Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 53(4); 2019 > Article
-
Original Article
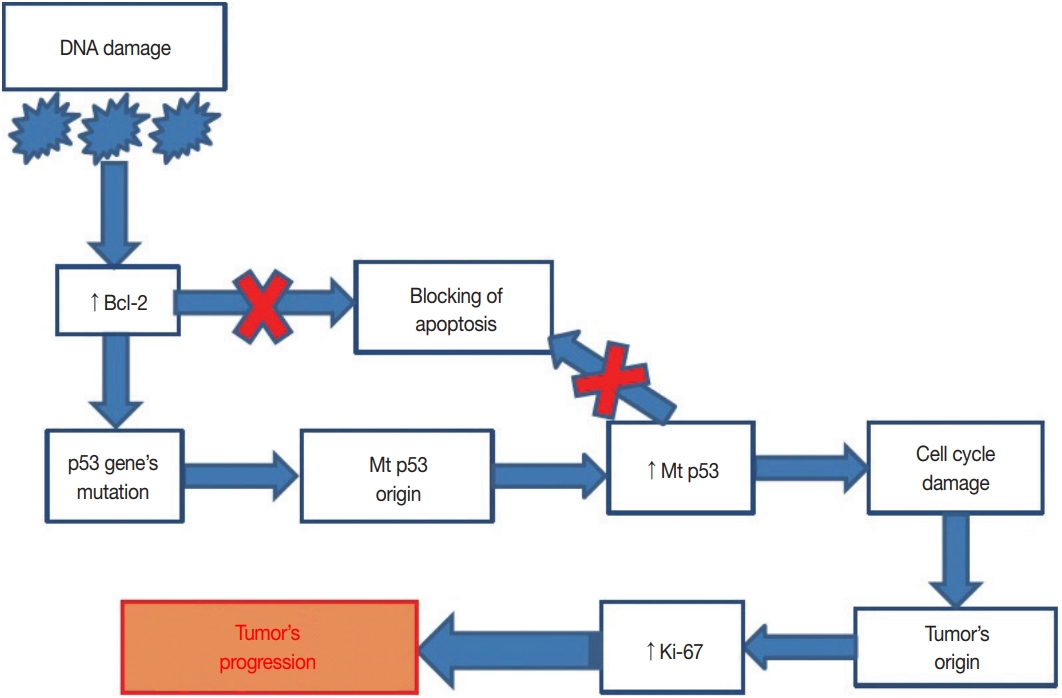
Serous Adenocarcinoma of Fallopian Tubes: Histological and Immunohistochemical Aspects -
Natalia Hyriavenko
 , Mykola Lyndin
, Mykola Lyndin , Kateryna Sikora1
, Kateryna Sikora1 , Artem Piddubnyi
, Artem Piddubnyi , Ludmila Karpenko
, Ludmila Karpenko , Olha Kravtsova1
, Olha Kravtsova1 , Dmytrii Hyriavenko2
, Dmytrii Hyriavenko2 , Olena Diachenko2
, Olena Diachenko2 , Vladyslav Sikora
, Vladyslav Sikora , Anatolii Romaniuk
, Anatolii Romaniuk
-
Journal of Pathology and Translational Medicine 2019;53(4):236-243.
