Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(4); 2017 > Article
-
Original Article
Prognostic Significance of a Micropapillary Pattern in Pure Mucinous Carcinoma of the Breast: Comparative Analysis with Micropapillary Carcinoma - Hyun-Jung Kim, Kyeongmee Park, Jung Yeon Kim, Guhyun Kang, Geumhee Gwak1, Inseok Park1
-
Journal of Pathology and Translational Medicine 2017;51(4):403-409.
DOI: https://doi.org/10.4132/jptm.2017.03.18
Published online: June 9, 2017
Department of Pathology, Inje University Sanggye Paik Hospital, Seoul, Korea
1Department of Surgery, Inje University Sanggye Paik Hospital, Seoul, Korea
- Corresponding Author Kyeongmee Park, MD, PhD Department of Pathology, Inje University Sanggye Paik Hospital, 1342 Dongil-ro, Nowon-gu, Seoul 01757, Korea Tel: +82-2-950-1263 Fax: +82-2-951-6964 E-mail: kpark@paik.ac.kr
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Pure mucinous adenocarcinoma of the breast with the rare lymphoplasmacytic infiltration: A case report with review of literature
Yash Hasmukhbhai Prajapati, Vishal Bhabhor, Kahan Samirkumar Mehta, Mithoon Barot, Husen Boriwala, Mohamed Omar
Clinical Case Reports.2024;[Epub] CrossRef - Clinicopathological features and prognosis of mucinous breast carcinoma with a micropapillary structure
Beibei Yang, Menglu Shen, Bo Sun, Jing Zhao, Meng Wang
Thoracic Cancer.2024; 15(36): 2530. CrossRef - Expression of autocrine motility factor receptor (AMFR) in human breast and lung invasive micropapillary carcinomas
Jing Xu, Hongfei Ma, Qi Wang, Hui Zhang
International Journal of Experimental Pathology.2023; 104(1): 43. CrossRef - The Spectrum of Mucinous Lesions of the Breast
Upasana Joneja, Juan Palazzo
Archives of Pathology & Laboratory Medicine.2023; 147(1): 19. CrossRef - Pure Mucinous Carcinoma of the Breast: Radiologic-Pathologic Correlation
Cherie M Kuzmiak, Benjamin C Calhoun
Journal of Breast Imaging.2023; 5(2): 180. CrossRef - On Ultrasonographic Features of Mucinous Carcinoma with Micropapillary Pattern
Wei-Sen Yang, Yang Li, Ya Gao
Breast Cancer: Targets and Therapy.2023; Volume 15: 473. CrossRef - Micropapillary Breast Carcinoma: From Molecular Pathogenesis to Prognosis
Georgios-Ioannis Verras, Levan Tchabashvili, Francesk Mulita, Ioanna Maria Grypari, Sofia Sourouni, Evangelia Panagodimou, Maria-Ioanna Argentou
Breast Cancer: Targets and Therapy.2022; Volume 14: 41. CrossRef - Mucinous carcinoma of the breast: distinctive histopathologic and genetic characteristics
Minjung Jung
Kosin Medical Journal.2022; 37(3): 176. CrossRef - Triple-Positive Breast Carcinoma: Histopathologic Features and Response to Neoadjuvant Chemotherapy
Jennifer Zeng, Marcia Edelweiss, Dara S. Ross, Bin Xu, Tracy-Ann Moo, Edi Brogi, Timothy M. D'Alfonso
Archives of Pathology & Laboratory Medicine.2021; 145(6): 728. CrossRef - HER2 positive mucinous carcinoma of breast with micropapillary features: Report of a case and review of literature
Dinesh Chandra Doval, Rupal Tripathi, Sunil Pasricha, Pankaj Goyal, Chaturbhuj Agrawal, Anurag Mehta
Human Pathology: Case Reports.2021; 25: 200531. CrossRef - Sonographic Features of Pure Mucinous Breast Carcinoma With Micropapillary Pattern
Wu Zhou, Yong-Zhong Li, Li-Min Gao, Di-Ming Cai
Frontiers in Oncology.2021;[Epub] CrossRef - Clinicopathologic characteristics of HER2-positive pure mucinous carcinoma of the breast
Yunjeong Jang, Hera Jung, Han-Na Kim, Youjeong Seo, Emad Alsharif, Seok Jin Nam, Seok Won Kim, Jeong Eon Lee, Yeon Hee Park, Eun Yoon Cho, Soo Youn Cho
Journal of Pathology and Translational Medicine.2020; 54(1): 95. CrossRef - Mucinous carcinoma with micropapillary features is morphologically, clinically and genetically distinct from pure mucinous carcinoma of breast
Peng Sun, Zaixuan Zhong, Qianyi Lu, Mei Li, Xue Chao, Dan Chen, Wenyan Hu, Rongzhen Luo, Jiehua He
Modern Pathology.2020; 33(10): 1945. CrossRef - Micropapillary pattern in pure mucinous carcinoma of the breast – does it matter or not?
