3+ | No | No |
| 17 | F | C | No | 3+ | No | No |
| 18 | F | C | No | 2+ | No | No |
| Variable | ALK-1 positive (n = 10) | ALK-1 negative (n = 8) | p-value |
|---|---|---|---|
| Male:Female | 1:1 | 1:1 | - |
| Age (yr) | |||
| Range | 3–44 | 18–41 | - |
| Mean | 23.8 | 27.1 | |
| Tumor type | |||
| Classical | 9 | 5 | .275 |
| Atypical | 1 | 3 | .275 |
| Multifocal (multicentric in origin) | 2 | 1 | > .99 |
| Biological behavior | |||
| Recurrence | 3 | 3 | > .99 |
| Metastasis | 2 | 0 | .485 |
ALK-1, anaplastic lymphoma kinase-1; SMA, smooth muscle actin, MSA, muscle-specific actin; EMA, epithelial membrane antigen; CK, cytokeratin; TLE-1, transducin-like enhancer protein-1.
F, female; SE, surgical excision; CT, chemotherapy; DOD, died of disease; M, male; NA, not available; ANED, alive with no evidence of disease; RT, radiotherapy; CBD, common bile duct.
Values are presented as number (%). ALK-1, anaplastic lymphoma kinase-1; SMA, smooth muscle actin, MSA, muscle-specific actin; EMA, epithelial membrane antigen; CK, cytokeratin; TLE-1, transducin-like enhancer protein-1.
ALK-1, anaplastic lymphoma kinase-1; F, female; C, cellular histological pattern; NA, not available; M, male; Fi, fibrous histological pattern; My, myxoid histological pattern; 0, negative; 1+, <10% positive cells; 2+, 10-50% positive cells; 3+, >50% of cells positive.
ALK-1, anaplastic lymphoma kinase-1.

 E-submission
E-submission