DOI: https://doi.org/10.4132/jptm.2019.03.21
Published online: April 11, 2019
Department of Pathology, Sumy State University, Sumy, Ukraine
1Sumy Regional Clinical Perinatal Center, Sumy, Ukraine
2Sumy State University, Sumy, Ukraine
- Corresponding Author Natalia Hyriavenko, MD, PhD Department of Pathology, Sumy State University, st. Privokzalnaya, 31, m. Sumy 40022, Ukraine Tel: +380-997466578 Fax: +380-542334058 E-mail: n.gyryavenko@med.sumdu.edu.ua
© 2019 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Clinical and Morphological Analysis of Odontogenic Tumors and Tooth Developmental Anomalies
Yе.V. Kuzenko, S.M. Hermanchuk, O.O. Mykhno, D.H. Tsepochko, O.V. Kuzenko, A.Yu. Olishkevych
Kharkiv Dental Journal.2025; : 275. CrossRef - Rare non-serous fallopian tube cancers: institutional experience and literature review
Dmitrii Sumtsov, Georgyi Sumtsov, Nataliia Hyriavenko, Mykola Lyndin, Kateryna Sikora, Nataliia Kalashnik, Svitlana Smiian, Igor Gladchuk
Wiener Medizinische Wochenschrift.2024; 174(9-10): 199. CrossRef - UŞAQLIQ BORULARININ BİRİNCİLİ XƏRÇƏNGİ: DİAQNOSTİKASI VƏ MÜALİCƏSİNİN NƏTİCƏLƏRİ