Xiaoli Xu, Rui Bi, Ruohong Shui, Baohua Yu, Yufan Cheng, Xiaoyu Tu, Wentao Yang
Histopathology.2019; 74(2): 248. CrossRef - An Update of Mucinous Lesions of the Breast
Beth T. Harrison, Deborah A. Dillon
Surgical Pathology Clinics.2018; 11(1): 61. CrossRef - The clinicopathological significance of micropapillary pattern in colorectal cancers
Jung-Soo Pyo, Mee Ja Park, Dong-Wook Kang
Human Pathology.2018; 77: 159. CrossRef - The sonographic findings of micropapillary pattern in pure mucinous carcinoma of the breast
Heqing Zhang, Li Qiu, Yulan Peng
World Journal of Surgical Oncology.2018;[Epub] CrossRef - Diagnostic dilemma of micropapillary variant of mucinous breast cancer
Geok Hoon Lim, Zhiyan Yan, Mihir Gudi
BMJ Case Reports.2018; 2018: bcr-2018-225775. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
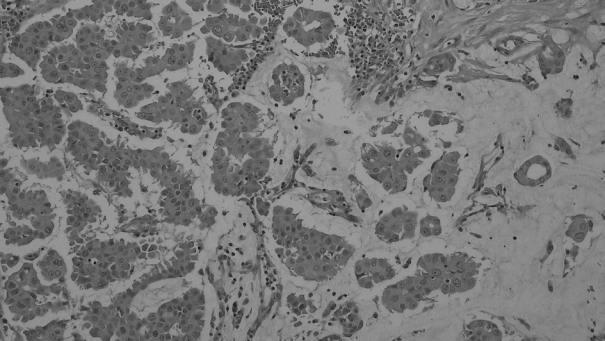
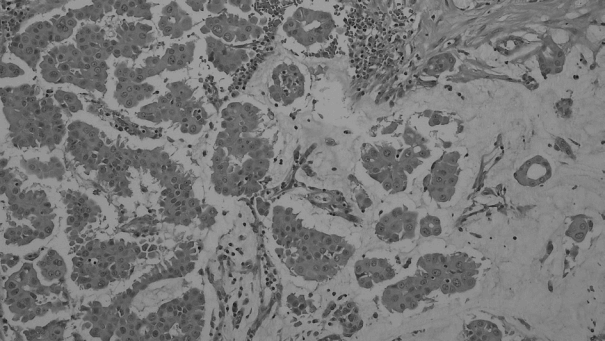
- Figure

Fig. 1.
| Parameter | MUC (n = 19) | MUMPC (n = 17) | IMPC (n = 15) | IDMPC (n = 13) | p-value |
|
|---|---|---|---|---|---|---|
| Age, mean (yr) | 52.7 ± 14.0 | 53.9 ± 11.8 | 45.9 ± 11.7 | 54.3 ± 12.6 | .22 |
|
| NG | Low | 6 (31.6) | 1 (5.9) | 0 | 0 | < .01 |
| Intermediate | 11 (57.9) | 14 (82.4) | 4 (26.7) | 4 (30.8) | ||
| High | 2 (10.5) | 2 (11.8) | 11 (73.3) | 9 (69.2) | ||
| Mitosis | Low | 16 (84.2) | 13 (76.5) | 2 (13.3) | 2 (15.4) | < .01 |
| Intermediate | 1 (5.3) | 4 (23.5) | 3 (20.0) | 3 (23.1) | ||
| High | 2 (10.5) | 0 | 10 (66.7) | 8 (61.5) | ||
| ER | Negative | 0 | 0 | 5 (33.3) | 4 (30.8) | .04 |
| Positive | 19 (100) | 17 (100) | 10 (66.7) | 9 (69.2) | ||
| PR | Negative | 1 (5.3) | 1 (5.9) | 7 (46.7) | 5 (38.5) | .04 |
| Positive | 18 (94.7) | 16 (94.1) | 8 (53.3) | 8 (61.5) | ||
| HER2 | Negative | 18 (94.7) | 16 (94.1) | 9 (60.0) | 8 (61.5) | .01 |
| Positive | 1 (5.3) | 1 (5.9) | 6 (40.0) | 5 (38.5) | ||
| Ki-67 | Low | 11 (57.9) | 7 (41.2) | 1 (6.7) | 1 (7.7) | < .01 |
| High | 8 (42.1) | 10 (58.8) | 14 (93.3) | 12 (92.3) | ||
| MS | LA | 11 (57.9) | 6 (35.3) | 1 (6.7) | 1 (7.7) | < .01 |
| LB | 8 (42.1) | 11 (64.7) | 9 (60.0) | 8 (61.5) | ||
| HER2 | 0 | 0 | 2 (13.3) | 2 (15.4) | ||
| Basal-like | 0 | 0 | 3 (20.0) | 2 (15.4) | ||
| T-stage | 1 | 11 (57.9) | 6 (35.3) | 6 (40.0) | 4 (30.8) | .62 |
| 2 | 7 (36.8) | 7 (41.2) | 6 (40.0) | 7 (53.8) | ||
| 3 | 1 (5.3) | 4 (23.5) | 3 (20.0) | 2 (15.4) | ||
| LNM | Absence | 17 (89.5) | 13 (76.5) | 3 (20.0) | 4 (30.8) | < .01 |
| Presence | 2 (10.5) | 4 (23.5) | 12 (80.0) | 9 (69.2) |
| Parameter | Alive | Dead | p-value |
|
|---|---|---|---|---|
| Age, mean (yr) | 51.6 ± 12.3 | 53.0 ± 16.0 | .75 |
|
| Category | MUC | 19 (100) | 0 | .20 |
| MUMPC | 14 (82.4) | 3 (17.6) | ||
| IMPC | 12 (80.0) | 3 (20.0) | ||
| IDMPC | 10 (76.9) | 3 (23.1) | ||
| MUC vs MUMPC | MUC | 19 (100) | 0 | .06 |
| MUMPC | 14 (82.4) | 3 (17.6) | ||
| NG | Low | 7 (100) | 0 | .34 |