D.G. Sumtsov, G.O. Sumtsov, N.I. Hyriavenko, S.A. Smiian, N.V. Kalashnyk, K.O. Sikora, N.M. Rozhkovska, I.Z. Gladchuk
Azerbaijan Medical Journal.2023; (4): 75. CrossRef - FEATURES OF ENDOMETRIUM STRUCTURE IN ALCOHOL-ABUSING HIV-INFECTED INDIVIDUALS
M. Lytvynenko
Inter Collegas.2021; 8(1): 52. CrossRef - Concurrent Clostridial Enteritis and Oviductal Adenocarcinoma with Carcinomatosis in an Adult Alpaca (Vicugna pacos)
Mandy Womble, Megan E. Schreeg, Allison Hoch, Enoch B. de Souza Meira, Derek Foster, Christopher Premanandan, Tatiane T. Negrão Watanabe
Journal of Comparative Pathology.2021; 189: 52. CrossRef - Problems of primary fallopian tube cancer diagnostics during and after surgery
D.G. Sumtsov, I.Z. Gladchuk, G.O. Sumtsov, N.I. Hyriavenko, M.S. Lyndin, V.V. Sikora, V.M. Zaporozhan
REPRODUCTIVE ENDOCRINOLOGY.2021; (59): 66. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



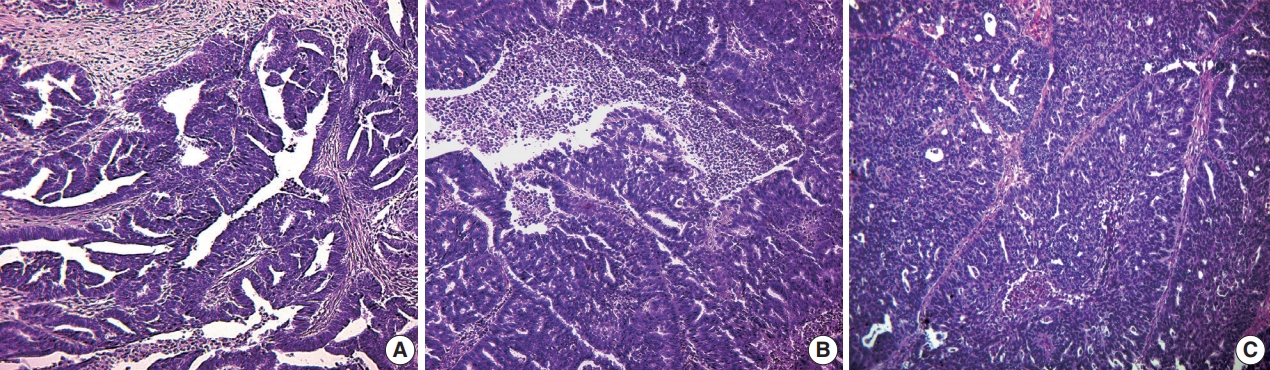
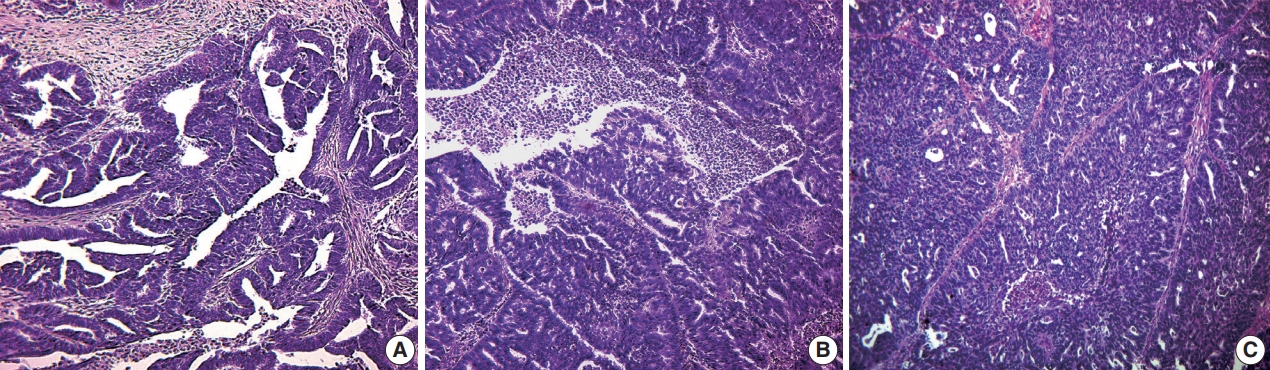
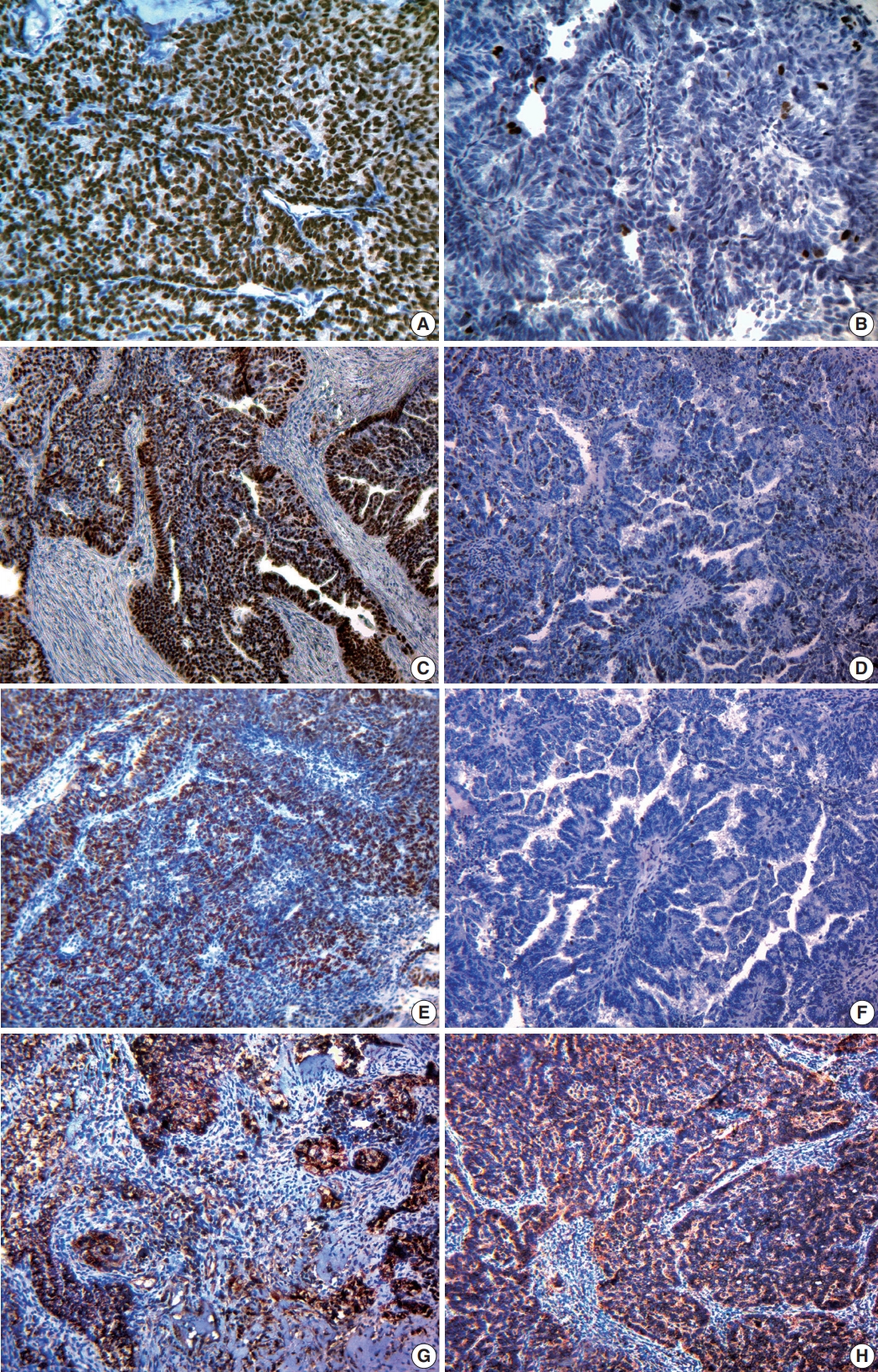
Fig. 1.