| Intermediate | 29 (87.9) | 4 (12.1) | ||
| High | 19 (79.2) | 5 (20.8) | ||
| Mitosis | Low | 31 (93.9) | 2 (6.1) | .14 |
| Intermediate | 8 (72.7) | 3 (27.3) | ||
| High | 16 (80.0) | 4 (20.0) | ||
| ER | Negative | 6 (66.7) | 3 (33.3) | .07 |
| Positive | 49 (89.1) | 6 (10.9) | ||
| PR | Negative | 9 (64.3) | 5 (35.7) | .01 |
| Positive | 46 (92.0) | 4 (8.0) | ||
| HER2 | Negative | 47 (92.2) | 4 (7.8) | .01 |
| Positive | 8 (61.5) | 5 (38.5) | ||
| Ki-67 | Low | 19 (95.0) | 1 (5.0) | .16 |
| High | 36 (81.8) | 8 (18.2) | ||
| MS | LA | 20 (82.9) | 0 | .05 |
| LB | 29 (82.9) | 6 (17.1) | ||
| HER2 | 2 (50.0) | 2 (50.0) | ||
| Basal-like | 4 (80.0) | 1 (20.0) | ||
| T-stage | 1 | 27 (100) | 0 | < .01 |
| 2 | 24 (88.9) | 3 (11.1) | ||
| 3 | 4 (40.0) | 6 (60.0) | ||
| LNM | Absence | 36 (97.3) | 1 (2.7) | < .01 |
| Presence | 19 (70.4) | 8 (29.6) |
| Univariate |
p-value | Multivariate |
p-value | ||
|---|---|---|---|---|---|
| RR (95% CI) | RR (95% CI) | ||||
| Category | MUMPC | 1.0 | Reference | - | - |
| IMPC vs MUMPC | 1.2 (0.2–6.9) | .87 | - | - | |
| IDMPC vs MUMPC | 1.4 (0.2–8.4) | .71 | - | - | |
| NG | 2.5 (0.7–8.7) | .15 | - | - | |
| Mitosis | 1.9 (0.8–4.1) | .13 | - | - | |
| ER | 0.3 (0.1–1.2) | .09 | - | - | |
| PR | 0.2 (0.1–0.7) | .02 | 0.7 (0.1–6.6) | .78 | |
| HER2 | 7.3 (1.6–33.0) | .01 | 1.1 (0.1–11) | .92 | |
| Ki-67 | 4.2 (0.5–36.0) | .19 | - | - | |
| MS | LA | 1.0 | Reference | - | - |
| LB vs LA | 0.0 | .10 | - | - | |
| HER2 vs LA | 4.8 (0.6–41.0) | .15 | - | - | |
| Basal-like vs LA | 1.2 (0.1–13.0) | .88 | - | - | |
| T-stage | 15 (3.2–72.0) | .001 | 14 (2.1–88) | .006 | |
| LNM | 15 (1.8–130.0) | .01 | 9 (0.6–149) | .11 |
Values are presented as number (%). MUC, mucinous carcinoma without micropapillary feature; MUMPC, mucinous carcinoma with micropapillary feature; IMPC, invasive micropapillary carcinoma; IDMPC, mixed invasive ductal and micropapillary carcinoma; NG, nuclear grade; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; MS, molecular subtype; LA, luminal A; LB, luminal B; LNM, lymph node metastasis. Chi-square test; ANOVA test.
MUC, mucinous carcinoma without micropapillary feature; MUMPC, mucinous carcinoma with micropapillary feature; IMPC, invasive micropapillary carcinoma; IDMPC, mixed invasive ductal and micropapillary carcinoma; NG, nuclear grade; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; MS, molecular subtype; LA, luminal A; LB, luminal B; LNM, lymph node metastasis. Chi-square test; t test (2-tailed).
RR, relative risk; 95% CI, 95% confidence interval; MUMPC, mucinous carcinoma with micropapillary feature; IMPC, invasive micropapillary carcinoma; IDMPC, mixed invasive ductal and micropapillary carcinoma; NG, nuclear grade; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; MS, molecular subtype; LA, luminal A; LB, luminal B; LNM, lymph node metastasis.

 E-submission
E-submission