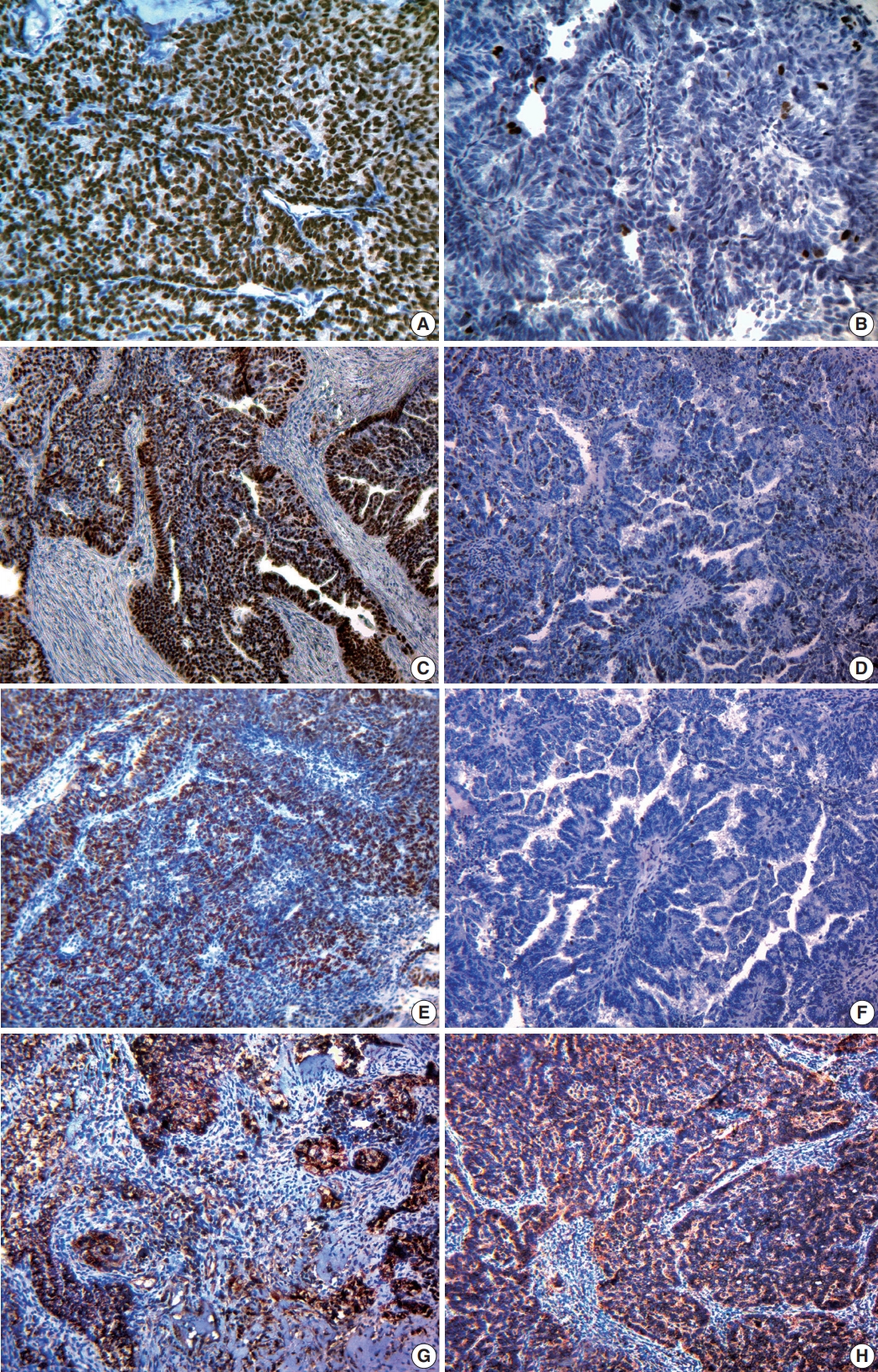
Fig. 2.
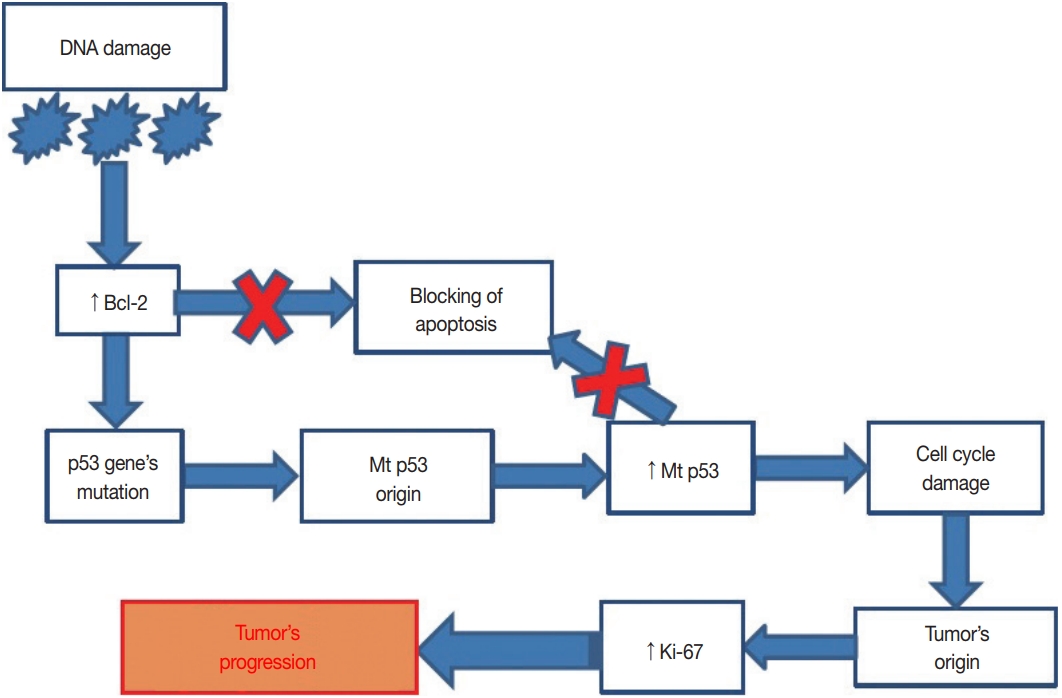
Fig. 3.
| Antibody | Immunized animal | Clone | Dilution | Pattern | Evaluation features |
|---|---|---|---|---|---|
| ER | Rabbit | SP1 | 1:200 | Nucleus | According to the recommendations of D.C. Allred and taking into account the proportion of colored nuclei and the intensity of their staining |
| PR | Rabbit | YR85 | 1:150 | Nucleus | |
| HER2/neu | Rabbit | SP3 | 1:100 | Membrane | In points (from 0 to 3), according to the manufacturer’s recommendations and taking into account the completeness and intensity of membrane staining |
| Ki-67 | Rabbit | SP6 | 1:100 | Nucleus | 0 point: negative reaction |
| 1 point: weakly positive reaction (n = 0%–30%) | |||||
| 2 points: moderately positive reaction (30% < n < 60%) | |||||
| 3 points: strong positive reaction (n > 60%) | |||||
| p53 | Mouse | SP5 | 1:100 | Nucleus | Weak reaction (10–25% of positively stained tumor cells), moderate positive |
| Bcl-2 | Mouse | 100/D5 | 1:100 | Cytoplasm | Weak expression (1+): weak cytoplasmic staining of more than 10% of tumor cells |
| Moderate (2+): staining of average intensity more than 10% | |||||
| Strong (3+): intense staining of more than 10% of tumor cells | |||||
| VEGF | Rabbit | Polyclon | 1:200 | Cytoplasm and membrane | 0 points: absence of cytoplasmic expression |
| 1 point: weak cytoplasmic staining less than 10% of cells | |||||
| 2 points: weak or moderate expression in 10%–50% of cells | |||||
| 3 points: strong or moderate expression in more than 50% of cells |
| Stage | No. of patients with PCFT (%) |
|---|---|
| 0 | - |
| I | 24 (33.8) |
| IA–T1aN0M0 | 5 (7.0) |
| IB–T1bN0M0 | 8 (11.3) |
| IC–11cN0M0 | 11 (15.5) |
| II | 19 (26.8) |
| IIA–T2aN0M0 | 10 (14.1) |
| IIB–T2bN0M0 | 3 (4.2) |
| IIC–T2cN0M0 | 6 (8.5) |
| III | 28 (39.4) |
| IIIA–T3aN0 M0 | 2 (2.8) |
| IIIB–T3bN0 M0 | 5 (7.0) |
| IIIC–T3c N0M0 | 2 (2.8) |
| IIIC–T1-3N1M0 | 19 (26.8) |
| IV–T1-3N0-1M1 | - |
| Total | 71 (100) |
| Average index of proliferation (%) | No. of female patients with SAcFT (%) |
|---|---|
| 0 | 0 |
| 0–30 | 12 (18.2) |
| 30–60 | 18 (27.3) |
| > 60 | 36 (54.5) |
| Evaluation’s criteria | Average No. of p53 (%) |
|---|---|
| Disease stage according to FIGO classification | |
| I | 10.5 ± 2.2 |
| II | 46.8 ± 5.2 |
| III | 71.7 ± 3.9 |
| Presence of lesion in regional lymph nodes | |
| N0 | 29.7 ± 3.6 |
| N1 | 80.6 ± 2.7 |
| Degree of tumor differentiation | |
| G1 | 5.6 ± 2.4 |
| G2 | 35.6 ± 6.2 |
| G3 | 62.2 ± 4.2 |
| Evaluation’s criteria | No. of Bcl-2–positive tumor cases |
|||
|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | |
| Disease stage according to FIGO classification | ||||
| I (n = 23) | - | 1 | 4 | 18 |
| II (n = 16) | 3 | 2 | 5 | 6 |
| III (n = 27) | 8 | 4 | 9 | 6 |
| The presence of lesion in regional lymph nodes | ||||
| N0 (n = 47) | 2 | 5 | 12 | 28 |
| N1 (n = 19) | 9 | 2 | 6 | 2 |
| Degree of tumor differentiation | ||||
| G1 (n = 10) | - | - | - | 100 |
| G2 (n = 23) | 3 | - | 5 | 15 |
| G3 (n = 33) | 8 | 7 | 13 | 5 |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor; VEGF, vascular endothelial growth factor.
PCFT, primary cancer of the fallopian tubes; FIGO, International Federation of Gynecology and Obstetrics.
SAcFT, serous adenocarcinoma of the fallopian tubes.
SAcFT, serous adenocarcinoma of the fallopian tubes; FIGO, International Federation of Gynecology and Obstetrics.
SAcFT, serous adenocarcinoma of the fallopian tubes; FIGO, International Federation of Gynecology and Obstetrics.

 E-submission
E-submission